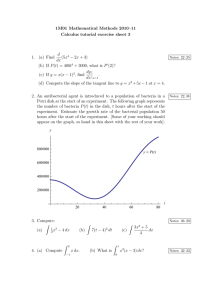
Detecting atmospheric pollutants: sulfur dioxide Supporting Practical Science, D&T and Art
advertisement
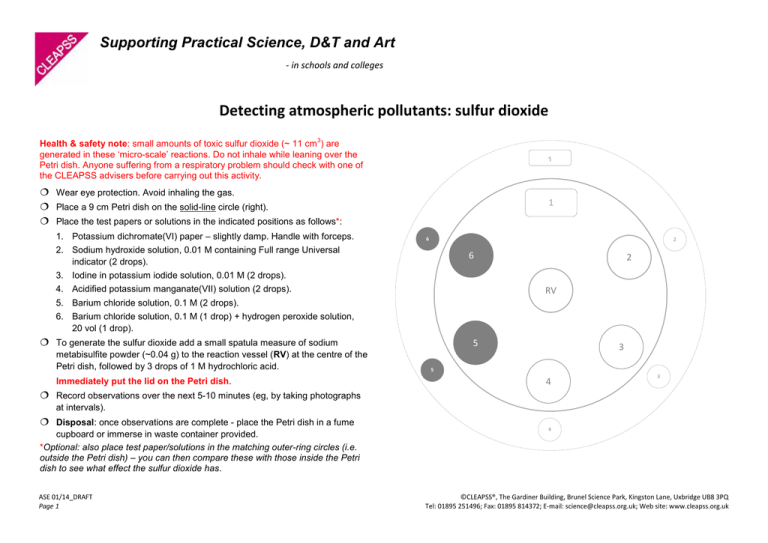
Supporting Practical Science, D&T and Art - in schools and colleges Detecting atmospheric pollutants: sulfur dioxide 3 Health & safety note: small amounts of toxic sulfur dioxide (~ 11 cm ) are generated in these ‘micro-scale’ reactions. Do not inhale while leaning over the Petri dish. Anyone suffering from a respiratory problem should check with one of the CLEAPSS advisers before carrying out this activity. 1 ¦ Wear eye protection. Avoid inhaling the gas. ¦ Place a 9 cm Petri dish on the solid-line circle (right). ¦ Place the test papers or solutions in the indicated positions as follows*: 1 1. Potassium dichromate(VI) paper – slightly damp. Handle with forceps. 6 2 2. Sodium hydroxide solution, 0.01 M containing Full range Universal indicator (2 drops). 6 2 3. Iodine in potassium iodide solution, 0.01 M (2 drops). RV 4. Acidified potassium manganate(VII) solution (2 drops). 5. Barium chloride solution, 0.1 M (2 drops). 6. Barium chloride solution, 0.1 M (1 drop) + hydrogen peroxide solution, 20 vol (1 drop). 5 ¦ To generate the sulfur dioxide add a small spatula measure of sodium metabisulfite powder (~0.04 g) to the reaction vessel (RV) at the centre of the Petri dish, followed by 3 drops of 1 M hydrochloric acid. Immediately put the lid on the Petri dish. 3 5 4 3 ¦ Record observations over the next 5-10 minutes (eg, by taking photographs at intervals). ¦ Disposal: once observations are complete - place the Petri dish in a fume cupboard or immerse in waste container provided. *Optional: also place test paper/solutions in the matching outer-ring circles (i.e. outside the Petri dish) – you can then compare these with those inside the Petri dish to see what effect the sulfur dioxide has. ASE 01/14_DRAFT Page 1 4 ©CLEAPSS®, The Gardiner Building, Brunel Science Park, Kingston Lane, Uxbridge UB8 3PQ Tel: 01895 251496; Fax: 01895 814372; E-mail: science@cleapss.org.uk; Web site: www.cleapss.org.uk