OXIDATIVE STRESS & SUPEROXIDE DISMUTASE Free Radicals
advertisement
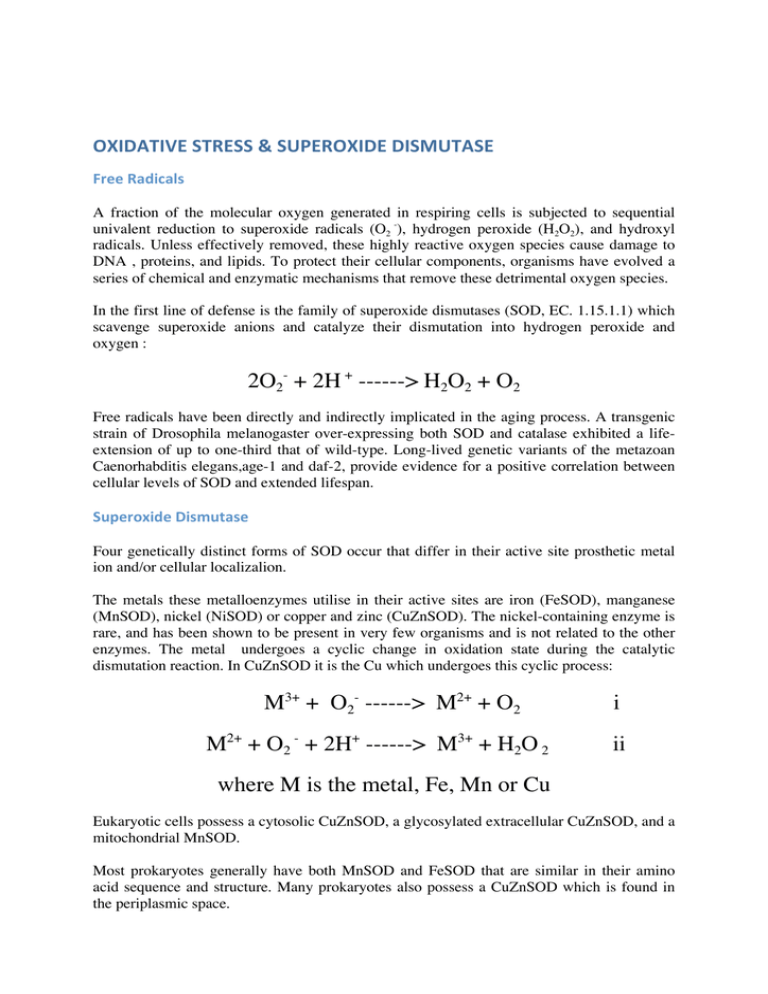
OXIDATIVE STRESS & SUPEROXIDE DISMUTASE Free Radicals A fraction of the molecular oxygen generated in respiring cells is subjected to sequential univalent reduction to superoxide radicals (O2 -), hydrogen peroxide (H2O2), and hydroxyl radicals. Unless effectively removed, these highly reactive oxygen species cause damage to DNA , proteins, and lipids. To protect their cellular components, organisms have evolved a series of chemical and enzymatic mechanisms that remove these detrimental oxygen species. In the first line of defense is the family of superoxide dismutases (SOD, EC. 1.15.1.1) which scavenge superoxide anions and catalyze their dismutation into hydrogen peroxide and oxygen : 2O2- + 2H + ------> H2O2 + O2 Free radicals have been directly and indirectly implicated in the aging process. A transgenic strain of Drosophila melanogaster over-expressing both SOD and catalase exhibited a lifeextension of up to one-third that of wild-type. Long-lived genetic variants of the metazoan Caenorhabditis elegans,age-1 and daf-2, provide evidence for a positive correlation between cellular levels of SOD and extended lifespan. Superoxide Dismutase Four genetically distinct forms of SOD occur that differ in their active site prosthetic metal ion and/or cellular localizalion. The metals these metalloenzymes utilise in their active sites are iron (FeSOD), manganese (MnSOD), nickel (NiSOD) or copper and zinc (CuZnSOD). The nickel-containing enzyme is rare, and has been shown to be present in very few organisms and is not related to the other enzymes. The metal undergoes a cyclic change in oxidation state during the catalytic dismutation reaction. In CuZnSOD it is the Cu which undergoes this cyclic process: M3+ + O2- ------> M2+ + O2 M2+ + O2 - + 2H+ ------> M3+ + H2O 2 i ii where M is the metal, Fe, Mn or Cu Eukaryotic cells possess a cytosolic CuZnSOD, a glycosylated extracellular CuZnSOD, and a mitochondrial MnSOD. Most prokaryotes generally have both MnSOD and FeSOD that are similar in their amino acid sequence and structure. Many prokaryotes also possess a CuZnSOD which is found in the periplasmic space. Mitochondrial MnSOD is encoded by a nuclear gene and is produced as a precursor protein targeted to the mitochondrial matrix during which it is processed into the mature form by cleavage of the transit peptide. The expression of MnSOD is usually inducible in both prokaryotic and eukaryotic cells, its levels increasing under conditions of elevated oxidative stress in the immediate environment . RESEARCH: We are investigating the differences between gene expression in the nematode C.elegans under a variety of conditions which produce oxidative stress in this multicelled, eukaryotic organism. Structure Information about the three dimensional structure of protein molecules is readily available and the number of structures solved as evidenced by the number of entries in the Brookhaven database is expanding at an exponential rate. Knowing the structure of a particular protein is of tremendous use when designing experiments to better understand its biological function or catalytic mechanism at a sub-molecular level. A minimum requirement to beginning structure-function studies is to determine the primary structure (sequence) of one's protein. If no X-ray or NMR structural data are available structures of proteins with homologous sequences are often adequate. MnSOD‐3 from Caenorhaditis elegans We have previously isolated and sequenced the gene and mRNA for both SOD-2 and SOD-3 of the nematode worm, C. elegans. In a collaboration with the University of Leeds, UK we have now solved their structures. The x-ray diffraction and data collection was performed at the new Diamond Light Source, Harwell Research Establishment, UK. human CuZnSOD The human CuZnSOD exists in a dimeric, cytosolic form (left figure) and a tetrameric extracellular form. The tertiary structure (right figure) is mainly beta sheet (shown in yellow), only one small helix is present (two helical regions are shown in red). Only dimers are active, although the equivalent homologous enzyme isolated from the E.coli periplasmic space is a monomeric enzyme. Investigators are currently trying to determine what makes the E. coli enzyme monomeric. The two metals (copper is green zinc is purple in the figure) are locked in place by seven amino acid residues (one aspartic acid and six histidines) but each metal is coordinated by four. Thus one residue, a histidine, bridges between the two metal ions and is an extremely rare (physiologically) fully deprotonated imidazolate form of histidine. RESEARCH: We are investigating the differences between human and E.coli CuZnSODs. By changing the protein structure of human CuZnSOD and targeting the expressed protein into the periplasmic space of E.coli cells, we aim to see if a monomeric SOD can be produced from the human enzyme. A chimaeric human-bacterial SOD has already been produced in our laboratory and is being characterized. human MnSOD The human mitochondrial enzyme MnSOD exists as a tetrameric protein (left figure) , each subunit of which binds one atom of manganese in the fully-metallated state. To be active, the metal must be in the Mn III state (see reactin i above) and in this state the protein is a bright pink. The tertiary structure (right figure) is one of alpha plus beta, mostly alpha helix (red) but with three beta strands making a beta sheet (yellow). The metal (purple sphere) is held in position by three histidine and one aspartic acid residue. Monomers of the protein are made in the cell cytosol from nuclear-encoded genes, and using a signal peptide (about 22 amino acids attached to the N-terminus of the protein) it is directed into the mitochondrial matrix where it must be refolded and combined with the manganese it requires for its activity. Some human diseases have been associated with mutations in the signal peptide, causing the import to stall, and the MnSOD to be withheld from the mitochondrion. RESEARCH: The effect of chaperone proteins on the imported precursor form of MnSOD is being compared with that of the mature form of the enzyme. We have already discovered different effects of chaperonins on the E.coli enzymes under a variety of conditions. E.coli MnSOD and FeSOD E.coli contains both MnSOD and FeSOD. As well as having a high degree of homology between themselves (in the left figure, FeSOD is yellow, MnSOD is blue), all mononuclear SODs like these are all very similar on a structural level, though some are active as dimers (as in E.coli) while others are tetramers (as in human, above). For example compare the folded shape of human MnSOD above with the FeSOD and MnSOD of E.coli (below left). Given this high degree of similarity, it has been difficult by examination of phylogenetic data using protein sequence information and structural data to ascertain the nature of metal specificity in these enzymes. Why does E.coli MnSOD only fold around manganese and FeSOD only around iron? Why are these active only when the correct metal is present in their active site? Active sites are compared in the right figure, below. The only major difference between them is the glutamine residue at the top of the figure. In FeSOD it derives from the N domain, in MnSOD it comes from the C domain. RESEARCH: We are investigating the nature of metal selectivity and specificity in these enzymes. Using site-directed mutagenesis we have produced an FeSOD with glutamine shown above in the characteristic orientation of MnSOD. We have also carried out the same reactions with MnSOD so that the glutamine residue resembles the FeSOD. We are continuing to carry out site-directed mutagenesis reactions in an effort to understand this phenomenon.









