THE CHIRAL SWITCH: THE DEVELOPMENT OF SINGLE ENANTIOMER DRUGS FROM RACEMATES
advertisement

ACTA FACULTATIS PHARMACEUTICAE UNIVERSITATIS COMENIANAE
Tomus L 2003
THE CHIRAL SWITCH:
THE DEVELOPMENT OF SINGLE ENANTIOMER DRUGS
FROM RACEMATES
1
Hutt, A.J. − 2Valentová, J.
1
Department of Pharmacy, King´s College London, London, UK
Department of Chemical Theory of Drugs, Faculty of Pharmacy,
Comenius University, Bratislava
2
Over the last ten to fifteen years drug chirality, particularly the use of single enantiomers
versus racemic mixtures, has become an area of considerable interest. As a result of advances in
the chemical technologies associated with the synthesis, separation and analysis of the individual
enantiomers present in a racemate, together with regulatory requirements the number of chiral
drugs presented for approval to regulatory authorities as single enantiomers rather than racemates
has increased. In addition to new chemical entities a number of „old“ racemates have been reevaluated as potential single enantiomer products with the possibility for an improved therapeutic
profile. These so-called Chiral Switches have resulted in a number of agents being commercially
available as both single enantiomer and racemic mixtures at the same time. However, not all these
re-evaluations have resulted in the expected therapeutic benefits and unpredicted adverse
reactions have resulted. The issues, both economic and therapeutic, associated with the Chiral
Switch process are addressed in this article.
Key words: chiral switch − stereoisomers − racemates − enantiomers
INTRODUCTION
In recent years there has been considerable interest in the biological activity, both
pharmacological and toxicological, of the enantiomers of chiral drugs. This interest in
drug stereochemistry has resulted from the considerable advances in the synthesis [1,2],
analysis and separation [3-9] of chiral molecules, together with an increased
appreciation of the potential significance of the differential biological properties of the
enantiomers of chiral drugs administered as racemates [10-16]. As a result of these
advances in technology and the potential benefits of single enantiomer drugs
7
(summarized in Table 1), drug stereochemistry became an issue for the pharmaceutical
industry and the regulatory authorities [17-20]. A number of scientific meetings,
involving academic, industrial and regulatory scientists, were held in the late 1980s –
early 1990s with the specific objective of discussing the new technologies and the
significance of chirality in pharmacology and therapeutics [21-23].
Table 1. Potential advantages of single enantiomer products [16]
Less complex, more selective pharmacodynamic profile
Potential for an improved therapeutic index
Less complex pharmacokinetic profile
Reduced potential for complex drug interactions
Less complex relationship between plasma concentration and effect
In 1992 the Food and Drug Administration (FDA) in the USA published a policy
statement for the development of new stereoisomeric drugs [24], which was closely
followed by European guidelines in 1993, which came into force in 1994 [25]. At
present there is no absolute requirement from any of the major regulatory authorities for
the development of single enantiomer drugs and the decision regarding the
stereoisomeric form, i.e. single enantiomer or racemic mixture, to be developed is left to
the compound sponsor. However, the decision taken requires scientific justification
based on quality, safety and efficacy, together with the risk-benefit ratio and may be
argued on a case-by-case basis [25].
As a result of regulatory attitudes, and associated technological developments the
number of new chiral chemical entities as single stereoisomers, rather than racemic
mixtures, submitted for approval to various regulatory bodies over the last ten years has
increased and the trends for future drug development are evident [25,26]. However, in
addition to new agents a number of established drugs marketed as racemates, have been
re-evaluated and re-marketed as single enantiomers [27]. As a result both single
enantiomer and racemic mixture products of the same „drug“ are both available in
a number of countries. It is obviously important that both pharmacists and physicians
are aware that both single stereoisomer and mixture products are available and that they
are able to compare their relative merits. The aim of this article is to examine these
so-called Chiral Switches.
THE CHIRAL SWITCH
A racemic or chiral switch may be defined as the development of a single
enantiomer from a previously marketed racemate [27]. Frequently the marketed single
enantiomers have the same, or very similar, therapeutic indications as the originally
marketed racemate but this may not always be the situation and novel indications for
8
„old“ compounds have been reported. The chiral switch process has resulted in
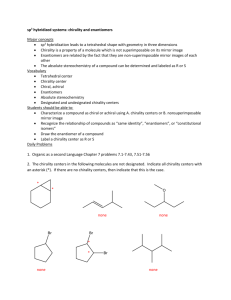
a number of agents being re-marketed as single enantiomer products (Fig. 1) and a
summary of these agents, together with their reported therapeutic advantages, is
presented in Table 2.
O
F
COOH
N
CH3
(CH 3)2CHCH2
N
N
O
H
CH 3
H
COOH
CH 2NHC(CH 3)3
(R)-Salbutamol
[levalbuterol]
Dexibuprofen
[(S)-ibuprofen]
CH3
H
OH
HO
CH3
Levofloxacin
[(S)-ofloxacin]
H
HOCH 2
NC
Cl
O
COOH
NHCH 3
O
CH2CH 2CH2N(CH 3)2
O
F
Dexketoprofen
[(S)-ketoprofen]
CH 3
(S)-Ketamine
N
H
C 4H 9
CH 3
N
H
COOCH 3
H
NH
Levobupivacaine
[(S)-bupivacaine]
CH3 O
N
H
CH 2CH2 OCH2 COOH
N
H
NHCO
CH3 O
Escitalopram
[(S)-citalopram]
Cl
(R,R)-Methylphenidate
Levocetirizine
[(R)-cetirizine]
OCH3
CH 3
CH 3
CH 2CH2 COO[CH2]5OCOCH2 CH 2
CH 2
N
CH 2
H
OCH3
CH3O
O
N
CH3
S
N
H
CH2
OCH3
N
OCH 3
OCH 3
OCH 3
2C6H5SO3
CH 3
OCH3
Cisatracurium
[(1R,2R,1'R,2'R)-atracurium besylate]
Esomeprazole
[(S)-omeprazole]
Fig. 1. Marketed single enantiomers of agents which have undergone the chiral switch
The idea of investigating single stereoisomers following the observation of
unacceptable adverse effects with the racemate, or developments in technology enabling
the production of a single stereoisomer is not new. For example D-penicillamine,
introduced originally for the treatment of Wilson´s disease [28] has been used by
rheumatologists for number of years [29]. The toxicity of the L-enantiomer to animals,
including weight loss, intermittent fitting and death, has been known since the 1950s
9
[29] and, more recently the greater mutagenic potency of L- compared to D-penicillamine has been reported [30]. In the initial clinical evaluation of the drug for the
treatment of Wilson´s disease in the USA, the use of the synthetic racemate resulted in
optic neuritis [31] and the drug was withdrawn. In the UK, D-penicillamine was
obtained as the single enantiomer, by the hydrolysis of penicillin, and the adverse effect
was not observed [32].
Similarly the initial use of racemic dopa for the treatment of Parkinson´s disease
resulted in a number of adverse effects, including nausea, vomiting, anorexia,
involuntary movements and granulocytopenia [33]. The use of L-dopa resulted in
halving the required dose, a reduction in adverse effects, granulocytopenia was not
observed with the single enantiomer, and an increased number of improved patients
[34].
The progestogen norgestrel, used as an oral contraceptive and in hormone
replacement therapy, the activity of which resides in the laevorotatory enantiomer was
initially marketed as a racemate in the mid-1970s. Subsequently, following
developments in the synthetic methodology, the single enantiomer levonorgestrel was
marketed in 1979. Both the single enantiomer and racemate are commercially available
and used either alone or as components of combination products.
When single enantiomers are developed from previously marketed racemates the
regulatory bodies permit bridging studies between the original and new submission [25].
Obviously potential difficulties may arise if the sponsor of the single enantiomer was
not responsible for the original submission [25]. These investigations should include
a comparison of the pharmacokinetic profile of the single enantiomer following
administration as such and as a component of the racemate. Such studies ensure that
interactions between the enantiomers present in the racemate do not occur resulting in
differences in the pharmacokinetic parameters of the selected stereoisomer. For example
in the case of citalopram the pharmacokinetic profile of both the drug and the desmethyl
metabolite of the S-enantiomer (escitalopram) have been shown to be bioequivalent
following oral administration of tablet formulations containing 40 mg of the racemate or
20 mg of escitalopram [35]. Similarly toxicological bridging studies in animals with
both the single enantiomer and racemate showed a similar profile and the data derived
with the racemate could be extrapolated to the stereoisomer [36].
In addition to the agents indicated in Table 2 a number of other compounds are
undergoing evaluation as single enantiomer products, some of which are at a fairly
advanced stage of development [27]. These include: (R,R)-formoterol for the treatment
of asthma with the reported advantage of reduced airway hyperreactivity; (S)-oxybutinin, for the treatment of urinary incontinence with a reduction in anticholingeric
side effects [37,38]; (S)-doxazosin for use in benign prostatic hyperplasia with the
advantage of a reduction in orthostatic hypotension; (S)-lansoprazole and (-)-pantoprazole for the treatment of gastro-oesophageal reflux; (+)-norcisapride for the
treatment of nocturnal heartburn with a reduction in cardiotoxicity; eszopiclone for the
treatment of insomnia; (S)-amlodipine for the treatment of hypertension with the
advantage of a reduction in side effects; (S)-fluoxetine for use in migraine prophylaxis,
phase II clinical trials indicating a reduction in attack frequency earlier and greater in
comparison to placebo controls [39].
10
Table 2. Racemate to single enantiomer: the chiral switch
Drug
Dexketoprofen
Dexibuprofen
Esomeprazole
Levofloxacin
Action/Indication
Comment
Non-steroidal
Inhibition of cyclooxygenase activity resides in the
anti-inflammatory drug S-enantiomer, unlike (R)-ibuprofen, (R)-ketoprofen undergoes minimal chiral inversion in humans
(less than 10%) [76]. Reduced dose requirement in
comparison to the racemate; formulation as the
trometamol salt results in more rapid absorption
and onset of action compared to the racemic free
acid; reduced potential for gastric ulceration in
animals [76].
Non-steroidal
Main activity, inhibition of cyclooxygenase,
anti-inflammatory drug resides predominantly in the S-enantiomer [72].
Following administration of the racemate (R)-ibuprofen undergoes partial chiral inversion
(approximately 60%) to the active S-enantiomer
[73]. A statistical evaluation of six clinical trials
involving a variety of conditions, including
backpain, joint pain, rheumatoid arthritis and
ankylosing spondylitis, indicated that treatment
with the single enantiomer (1200 mg daily) to be
equivalent to, or better than, the racemate (2400
mg daily) [74]. The mean daily dose of
dexibuprofen in patients with rheumatoid arthritis
was reduced by approximately one third compared
to the racemate [75]. A post marketing
surveillance study in 1400 patients has indicated
that dexibuprofen is a highly effective NSAID
with a low adverse effect profile [102]. Single
enantiomer preparations are available in Austria
and Switzerland.
Proton pump inhibitor S-Enantiomer of omeprazole; lower first pass
metabolism, slower plasma clearance and
increased systemic availability compared to the R-enantiomer [65]. Clinical evidence that the single
enantiomer maintains intragastric pH above 4 in
patients with gastro-oesophageal reflux disease
significantly longer, with a 24 median intragastric
pH greater than an equal dose of the racemate
[65,86,87]. Reduction in interpatient variability in
response [88].
Antimicrobial
Quinolone derivative the activity of which resides
in the S-enantiomer [67-70] modest differences in
enantiomeric disposition favour the single
enantiomer [70,71].
11
Levobupivacaine Local anaesthetic
Escitalopram
Selective serotonin
reuptake inhibitor
(S)-Ketamine
Anaesthetic
Levocetirizine
H1-Antihistamine
12
Enantiomers of bupivacaine exhibit stereoselectivity with respect to blockade of sodium and
potassium ion channels, the R-enan-tiomer being
more potent [77,78]. The cardiotoxicity of the drug
appears to be predominantly associated with the Renantiomer, in vitro investigations have indicated
smaller conduction changes following treatment
with the S-enantiomer in comparion to either (R)-bupivacaine or the racemate [79]. Following
intravenous infusion of the racemate or S-enantiomer to healthy volunteers a significantly reduced
negative inotropic effect (approximately half) was
observed with the single enantiomer compared to
the mixture [80]. Clinical studies have indicated
that sensory block and the clinical profile
following the single enantiomer are essentially the
same as the racemate [81]. Thus, the single
enantiomer results in a similar clinical profile with
a reduction in cardiotoxicity [82].
S-Enantiomer of citalopram a potent selective
serotonin reuptake inhibitor which in in vitro test
systems is between 130 and 160 fold more potent
than the R-enantiomer [94]. Clinical studies in
depressed patients have indicated as much
improvement with 10 mg daily of the single
enantiomer as achieved following 40 mg daily of
the racemate (similar trends were observed with 20
mg daily of the single enantiomer) [95], together
with a faster onset of action, reduction in side
effects and improved tolerability profile [95,96].
The single enantiomer is available in the UK and
USA.
(S)-Ketamine has a greater analgesic and
anaesthetic potency than the R-enantiomer in both
animals and man [83,84]; post anaesthetic
emergence reactions (hallucinations and agitation)
are predominantly associated with the R-enantiomer [84,85]. The single S-enantiomer is available
in Germany [85].
R-Enantiomer of cetirizine; Ki values against H1-receptors 3.2 and 6.3 nM for the R-enantiomer
and racemate respectively. Clinical studies have
indicated the equivalence of a 2.5 mg dose of the
single enantiomer compared to 5 mg of the
racemate, the S-enantiomer being essentially
inactive [97,98].
Cisatracurium
(R)-Salbutamol
[levalbuterol]
(R,R)Methylphenidate
Neuromuscular blocker Developed from atracurium, a compound
containing four chiral centres in its structure which
due to its symmetrical nature exists as a mixture of
ten stereoisomeric forms [89]; cisatracurium, the
1R,2R,1’R,2’R-stereoisomer comprises approximately 15 % of the mixture [90,91]. The single
stereoisomer is approximately three fold more
potent than the mixture, with a slightly slower
onset of action and reduced histamine releasing
properties [90,91]. As a result of the lower dose
requirement of the single stereoisomer the
formation of laudanosine (a metabolite reported to
induce seizures in animals) is reduced compared to
atracurium [91].
Racemic and (S)-salbutamol induce airway
β2-agonist
hyperresponsiveness in sensitised animals; use of
the racemate is associated with some loss of
bronchodilator potency, decreased protection
against bronchoprovocation and increased sensitivity to allergen challenge and bronchoconstrictor
stimuli [92]. Studies in humans have indicated that
inhalation of (R)-salbutamol produces significantly
greater bronchodilatation than the equivalent dose
of the racemate [93]. The single enantiomer is
available in the USA
Attention-deficit
The R,R-enantiomer is approximately ten fold
hyperactivity disorder more potent than (S,S)-methylphenidate in the
inhibition of dopamine and noradrenaline into
striatal and hypothalamic synaptosomes respectively [99]. The drug undergoes enantioselective
disposition, due to presystemic metabolism, the
absolute bioavailability being ∼0.23 and ∼0.05 for
the R,R- and S,S-enantiomers respectively [100].
Reported to be equally effective as the racemate at
half-the-dose with the advantages of a more rapidonset of action and possible improved side effect
profile [101]. Available in the USA.
The activity of racemic sibutramine, a monoamine reuptake inhibitor used for the
treatment of obesity, is associated with the formation of both its desmethyl and
didesmethyl metabolites. Examination of the pharmacological activity of the
enantiomers of the metabolites in vitro has indicated the greater potency of the
R- compared to the S-enantiomers and the racemic drug, in the inhibition of
noradrenaline, serotonin and dopamine uptake. Also, in vivo studies have revealed the
greater anorexic potency and locomotor activity of the R-enantiomer metabolites in rats
[40]. Interestingly, the in vivo investigations indicated that the anorexic effects could be
dissociated from the locomotor activity. These observations have resulted in the
suggestion that single enantiomer versions of the metabolites could provide safe and
13
effective treatments for both obesity and depression [40]. According to the Sepracor
website (http://www.sepracor.com) one of the R-metabolites (which is not specified) is
undergoing evaluation for the treatment of refractory depression, whereas an S-enantiomer metabolite (again not specified) is being evaluated for treatment of sexual
dysfunction [41].
The R-enantiomer of the racemic nonsteroidal anti-inflammatory drug flurbiprofen
has been evaluated in clinical trials for the treatment of late-stage prostate cancer [42].
This trial followed investigations indicating the activity of the single enantiomer in
animal models of prostate (TRAMP mouse) and colon (Min mouse) cancer [43,44].
Additionally, recent evidence has indicated the potential of NSAIDs for the prevention
of Alzheimer’s disease [45,46]. As (R)-flurbiprofen is essentially inactive with respect
to inhibition of cyclooxygenases 1 and 2, it has been suggested that it may be a useful
candidate for clinical development [47] without the gastrointestinal side effects
observed with the racemic drug [48,49]. An Investigational New Drug Application has
recently been submitted to the US FDA, by Encore Pharmaceuticals, in order to start
clinical trials with (R)-flurbiprofen for the treatment and prevention of Alzheimer’s
disease. In both the above examples the evaluation of the single enantiomers has the
potential to provide new therapeutic indications for „old“ drugs.
Such re-evaluations of single enantiomers are not without problems and examples
may be cited where removal of the so-called isomeric impurity has not resulted in the
supposed
or unexpected adverse reactions have resulted. The
advantages
being
realised
-blocking R,R-stereoisomer, named dilevalol, of the
"!$#&%'(*) + - ,-.0/ -blocking drug labetalol was terminated due to adverse effects
associated with hepatotoxicity [50,51]. Sotalol is a 12*34563879
7&:4;43<=6>4@? -blocking
A*BCDEFHGEIKJ*LAMNMPOQOROSADE=GATRT=UEIVGJA*JEWGYXGEU[Z]\
^_a`cbdI&Ce -blocking activity resides
predominantly in the (-)-enantiomer, with (+)-sotalol being 14 to 50 fold less active
depending on the test system used , whereas the enantiomers are equipotent with respect
to their antiarrhythmic activity [52,53]. The (+)-enantiomer was evaluated in patients
with depressed ventricular function following myocardial infarction in the SWORD trial
(Survival With Oral d-Sotalol) [54]. The investigation was terminated prematurely due
to increased mortality in the drug treatment group compared to the placebo control
group [54]. It has been proposed that the combinAE=Gf
Dgfhie -blockade and class III
antiarrhythmic activity present in the racemate provides a more effective therapy than
the class III antiarrhythmic activity alone [51]. More recently the development of (R)-fluoxetine was terminated due to a small, but significant increase in QTc prolongation
during clinical evaluation at the highest dose level examined [55,56]. The above
examples indicate that the removal of the isomeric ballast present in a racemate is not a
trivial matter and additionally may have considerable financial consequences (see
below).
An additional point associated with these single enantiomer „failures“ is that
racemic mixture formulations are still available and widely used. In the present
regulatory climate with respect to chiral drugs if the single enantiomers had been
developed initially and „failed“ during development it is highly unlikely that any major
pharmaceutical company would have attempted to re-evaluate and develop the
racemates. Thus, therapeutically useful agents would have been lost. Similarly it is
possible that therapeutically useful compounds may have been lost as the racemates
14
were evaluated and thought to be unsuitable for development, whereas their individual
enantiomers may have had pharmacologically useful properties.
In addition to the single enantiomer „failures“ outlined above there is also an
instance where following the successful launch of a single stereoisomer both it, and the
original racemate, were withdrawn some years later. The anorectic agent fenfluramine
was one of the first compounds to undergo the chiral switch process with the marketing
of the single S-enantiomer, dexfenfluramine. Following an association of the drug with
valvular heart disease, initially involving the fenfluramine-phentermine combination
(the so-called fen-phen combination) in the USA [57] and the rare but serious risk of
pulmonary hypertension, both the single enantiomer and racemate were voluntarily
withdrawn worldwide in September 1997 [57,58].
ECONOMIC CONSIDERATIONS
The global market for pharmaceuticals is enormous, estimated to be worth
approximately $ 300 billion US in 1997 [59]. However, over the next five years the
patents on a number of drugs, the market worth of which is quoted as $ 40 billion, are
due to expire [60]. There is also evidence that the rate of development of new agents is
declining [60-62], for example the US FDA approved only 28 new chemical entities in
2001 which is reported to be the lowest number for 30 years [60]. The situation is
compounded by the fact that research and development costs are increasing, having
doubled over the last fifteen years [61], and were estimated to be between $ 300 and
400 million in 1997 [63]. The current cost estimates of bringing a drug to market are of
the order of $ 600 million [60] and may be as high as $ 800 million [61]. Additionally,
drug development time is now so long that the average effective patent life of a „new“
agent is only 10-12 years [60].
As a number of commercially highly successful drugs are chiral the economic
significance of stereochemistry to the pharmaceutical industry is obvious. The chiral
switch process provides a strategy to extend the profitable life of a pharmaceutical
„bestseller“, and may result in extended patent protection and provide an advantage
against generic competition.
In some instances it could be argued that the development of a single stereoisomer
from a racemate would not result in a significant therapeutic advantage [59]. In the case
of omeprazole, the biggest selling drug worldwide in 1997, sales the USA alone being
greater than $ 5 billion [59], there is some controversy regarding the relative merits of
the single enantiomer [64]. The enantiomers of omeprazole, and the related proton
pump inhibitors, lansoprazole and pantoprazole, have been reported to be essentially
equipotent both in in vitro test systems and in vivo in the rat [64]. As these agents
undergo acid catalysed transformation into an achiral cyclic sulphenamide which then
covalently binds with the proton pump it is difficult to see a pharmacodynamic rationale
for the development of a single enantiomer [64]. An additional complication in the case
of esomeprazole/omeprazole is that a number of clinical studies compare 40 mg doses
of the single enantiomer with a 20 mg dose of the racemate which makes comparison
between the two agents difficult. However, as pointed out in Table 2, there are
15
pharmacokinetic differences between the enantiomers and some clinical studies, based
on equal doses, do indicate potential therapeutic advantages of the single enantiomer
[65].
There are however financial risks associated with the development of single
enantiomers from racemates and it has been estimated that the research and
development costs of dilevalol were $ 100 million [66]. Similarly the recent termination
of the licensing agreement between Eli Lilly and the specialist chiral chemical
company Sepracor, for the development of (R)-fluoxetine, resulted in the latter
company receiving only $ 23 million of what was to have been a $ 90 million deal,
additionally the stock value of Sepracor fell by 28% [56]. Such costs within the industry
may be regarded as relatively modest in comparison to the cost associated with taking a
novel therapeutic agent to market, particularly as by the nature of the process the
possibility of success is that much greater.
CONCLUDING COMMENT
This article has attempted to provide an overview of the current situation regarding
the Chiral Switches. Re-evaluation of the enantiomers of racemic drugs will
undoubtedly continue and, in some instances, result in the introduction of single
enantiomer versions of established drugs. Hopefully, such re-evaluations will provide
agents with a “cleaner” pharmacological profile, improved safety and efficacy, and
ultimately therapeutic benefits in addition to commercial advantages. Since the
completion of this article a comprehensive review of the Chiral Switch, including a
discussion of the patentability and intellectual property situation associated with the
development of single stereoisomers from racemates has been published [41]. The
attention of interested readers is also drawn to the articles by Agranat and Caner [103]
concerning intellectual property and drug stereochemistry and that of Strong [104]
which reviews the FDA policy on stereoisomer regulation and associated exclusivity
law.
Acknowledgements: The financial support provided by a joint grant from the British Council,
Slovakia and Comenius University is gratefully acknowledged. The authors thank Professor F.
Devínsky of the Faculty of Pharmacy, Comenius University for this continued support and
encouragement.
REFERENCES
111. COLLINS, A.N. – SHELDRAKE, G.N. – CROSBY, J.: Chirality in Industry. New York,
Wiley 1992.
112. COLLINS, A.N. – SHELDRAKE, G.N. – CROSBY, J.: Chirality in Industry II. New York,
Wiley 1997.
113. KRSTULOVIC, A.M.: Chiral Separations by HPLC. Chichester, Ellis Horwood 1989.
114. WAINER, I.W.: Drug Stereochemistry. Analytical Methods and Pharmacology. 2nd Edition.
New York, Marcel Dekker 1993.
16
115. SUBRAMANIAN, G.: A Practical Approach to Chiral Separations by Liquid
Chromatography. Weinheim, VCH 1994.
116. CHANKVETADZE, B.: Capillary Electrophoresis in Chiral Analysis. Chichester, Wiley
1997.
117. ABOUL-ENEIN, H.Y. – WAINER, I.W.: The Impact of Stereochemistry on Drug
Development and Use. New York, Wiley, 1997.
118. SUBRAMANIAN, G.: Chiral Separation Techniques. A Practical Approach. 2nd Edition.
Weinheim, Wiley-VCH 2001.
119. CHALLENER, C.A.: Chiral Drugs. Aldershot, Ashgate 2001.
110. ARIËNS, E.J. – SOUDIJN, W. – TIMMERMANS, P.B.H.W.H.: Stereochemistry and
Biological Activity of Drugs. Oxford, Blackwell 1983.
111. SMITH, D.F.: Handbook of Stereoisomers: Therapeutic Drugs. Boca Raton, CRC Press
1989.
112. CROSSLEY, R.: Chirality and the Biological Activity of Drugs. Boca Raton, CRC Press
1995.
113. EICHELBAUM, M. – GROSS, A.S.: Stereochemical aspects of drug action and disposition.
In: Advances in Drug Research, Vol. 28. (TESTA, B. – MEYER, U.A. – eds.). London,
Academic Press, 1996, p. 1-64.
114. HUTT , A.J. – TAN, S.C.: Drug chirality and its clinical significance. Drugs 52 (Suppl 5),
1996, p. 1-12.
115. HUTT, A.J.: Drug chirality and its pharmacological consequences. In : Introduction to the
Principles of Drug Design and Action. 3rd Edition (SMITH, H.J. – ed.). Reading, Harwood
Academic, 1998, p. 97-166.
116. SLOVÁKOVÁ, A. – HUTT, A.J.: Chiral compounds and their pharmacologic effects (in
Slovak). jlkmonqp Slov. Farm. 48, 1999, p. 107-112.
117. DE CAMP, W.H.: The FDA perspective on the development of stereoisomers. Chirality 1,
1989, p. 2-6.
118. CAYEN, M.N.: Racemic mixtures and single stereoisomers: industrial concerns and issues
in drug development. Chirality 3 , 1991, p. 94-98.
119. NATION, R.L.: Chirality in new drug development. Clinical pharmacokinetic
considerations. Clin. Pharmacokin. 27, 1994, p. 249-255.
120. RAUWS, A.G. – GROEN, K.: Current regulatory (draft) guidance on chiral medicinal
products: Canada, EEC, Japan, United States. Chirality 6, 1994, p. 72-75.
121. CALDWELL, J.: St Mary´s Discussion Forum: Racemates and enantiomers: scientific and
regulatory aspects. Chirality 1, 1989, p. 249-250.
122. HUTT, A.J.: Drug chirality: impact on pharmaceutical regulation. Chirality 3, 1991, p. 161164.
123. GROSS, M. – CARTWRIGHT, A. – CAMPBELL, B. – BOLTON, R. – HOLMES, K. –
KIRKLAND, K. – SALMONSON, T. – ROBERT, J.-L.: Regulatory requirements for chiral
drugs. Drug Info. J. 193, 1993, p. 453-457.
124. FDA´s Policy statement for the development of new stereoisomeric drugs. Chirality 4,
1992, p. 338-340.
125. BRANCH, S.: International regulation of chiral drugs. In: Chiral Separation Techniques. A
Practical Approach. 2nd Edition (SUBRAMANIAN, G. – ed.). Weinheim, Wiley-VCH
2001, p. 319-342.
126. SHINDO, H. – CALDWELL, J.: Development of chiral drugs in Japan: an update on
regulatory and industrial opinion. Chirality 7, 1995, p. 349-352.
127. TUCKER, G.: Chiral switches. Lancet. 355, 2000, p. 1085-1087.
17
128.
129.
130.
131.
132.
133.
134.
135.
136.
137.
138.
139.
140.
141.
142.
143.
144.
18
WALSHE, J.M.: Penicillamine, a new oral therapy for Wilson´s disease. Am. J. Med 21,
1956, p. 487-495.
WILLIAMS, K.M.: Enantiomers in arthritic disorders. Pharmacol. Ther. 46, 1990, p. 273295.
GLATT, H. – OESCH, F.: Mutagenicity of cysteine and penicillamine and its enantiomeric
selectivity. Biochem. Pharmacol 34, 1985, p. 3725-3728.
TU, J.B. – BLACKWELL, R.Q. – LEE, P.F.: D,L-Penicillamine as a cause of optic axial
neuritis. J. Am. Med. Assoc. 185, 1963, p. 83-86.
WALSHE, J.M.: Chirality of penicillamine. Lancet 339, 1992, p. 254.
COTZIAS, G.C. – VAN WOERT, M.H. – SCHIFFER, L.M.: Aromatic amino acids and
modification of Parkinsonism. New Engl. J. Med. 276, 1967, p. 374-379.
COTZIAS, G.C. – PAPAVASILIOU, P.S. – GELLENE, R.: Modification of Parkinsonism
– chronic treatment with L-dopa. New Engl. J. Med. 280, 1969, p. 337-345.
DEWES, P. – THIJSSEN, I. – MENGEL, A.: A single-dose cross-over pharmacokinetic
study comparing racemic citalopram (40 mg) with the S-enantiomer of citalopram
(Escitalopram, 20 mg) in healthy male volunteers. Poster II – 45 presented at the 41st
Annual NCDEU Meeting, Phoenix, USA 2001.
SUMMARY OF PRODUCT CHARACTERISTICS: Cipralex (Escitalopram), Lundbeck,
2002.
SMITH, E.R. – WRIGHT, S.E. – ABERG, G. – FANG, Y. – McCULLOUGH, J.R.:
Comparison of the antimuscarinic and antispasmodic actions of racemic oxybutynin and
desethyloxybutynin and their enantiomers with those of racemic terodiline. Arzneim.Forsch. 48, 1998, p. 1012-1018.
JONES, S.E. – SHUBA, L.M. – ZHABYEYEV, P. – McCULLOUGH, J.R. –
McDONALD, T.F.: Differences in the effects of urinary incontinence agents S-oxybutynin
and terodiline on cardiac K(+) currents and action potentials. Br. J. Pharmacol. 131, 2000,
p. 245-254.
STEINER, T.J. – AHMED, F. – FINDLEY, L.J. – MacGREGOR, E.A. – WILKINSON,
M.: S-Fluoxetine in the prophylaxis of migraine: a phase II double-blind randomized
placebo-controlled study. Cephalagia 18, 1998, p. 283-286.
GLICK, S.D. – HASKEW, R.E. – MAISONNEUVE, I.M. – CARLSON, J.N. – JERUSSI,
T.P.: Enantioselective behavioral effects of sibutramine metabolites. Eur. J. Pharmacol. 397,
2000, p. 93-102.
AGRANAT, I. – CANER, H. – CALDWELL, J.: Putting chirality to work: the strategy of
chiral switches. Nature Reviews: Drug Discovery 1, 2002, p. 753-768.
KEEGAN, P. – LOUGHMAM, B.E.: Early clinical trials of chemopreventive and biologic
agents: designs, populations and end points. Urology 57 (Suppl 4A), 2001, p. 216-219.
WECHTER, W.J. – KANTOCI, D. – MURRAY, E.D. – QUIGGLE, D.D. – LEIPOLD,
D.D. – GIBSON, K.M. – McCRACKEN, J.D.: R-Flurbiprofen chemoprevention and
treatment of intestinal adenomas in the APC Min/+ mouse model: implications for
prophylaxis and treatment of colon cancer. Cancer Res. 57, 1997, p. 4316-4324.
WECHTER, W.J. – LEIPOLD, D.D – MURRAY, E.D. – QUIGGLE, D.D. –
McCRACKEN, J.D. – BARRIOS, R.S. – GREENBERG, N.M.: E-7869 (R-flurbiprofen)
inhibits progression of prostate cancer in the TRAMP mouse. Cancer Res. 60, 2000,
p. 2203-2208.
145.
146.
147.
148.
149.
150.
151.
152.
153.
154.
155.
156.
157.
158.
159.
160.
IN’T VELD, B.A. – RUITENBERG, A. – HOFMAN, A. – LAANNER, L.J. – VAN DUIJI,
C.M. – STIJNEN, T. – BRETELER, M.M.B. – STRICKER, B.H.C.: Nonsteroidal
antiinflammatory drugs and the risk of Alzheimer’s disease. New Engl. J. Med. 345, 2001,
p. 1515-1521.
WEGGEN, S. – ERIKSEN, J.L. – DAS, P. – SAGI, S.A. – WANG, R. – PIETRZIK, C.U. –
FINDLAY, K.A. – SMITH, T.E. – MURPHY, MP – BUTLER, T. – KANG, D.E. –
MARQUEZ-STERLING, N. – GOLDE, T.E. – KOO, E.H.: A subset of NSAIDs lower
rasutwvxwyzwx*{w|s}y~ $
"wa"a
"wa
* *do"Nwq*w
RN "
oYw" "¡w¡N " 216.
MORIHARA, T. – CHU, T. – UBEDA, O. – BEECH, W. – COLE, G.M.: Selective
¢ £w¤"¢¦¥"¢ §]¢¨q£©¨ª«u¬ ­w®¯w°±w²q³
´oµ]¶±q·}¸¹ºH»¼½]¾
R-enantiomers. J. Neurochem. 83, 2002, p. 10091012.
MAHMUD, T. – SOMASUNDARAM, S. – SIGTHORSSON, G. – SIMPSON, R.J. –
RAFI, S. – FOSTER, R. – TAVARES, I.A. – ROSETH, A. – HUTT, A.J. – JACOB, M. –
PACY, J. – SCOTT, D.L. – WRIGGLESWORTH, J.M. – BJARNSON, I.: Enantiomers of
flurbiprofen can distinguish key pathophysiological steps of NSAID entropathy in the rat.
Gut 43, 1998, p. 775-782.
JERUSSI, T.P. – CAUBET, J-F. – McCRAY, J.E. – HANDLEY, D.A.: Clinical endoscopic
evaluation of the gastroduodenal tolerance to (R)-ketoprofen, (R)-flurbiprofen, racemic
ketoprofen and paracetamol: a randomized, single-blind, placebo-controlled trial. J. Clin.
Pharmacol. 38, 1998, p. 19S-24S.
ANONYMOUS : Schering-Plough withdraws dilevalol. Scrip Number 1540, 1990, p. 24.
SHAH, R.R. – MIDGLEY, J.M. – BRANCH, S.K.: Stereochemical origin of some
clinically significant drug safety concerns: lessons for future drug development. Adverse
Drug React. Toxicol. Rev. 17, 1998 , p. 145-190.
ADVANI, S.V. – SINGH, B.N.: Pharmacodynamic, pharmacokinetic and antiarrhythmic
properties of d-sotalol, the dextro-isomer of sotalol. Drugs 49, 1995, p. 664-679.
KATO, R. – IKEDA, N. – YABEK, S. – KANNEN, R. – SINGH, B.N.: Electrophysiologic
effects of the levo- and dextrorotatory isomers of sotalol in isolated cardiac muscle and their
in vivo pharmacokinetics. J. Am. Coll. Cardiol. 7, 1986, p. 116-125.
WALDO, A.L. – CAMM, A.J. – DE RUYTER, H. – FRIEDMAN, P.L. – MacNEIL, D.J. –
PAULS, J.F. – PITT, B. – PRATT, C.M. – SCHWARTZ, P.J. – VALTRI, E.P.: Effect of
d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote
myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet
348, 1996, p. 7-12.
ANONYMOUS : Side effects kill „new Prozac“. Chem. Br. 36 , 2000, p. 11.
THAYER, A.: Lilly pulls the plug on prozac isomer drug. Chem. Eng. News, October 30,
2000, p. 8.
CONNOLLY, M.H. – CARY, J.L. – McGOON, M.D.: Valvular heart disease associated
with fenfluramine-phentamine. New Engl. J. Med. 337, 1997, p. 581-588.
ANONYMOUS: Fenfluramine and dexfenfluramine withdrawn. Further cases of valvular
heart disease. Current problems in Pharmacovigilance 23, 1997, p. 13-14.
MAIER, N.M. – FRANCO, P. – LINDNER, W.: Separation of enantiomers: needs,
challenges, perspectives. J. Chromatogr. A 906, 2001, p. 3-33.
LÉGER, B.: New blockbusters urgently needed. Drug Plus International 1, 2002, p. 4.
19
161.
162.
163.
ANONYMOUS: Bigger isn´t always better. Nature 418 , 2002, p. 353.
167.
ATARASHI, S. – YOKOHAMA, S. – YAMAZAKI, K. – SAKANO, K. – IMAMURA, M.
– HAYAKAWA, I.: Synthesis and antibacterial activities of optically active ofloxacin and
its fluoromethyl derivative. Chem. Pharm. Bull. 35, 1987, p. 1896-1902.
HAYAKAWA, I. – ATARASHI, S. – YOKOHAMA, S. – IMAMURA, M. – SAKANO,
K.-I. – FURUKAWA, M.: Synthesis and antibacterial activities of optically active
ofloxacin. Antimicrob. Agents Chemother. 29, 1986, p. 163-164.
IMAMURA, M. – SHIBAMURA, S. – HAYAKAWA, I. – OSADA, Y.: Inhibition of DNA
gyrase by optically active ofloxacin. Antimicrob. Agents Chemother. 31, 1987, 325-327.
DAVIS, R. – BRYSON, H.M.: Levofloxacin. A review of its antibacterial activity,
pharmacokinetics and therapeutic efficacy. Drugs 47, 1994, p. 677-700.
HUTT, A.J. – O´GRADY, J.: Drug chirality: a consideration of the significance of the
stereochemistry of antimicrobial agents. J. Antimicrob. Chemother. 37, 1996, p. 7-32.
ADAMS, S.S. – BRESLOFF, P. – MASON, C.G.: Pharmacological differences between the
optical isomers of ibuprofen: evidence for metabolic inversion of the (-)-isomer. J. Pharm.
Pharmacol. 28, 1976, p. 256-257.
CALDWELL, J. – HUTT, A.J. – FOURNEL-GIGLEUX, S.: The metabolic chiral inversion
and dispositional enantioselectivity of the 2-arylpropionic acids and their biological
consequences. Biochem. Pharmacol. 37, 1988, p.105-114.
RAHLFS, V.W.: Reevaluation of some double-blind, randomized studies of dexibuprofen
(Seractil): a state-of-the-art overview. Studies in patients with lumbar vertebral column
syndrome, rheumatoid arthritis, distortion of the ankle joint, gonarthrosis, ankylosing
spondylitis, and activated coxarthrosis. J. Clin. Pharmacol. 36, 1996, p. 33S-40S.
STOCK, K.P. – GEISSLINGER, G. – LOEW, D. – BECK, W.S. – BACH, G.L. – BRUNE,
K.: S-Ibuprofen versus ibuprofen-racemate. A randomized double-blind study in patients
with rheumatoid arthritis. Rheumatol. Int. 11, 1991, p. 199-202.
MAULEÓN, D. – ARTIGAS, R. – GARCIA, M.L. – CARGANICO, G.: Preclinical and
clinical development of dexketoprofen. Drugs 52 (Suppl 5), 1996, p. 24-45.
NAU, C. – VOGEL, W. – HEMPELMANN, G. – BRÄU, M.R.: Stereoselectivity of
bupivacaine in local anesthetic-sensitive ion channels of peripheral nerve. Anesthesiology
91, 1999, p. 786-795.
VALENZUELA, C. – DELPÓN, E. – TAMKUN, M.M. – TAMARGO, J. – SNYDERS,
D.J.: Stereoselective block of a human cardiac potassium channel (Kv1.5) by bupivacaine
enantiomers. Biophys. J. 69, 1995, p. 418-427.
ROUHI, A.M.: Chiral roundup. Chem. Eng. News 80, 2002, p. 43-50.
MOLONY, J.L.: Drug discovery: design and serendipity. In: Principles and Practice of
Pharmaceutical Medicine (FLETCHER, A.J. – EDWARDS, L.D. – FOX, A.W. –
STONIER, P. – eds.). Chichester, Wiley 2002, p. 31-44.
164. KROMER, W.: Relative efficacies of gastric proton-pump inhibitors on a milligram basis:
desired and undesired SH reactions. Impact of chirality. Scand. J. Gastroenterol. 36 (Suppl
243), 2001, p. 3-9.
165. ANDERSSON, T. – HASSAN-ALIN, M. – HASSELGREN, G. – ¿ À ÁÃÂÂ
ÄÆÅÇ –
WEIDOLF, L.: Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole.
Clin. Pharmacokin. 40, 2001, p. 411-426.
166. ANONYMOUS: Dilevalol cost Schering $ 100 million. Scrip Number 1543, 1990, p. 19.
168.
169.
170.
171.
172.
173.
174.
175.
176.
177.
178.
20
179.
180.
181.
182.
183.
184.
185.
186.
187.
188.
189.
190.
191.
192.
193.
194.
195.
GRAF. B.M. – MARTIN, E. – BOSNJAK, Z.J. – STOWE, D.F.: Stereospecific effect of
bupivacaine isomers on atrioventricular conduction in the isolated perfused guinea pig
heart. Anesthesiology 86, 1997, p. 410-419.
BARDSLEY, H. – GRISTWOOD, R. – BAKER, H. – WATSON, N. – NIMMO, W.: A
comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following
intravenous administration to healthy volunteers. Br. J. Clin. Pharmacol. 46, 1998, p. 245249.
BURKE, D. – BANNISTER, J.: Left-handed local anaesthetics. Curr. Anaest. Crit. Care 10,
1999, p. 262-269.
FOSTER, R.H. – MARKHAM, A.: Levobupivacaine: a review of its pharmacology and use
as a local anaesthetic. Drugs 59, 2000, p. 551-579.
MARIETTA, M.P. – WAY, W.L. – CASTAGNOLI, N. – TREVOR, A.J.: On the
pharmacology of the ketamine enantiomorphs in the rat. J. Pharmacol. Exp. Ther. 202,
1977, p. 157-165.
WHITE, P.F. – HAM, J. – WAY, W.L. – TREVOR, A.J.: Pharmacology of ketamine
isomers in surgical patients. Anesthesiol. 52, 1980, p. 231-239.
KOHRS, R. – DURIEUX, M.E.: Ketamine: teaching an old drug new tricks. Anesth. Analg.
87, 1998, p. 1186-1193.
LIND, T. – RYDBERG, L. – KYLEBÄCK, A. – JONSSON, A. – ANDERSON, T. –
HASSELGREN, G. – HOLMBERG, J. – ÈÊÉ ËÌÌÍÏÎÃÐYÑÓÒÏÔRÕqÖ}×ØwÙÚaÛÕwÜ×ØÙÕ*ÝwÞßw×Ô}Þ ÖÃØwÙÕqÝ"×Nß
acid control vs. omeprazole in patients with symptoms of gastro-oesophageal reflux disease.
Aliment Pharmacol. Ther. 14, 2000, p. 861-867.
SPENCER, C.M. – FAULDS, D.: Esomeprazole. Drugs. 60, 2000, p. 321-329.
SCOTT, L.J. – DUNN, C.J. – MALLARKEY, G. – SHARPE, M.: Esomeprazole: a review
of its use in the management of acid-related disorders in the US. Drugs 62, 2002, p. 10911118.
STENLAKE, J.B. – WAIGH, R.D. – DEWAR, G.H. – DHAR, N.C. – HUGHES, R. –
CHAPPLE, D.J. – LINDON, J.C. – FERRIGE, A.G. – COBB, P.H.: Biodegradable
neuromuscular blocking agents. Part 6. Stereochemical studies on atracurium and related
polyalkylene di-esters. Eur. J. Med. Chem. 19 , 1984, p. 441-450.
SUN WAI, W.Y.S. – FLYNN, P.J.: 51W89, the 1R cis – 1´R cis isomer of atracurium.
Anaesth. Pharmacol. Rev. 3, 1995, p. 218-221.
BRYSON, H.M. – FAULDS, D.: Cisatracurium besilate. A review of its pharmacology and
clinical potential in anaesthetic practice. Drugs 53, 1997, p. 848-866.
PAGE, C.P. – MORLEY, J.: Contrasting properties of albuterol stereoisomers.
J. Allergy Clin. Immunol. 104, 1999, p. S31-S41.
NELSON, H.S.: Clinical experience with levalbuterol. J. Allergy Clin. Immunol. 104, 1999,
p. S77-S84.
HYTTEL, J. – BØGESØ, K.P. – PERREGAARD, J. – SÁNCHEZ, C.: The
pharmacological effect of citalopram resides in the (S)-(+)-enantiomer. J. Neural Trans. 88,
1992, p. 157-160.
BURKE, W.J. – GERGEL, I. – BOSE, A.: Fixed-dose trial of the single isomer SSRI
escitalopram in depressed outpatients. J. Clin. Psychiatry 63, 2002, p. 331-336.
21
196.
197.
198.
199.
100.
101.
102.
103.
104.
MONTGOMERY, S.A. – LOFT, H. – SÁNCHEZ, C. – REINES, E.H. – PAPP, M.:
Escitalopram (S-enantiomer of citalopram): clinical efficacy and onset of action predicted
from a rat model. Pharmacol. Toxicol. 88, 2001, p. 282-286.
DEVALIA, J.L. – DE VOS, C. – HANOTTE, F. – BALTES, E.: A randomized, doubleblind, crossover comparison among cetirizine, levocetirizine, and ucb 28557 on histamineinduced cutaneous responses in healthy adult volunteers. Allergy 56, 2001, p. 50-57.
WANG, D.Y. – HANOTTE, F. – DE VOS, C. – CLEMENT, P.: Effect of cetirizine,
levocetirizine, and dextrocetirizine on histamine-induced nasal response in healthy adult
volunteers. Allergy 56, 2001, p. 339-343.
PATRICK, K.S. – CALDWELL, R.W. – FERRIS, R.M. – BREESE, G.R.: Pharmacology
of the enantiomers of threo-methylphenidate. J. Pharmacol. Exp. Ther. 241, 1987, p. 152158.
SRINIVAS, N.R. – HUBBARD, J.W. – KORCHINSKI, E.D. – MIDHA, K.K.:
Enantioselective pharmacokinetics of dl-threo-methylphenidate in humans. Pharm. Res. 10,
1993, p. 14-21.
ANONYMOUS: Focalin approved in the US. Scrip Number 2698, 2001, p. 20.
CHLUD, K.: Evaluation of tolerance and efficacy of S(+)-ibuprofen (Seractil) in daily
practice: a post-marketing-surveillance study in 1400 patients. J. Clin. Pharmacol. 35, 1995,
p. 938.
AGRANAT, I. – CANER, H.: Intellectual property and chirality of drugs. Drug Discovery
Today 4, 1999, p. 313-321.
STRONG, M.: FDA policy and regulation of stereoisomers: paradigm shift and the future of
safer more effective drugs. Food Drug Law J. 54, 1999, p. 463-487.
Registred: March 18, 2003
Accepted: April 3, 2003
PharmDr. Jindra Valentová, PhD.
Faculty of Pharmacy
Comenius University
Odbojárov 10
832 32 Bratislava
Slovakia
àHáHàãâ$äæå}çèéêáãëìîíïHðHCHIRÁLNY
ïãéçâæñòóãâ$„SWITCH“:
àôçaíuå}çaà@õöçNëìSóHðãë}íñçë}÷æáãëì
ZMESÍ
1
Hutt, A. J. − 2Valentová, J.
1
2
Department of Pharmacy, King´s College London, London, UK
øùúûüqýùÿþ
ûþwûÓúû
qýûû
ùaýùþNû*úþWù úùø
!lýùoú"$#%Yù"ù
&(')*,+-
./0123.4-5*,678"61297:;'<8=/>
*?8"6 12A@)B)12C*,7;*?8"7
+7D'4@-
.E-8")FEHG>:/-2)JI
>KL,EMK9126 @7
+6 8"7
N=OPQ
O RSTNURVPXWYZYRVUVOP[QO$\]=^_T`PVUV4]"OYabZYFRcUXZU_
PadO_eF^_TcfadP
\,g$hMijYFe4ZYFekR
UVUN ^f
YlJQ
O=\?]^_TJPVUV]"O=YFadbZYRmf
technológiách spojených so syntézou, separáciou a
22
chemických
racemátov,
spolu
s nopFqrs
tu4vwx;y z{|=}~{=Dz,
F,~~Xz
|=
F}~=?|=}
{` }
4
=
HFd
= ?¡¢£¤¥¦§¦4¡"¨Fª©J«­¬4®¦F¢£¤¯£¤£¬F¢£¤¯¦4¡"° ¡[ ,§²±4¤4³¦£´¶µ¶
·¹¸º4»½¼¾$¿ºÁÀ5¼?¾"ÂÃÄÅÆÇ;ÃÂ
Åȷɾ"º¿AÂʺJ˺½¾È¸ÅÌ=ÉÍ ¸È¸º¿ÎDËú4ÏÐ4ʾÑÒ¿ºÒӺ÷È;ÔÌ=¼?¾ÄÅÆ9ȸ¸¾"Ì=ºF·dÎú¿Õ
ktoré by mali Ö ÍÈË×,Ì Ø Ù"ÚÛÜÝÚÞ4Ù"ßàáFâÝ4Ûã4äßå$æDçèßÚÙ"ãcÙ"éêªæDàëß ÛìåíÚïî5ðñòß Ù"àëÚóôêßÚõåß;ácÙãFö¹Þ÷ø
Ú
düý =ù
4ú
4ü
ûýûú úFùÿ ­ùý ÿ
ªûú úFùÿ
üýü =úFÿ ùúû
v ùúFû4üýþúÿ ý
û ,ýþÒü Dû
,ý 4ú ý ú
ý ýü
ùý
þ
þ ý û þ
,ýý ¹ü ú ýþ ûýü
ü
ý
ü4þ
üþ
5ý ùü
ùý
þ ý þúüúÿ þ ùú
ÿ
ú ü
ùú
,úFÿ
ù ü ú
û
) * ,+.-/ 0 1 % 2 + &$34 %%,'5 $6 & &-!"5-5 4
47 # $%! &' %+ 5(
A &-(589! &: ; &'2' ;;,' 2 5< =- >7" #? @% ! %
B ';CD (
Acta Facult. Pharm. Univ. Comenianae 50, 2003, p. 7-23.
23