Unit 12 Practice FRQ
advertisement

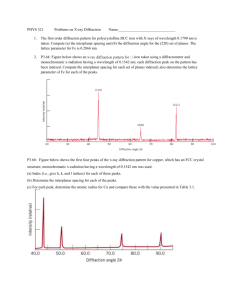
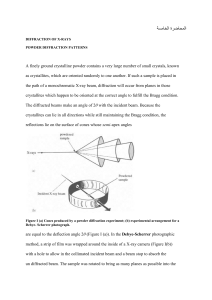
In their study of X-ray diffraction, William and Lawrence Bragg determined that the relationship among the wavelength of the radiation 1l2, the angle at which the radiation is diffracted 1u2, and the distance between planes of atoms in the crystal that cause the diffraction (d) is given by nλ = 2d sin ϴ. X rays from a copper X-ray tube that have a wavelength of 1.54 Å are diffracted at an angle of 14.22 degrees by crystalline silicon. Using the Bragg equation, calculate the distance between the planes of atoms responsible for diffraction in this crystal, assuming n = 1 (first-order diffraction). Tin exists in two allotropic forms: Gray tin has the diamond structure, and white tin has a closepacked structure. One of these allotropic forms is a semiconductor with a small band gap, while the other is a metal. (a) Which one is which? (b) Which form would you expect to have the longer Sn¬Sn bond distance? Unlike metals, semiconductors increase their conductivity as you heat them (up to a point). Suggest an explanation.