DNA assembly methods
advertisement
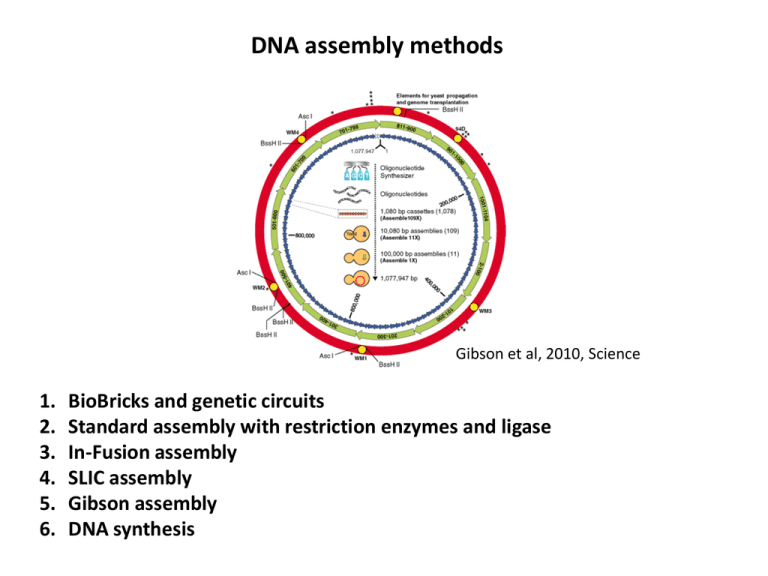
DNA assembly methods Gibson et al, 2010, Science 1. 2. 3. 4. 5. 6. BioBricks and genetic circuits Standard assembly with restriction enzymes and ligase In-Fusion assembly SLIC assembly Gibson assembly DNA synthesis Registry of Standard Biological Parts (BioBricks) partsregistry.org T9002 circuit RBS (Ribosome Binding Site) Promoter BioBrick part Transcriptional Terminator Coding sequence Scar sequence Canton et al, 2008, Nature Biotech. Standard Assembly Sleight et al, 2010, Nucleic Acids Research Restriction Enzymes http://www.accessexcellence.org/RC/VL/GG/restriction.php Why Standard Assembly is not ideal... 1. Does not allow for the creation of fusion proteins due to scar sequence 2. Requires restriction sites be removed before assembly 3. Requires an initial amplification of the BioBrick through transformation, overnight growth, and plasmid extraction 4. Requires tedious extraction of restricted DNA fragments from a gel, more intermediate enzymatic reactions, and extra time to quantitate and optimize these reactions In-Fusion PCR Cloning Kit http://www.clontech.com In-Fusion BioBrick Assembly Sleight et al, 2010, Nucleic Acids Research PCR (Polymerase Chain Reaction) 95C 55C 68C Taq Polymerase • Primers are single-stranded DNA sequences that specify where to start extension/polymerization • The primer sequence will be built into the PCR product PCR Primers Reverse primer Forward primer PCR Reaction Combine: Supermix • Phusion polymerase • dNTPs • Buffer Water Forward primer Reverse primer Template DNA (0.5 ng plasmid) Thermocycler In-Fusion BioBrick Assembly Sleight et al, 2010, Nucleic Acids Research PCR Products E0240 = E B0032 R0011 + Vector = R+V R0011 p(lacI) E0040 GFP B0010 B0032 1kb 100bp R V R+V E B0012 Purified PCR Products 1kb 100bp R+V E In-Fusion reaction R+V PCR Product (~2000 bp) 33.79 ng/ul E PCR Product (~1000 bp) 64.12 ng/ul Want a 2:1 insert:vector molar ratio and 100 ng vector. ng E = 2 X (1000/2000) X 100 = 100 ng R+V = 100 ng / 33.79 ng/ul = 3 ul E = 100 ng / 64.12 ng/ul = 1.5 ul In-Fusion Assembly reaction: R+V = 3 ul E = 1.5 ul Water = 5.5 ul Total = 10 ul Add to In-Fusion tube and mix thoroughly In-Fusion reaction (continued) 10 ul reaction tube 15 min 37C, 15 min 50C Dilute in TE Buffer Transformation Transformation via electroporation Transformants In-Fusion reaction (continued) Colony PCR Overnight culture of successful transformants Extract plasmid Submit for DNA sequencing DNA sequencing • Normally a good miniprep gives about 300 ng/ul • Add 4 ul miniprep, 1 ul primer, and 7 ul water for 12 total ul into eppy tube • Submit labeled tube to sequencing facility with filled out form ~100 bp ~100 bp VF2 R0011 p(lacI) B0032 E0040 GFP B0010 B0012 VR Analyzing sequence data VF2 VR A Plasmid Editor (APE): http://www.biology.utah.edu/jorgensen/wayned/ape/ Circuit characterization Population level Single cell level SLIC (Sequence and Ligation Independent Cloning) Li and Elledge, 2007, Nature Methods Gibson assembly Gibson et al, 2009, Nature Methods DNA synthesis Czar et al, 2009, Trends in Biotech. DNA synthesis of an artificial chromosome Gibson et al, 2010, Science









