PHY 335 Spring 2011 QUIZ #6 Assignment: due on Wednesday (9
advertisement

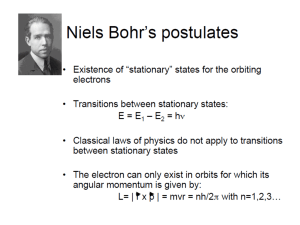
PHY 335 Spring 2011 QUIZ #6 Assignment: due on Wednesday (9:59pm), 6 April 2011. 1. The spectral lines of atomic hydrogen are given by the Balmer-Rydberg formula. a) Calculate the five longest wavelengths of the Lyman series. Mark corresponding transitions on the energy diagram of H-atom. b) Prove that the successive lines in the series get closer and closer together, approaching a definite limit (the series limit) as n∞. Find the wavelength of the series limit for the Lyman series. 2. Problem 39.40. 3. A hydrogen atom is in its ground state (n=1). Using the Bohr theory of the atom, calculate (a) the radius of the orbit, (b) the linear momentum of the electron, (c) the angular momentum of the electron, (d) the kinetic energy, (e) the potential energy, (f) the total energy. 4. Calculate the frequency of the photon emitted by a hydrogen atom making a transition from the n=4 to the n=3 state. Compare your result with the frequency of revolution for the electron in these two Bohr orbits. 5. The negative muon is a subatomic particle with the same charge as the electron but a mass that is about 207 times greater: mµ ≈ 207 me. A muon can be captured by a proton to form a “muonic hydrogen atom”, with radius and energy given by the Bohr model (except me has to be replaced by mµ) a) What is the radius and energy of the first Bohr orbit in a muonic hydrogen atom? b) What is the wavelength of the Lyman α line in muonic hydrogen? What sort of electromagnetic radiation is this? (Visible? IR? etc) 6. Problem 40.36. 7. Problem 40.38.