1-2 PLASTIC DEFORMATION
advertisement

ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
1-2 PLASTIC DEFORMATION
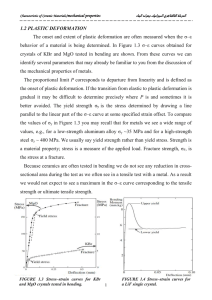
The onset and extent of plastic deformation are often measured when the σ–
εbehavior of a material is being determined. In Figure 1.3 σ–ε curves obtained for
crystals of KBr and MgO tested in bending are shown. From these curves we can
identify several parameters that may already be familiar to you from the discussion of
the mechanical properties of metals.
The proportional limit P corresponds to departure from linearity and is defined as
the onset of plastic deformation. If the transition from elastic to plastic deformation is
gradual it may be difficult to determine precisely where P is and sometimes it is
better avoided. The yield strength σy is the stress determined by drawing a line
parallel to the linear part of the σ–ε curve at some specified strain offset. To compare
the values of σy in Figure 1.3 you may recall that for metals we see a wide range of
values, e.g., for a low-strength aluminum alloy σy ~35 MPa and for a high-strength
steel σy ~ 400 MPa. We usually say yield strength rather than yield stress. Strength is
a material property; stress is a measure of the applied load.
Fracture strength, σF, is the stress at a fracture. Because ceramics are often tested in
bending we do not see any reduction in cross-sectional area during the test as we
often see in a tensile test with a metal. As a result we would not expect to see a
maximum in the σ–ε curve corresponding to the tensile strength or ultimate tensile
strength. Figure 1.4 shows a σ- ε curve for LiF that illustrates an abrupt elastic–
plastic transition. Plastic deformation begins at the upper yield point and there is a
decrease in stress. At the lower yield point deformation continues at lower stress
levels. This type of behavior is similar to that of some low-carbon steels as well as
aluminum oxide and magnesium oxide at high temperatures.
1
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
FIGURE 1.3 Stress–strain curves for KBr
and MgO crystals tested in bending.
FIGURE 1.4 Stress–strain curves for
a LiF single crystal.
1-2.1 Dislocations
Dislocations are line defects, but like all crystal defects, they are actually
volume defects; i.e., we should think of them as tubes, or pipes, whose properties
change across the tube radius and that generally do not have cylindrical symmetry.
Two vectors define the fundamental properties of any dislocation:
- The line direction
- The Burgers vector
The glide plane of the dislocation is the plane that contains both vectors. To
summarize:
●Geometry Burgers vector and line direction define the glide plane.
●Displacement When a dislocation is present, atoms are displaced from their
positions in the perfect crystal; the material is strained so there must be a stress.
●Movement Dislocations move and interact (they even intersect).
●Reacting We generate dislocations; they multiply and combine (by intersecting).
What is special about dislocations in ceramics?
●Complex and large unit cells are the norm rather than the exception.
●Charge-if you insert an extra half plane to make an edge dislocation, you must
consider the charge.
●Directional bonds-if you break a bond, does it reform?
2
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
In contrast to point defects, dislocations never exist in thermodynamic equilibrium
because they have formation energies of ~1 eV (or more) per atom along the line and
there is no significant balancing entropy contribution as there is for point defects.
They are almost always present in crystals because of how the crystal grew or
because it was deformed. Therefore dislocations usually form due to non equilibrium
conditions, such as thermal and mechanical processing, or for thin films and single
crystals, during growth. There are two special types of dislocation.
- Edge dislocations
- Screw dislocations
All other dislocations are referred to as “mixed.”
*Defining the Burgers Vector and the Glide Plane
The Burgers vector is defined by constructing a closed circuit around the
dislocation line. We first draw a circuit around the dislocation in a clockwise (righthanded screw) direction from the start (S) to the finish (F) as shown in Figure 1.5a.
We then transfer this circuit to a perfect crystal as shown in Figure 1.5b. If there is a
dislocation, this second loop will not close on itself. We then define the vector FS,
which is required to close the loop in the perfect crystal, as the Burgers vector. This
method of defining the Burgers vector, b, is known as the FS/RH perfect-crystal
convention. The Burgers vector is then defined with respect to the perfect crystal; you
would not want to define it in the imperfect crystal! It is important that you are
consistent in using this convention. Some other texts, and even some of the classic
papers, use a convention that produces the opposite sign for the Burgers vector. For
example, they might use an anticlockwise circuit, set b = SF, or draw the circuit in
the perfect crystal first. You must use a convention consistently.
- Edge dislocation: An extra half-plane of atoms is inserted above the glide plane as
illustrated in Figure 1.5a. The Burgers vector is perpendicular to the dislocation line,
u. The Burgers vector will be opposite if the extra half plane is below the glide plane.
- Screw dislocation: Successive atomic planes are connected to form the surface of a
helix (or screw) around the dislocation line where the dislocation is perpendicular to
3
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
the planes: like the core of a spiral, parking ramp. The Burgers vector is parallel to
the dislocation line and can point up or down for a left- or right-handed screw.
- Glide plane: This is the plane containing both the dislocation line and the Burgers
vector.
FIGURE 1.5 the Burgers circuit in the imperfect and perfect lattices for an edge dislocation in a
simple-cubic crystal.
1-2.2 Summary of Dislocation Properties:
We have not reviewed all the properties of dislocations, but have concentrated
on those you should know when considering dislocations in ceramics.
- Dislocations cannot end inside the crystal lattice.
- We almost always assume that strains are so small that linear elasticity is a good
approximation; so, Hooke’s law holds.
- The displacement field for any dislocation falls off as r
−1
as we move away from
the dislocation.
- The strain energy (or self energy) of a dislocation actually depends on the character
of the dislocation, but setting E =αGb2 is a good estimate, where α is ~0.5.
- Parallel dislocations repel one another if the angle between their Burgers vector is
less than 90°.
-A dislocation can always lower its strain energy by spreading its core or
dissociating. Whether this will lower the total energy depends on the energy required
4
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
to form the distorted region between the “partial dislocations.” If dissociation occurs
(forming identifiable partial dislocations) then this region is called a stacking fault.
- Dislocations glide by the movement of kinks and climb by the movement of jogs.
Since climb requires changing the number of point defects.
1-2.3 Dislocation in Ceramics:
In discussing dislocations in ceramic materials, the principle is always the
same. We deduce the possible Burgers vectors first, then the glide planes. We will
begin by asking if there is anything special about dislocations in ceramics; we can
preempt the answer by saying yes, as usual, the bonding and charge can add their
own effects. The second best-known special feature of dislocations in ceramics is
actually that the unit cell of such materials is usually larger than for the simple
metals. The structure of the dislocation core in ceramics depends on three factors
1. Charge of the ions
2. Size of ions
3. Presence of directional bonds.
Perhaps the more important question is: why learn about dislocations in ceramics
when ceramics do not deform plastically as easily as metals?
* Structure of the Core:
Not much is really known about the core of dislocations in ceramics. For
understand that chosen one Burgers vector (usually the most important one), one line
direction, and thus one glide plane. Furthermore, we usually draw the edge
dislocation because it is easiest to draw, not because it is the most important. In this
section, we will assume that the dislocation core is compact. The examples are
chosen to illustrate particular features.
- NaCl: it is relatively simple and illustrates the effect of charge.
- Si: it illustrates the effect of directional (covalent) bonding.
- Al2O3 and olivine: they are non cubic materials.
5
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
Although NaCl and MgO structures are both based on the cubic-F lattice, like Cu,
there is no evidence for any dislocation dissociation. A schematic diagram of such a
dislocation viewed along the [001] direction is shown in Figure 1.6. All the ions seen
here lie in the same (001) plane. If we remove this plane of atoms the structure looks
the same, but all the ions are reversed in sign. So charge is balanced as long as there
are no jogs or kinks on the dislocation.
The glide plane for dislocations in NaCl and MgO is {110} rather than the {111}
you might expect for fcc. You may read that the glide plane is {110} because this
plane is electrically neutral and motion on this plane avoids charged layers gliding
over one another. PbS, a semiconductor with this structure, shows glide occurring on
{001} planes and in TiC dislocations glide occurs on {111} planes. The real reason
the suspicion is that the core actually does spread on different planes. The simplest
covalently bonded ceramics are Si and Ge. The covalent bond formed by two atoms
sharing electrons is a localized and directional bond. Cubic ZnS has the same
structure, but the Si–Si basis is replaced by Zn–S, although the bond still has a large
covalent component. This feature is important in determining the characteristics of
dislocations in covalent materials. Dislocations in these materials tend to be immobile
at low temperatures.
Extensive slip occurs only at elevated temperatures of the many covalent crystals,
the diamond-cubic and c-ZnS structures are among the simplest and most widely
studied. Since the crystal lattice is fcc, perfect dislocations have the fcc Burgers
vector 1/2 <110>. Like dislocations in fcc metals, dislocations in Si, Ge, and c- ZnS
glide on {111} planes. These two dislocations are fundamentally different because
the zinc blende structure does not have a center of symmetry. Similar considerations
will hold for materials such as AIN and GaN, which also lack a center of symmetry
but have the wurtzite structure crystal structure. The stacking fault in the diamondcubic structure is described: the pair of planes behaves just as if it were one fcc plane
in this case.
6
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺜﺎﻟﺜﺔ
Properties of Ceramic Materials
Many ceramic materials are neither cubic nor hcp. Olivine and sapphire both have
oxygen sublattices that can be thought of as distorted hexagonal close packed (hcp),
but the distribution of cations makes them very different.
Olivine: In the olivine group of minerals (important orthorhombic silicates), the
energies of the [100], [010], and [001] dislocations are all different. The added
complication is that olivine is not an isotropic material.
Sapphire: Al2O3 is also very anisotropic although the perfect dislocations do have the
shortest Burgers vector 1/3
but other perfect dislocations have been reported
including [0001].Even when they have the shortest b, they may glide on other planes
such as.
FIGURE 12.10 the core of a dislocation in NaCl.
7