ةسماخلا ةرضاحملا
ةسماخلا ةرضاحملا
DIFFRACTION OF X-RAYS
POWDER DIFFRACTION PATTERNS
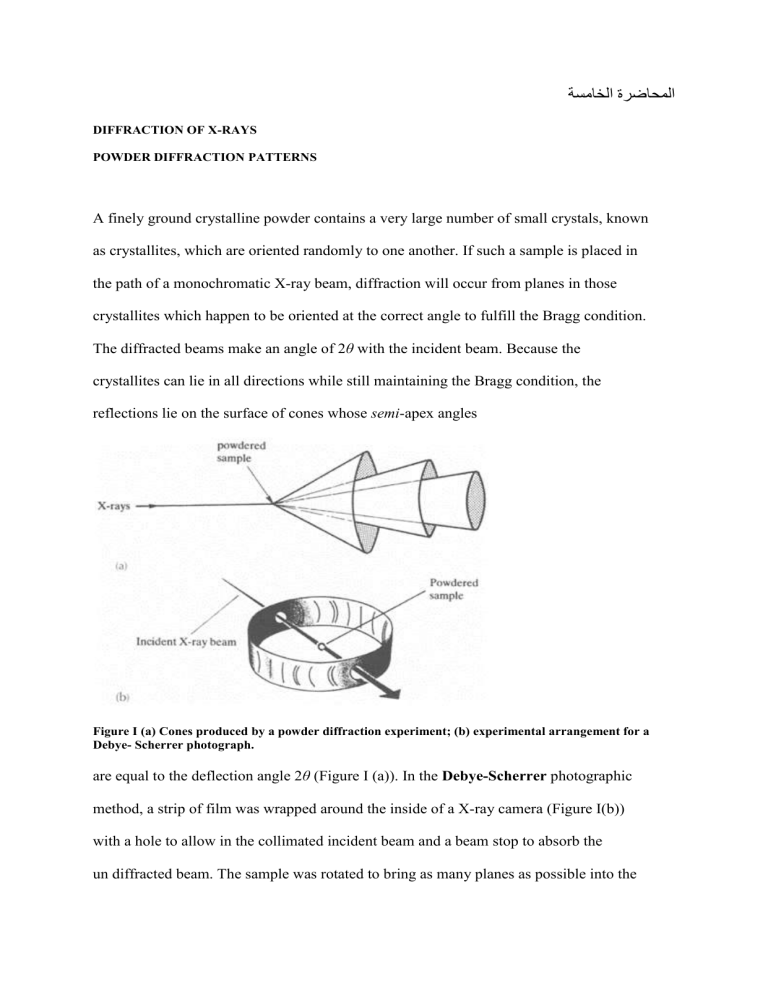
A finely ground crystalline powder contains a very large number of small crystals, known as crystallites, which are oriented randomly to one another. If such a sample is placed in the path of a monochromatic X-ray beam, diffraction will occur from planes in those crystallites which happen to be oriented at the correct angle to fulfill the Bragg condition.
The diffracted beams make an angle of 2 θ with the incident beam. Because the crystallites can lie in all directions while still maintaining the Bragg condition, the reflections lie on the surface of cones whose semi -apex angles
Figure I (a) Cones produced by a powder diffraction experiment; (b) experimental arrangement for a
Debye- Scherrer photograph. are equal to the deflection angle 2
θ
(Figure I (a)). In the Debye-Scherrer photographic method, a strip of film was wrapped around the inside of a X-ray camera (Figure I(b)) with a hole to allow in the collimated incident beam and a beam stop to absorb the un diffracted beam. The sample was rotated to bring as many planes as possible into the
diffracting condition, and the cones were recorded as arcs on the film. Using the radius of the camera and the distance along the film from the centre, the Bragg angle 2 θ, and thus the dhkl spacing for each reflection can be calculated. Collection of powder diffraction patterns is now almost always performed by automatic diffractometers (Figure 2(a)), using a scintillation or CCD detector to record the angle and the intensity of the diffracted beams, which are plotted as intensity against 2
θ
(Figure 2(b)). The resolution obtained using a diffractometer is better than photography as the sample acts like a mirror helping to refocus the X-ray beam. The data, both position and intensity, are readily measured and stored on a computer for analysis.
Figure 2 Diagram of a powder diffractometer; (b) a powder
The difficulty in the powder method lies in deciding which planes are responsible for each reflection; this is known as ‘indexing the reflections’ (i.e., assigning the correct hkl index to each reflection). Although this is often possible for simple compounds in high symmetry systems, it is extremely difficult to do for many larger and/or less symmetrical systems.
Q I - An orthorhombic unit cell of a compound of molar mass 135.01 g mol -I has the dimensions a = 589 pm, b = 822 pm, and c = 798 pm. The density of the solid is estimated as 2.9 g cm - 3 . Determine the number of formula units per unit cell and calculate a more precise value of the density.
Answer
Q2 The powder diffraction patterns of (a) tungsten, (b) copper obtained in a camera of radius 28.7 mm are shown in the figure. Both were obtained with
154 pm X-rays and the scales are marked. Identify the unit cell in each case, and calculate the lattice spacing. Estimate the metallic radii of Wand Cu.







