Daniel Goldberg, M.D., Ph.D. Protein Export in Intraerythrocytic Malaria Parasites
advertisement

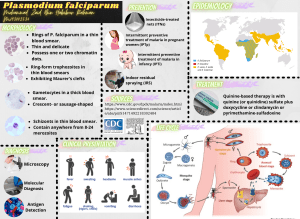
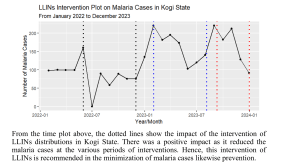
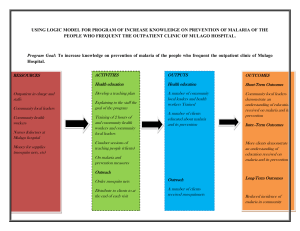
Ackert Hall, Room 120 Wednesday, March 2, 2016 4:00 P.M. Coffee and Cookies Chalmers Hall, Room 168 3:45 P.M. iochemistry olecular iophysics Protein Export in Intraerythrocytic Malaria Parasites Daniel Goldberg, M.D., Ph.D. Infectious Diseases Division Department of Medicine Washington University in St. Louis School of Medicine To mediate its survival and virulence the malaria parasite Plasmodium falciparum exports hundreds of proteins into the host erythrocyte. We have found that the export process requires a ClpB-like AAA+ ATPase called heat shock protein 101 (HSP101). We are defining the cellular and biochemical function of this key chaperone. We are also interested in establishing the function of the exported effector proteins. One such protein is histidine-rich protein II (HRPII). Plasma HRPII accumulates in the blood of patients with falciparum malaria and is a diagnostic and prognostic marker. Using a human cerebral microvascular endothelial blood-brain barrier (BBB) model, we have found that HRPII activates the inflammasome, resulting in decreased integrity of tight junctions and increased permeability. Intravenous administration of HRPII induces vascular leakage in the brains of mice, tight junction protein redistribution and increased early mortality from P. berghei experimental cerebral malaria. We propose that HRPII is a virulence factor that contributes to cerebral malaria by compromising BBB integrity.