The Chemistry Behind Antibody-Drug Conjugation Baran Lab Eran Sella Group Meeting
advertisement
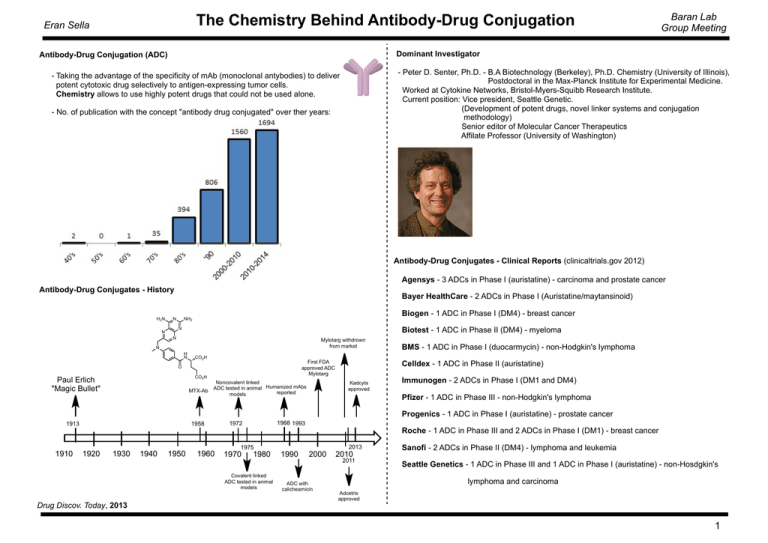
The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting Dominant Investigator Antibody-Drug Conjugation (ADC) - Peter D. Senter, Ph.D. - B.A Biotechnology (Berkeley), Ph.D. Chemistry (University of Illinois), Postdoctoral in the Max-Planck Institute for Experimental Medicine. Worked at Cytokine Networks, Bristol-Myers-Squibb Research Institute. Current position: Vice president, Seattle Genetic. (Development of potent drugs, novel linker systems and conjugation methodology) Senior editor of Molecular Cancer Therapeutics Affilate Professor (University of Washington) - Taking the advantage of the specificity of mAb (monoclonal antybodies) to deliver potent cytotoxic drug selectively to antigen-expressing tumor cells. Chemistry allows to use highly potent drugs that could not be used alone. - No. of publication with the concept "antibody drug conjugated" over ther years: Antibody-Drug Conjugates - Clinical Reports (clinicaltrials.gov 2012) Agensys - 3 ADCs in Phase I (auristatine) - carcinoma and prostate cancer Antibody-Drug Conjugates - History H2N Bayer HealthCare - 2 ADCs in Phase I (Auristatine/maytansinoid) N Biogen - 1 ADC in Phase I (DM4) - breast cancer NH2 Biotest - 1 ADC in Phase II (DM4) - myeloma N N N Mylotarg withdrown from market N H N CO2H First FDA approved ADC Mylotarg O CO2H Paul Erlich "Magic Bullet" MTX-Ab Noncovalent linked ADC tested in animal Humanized mAbs reported models BMS - 1 ADC in Phase I (duocarmycin) - non-Hodgkin's lymphoma Celldex - 1 ADC in Phase II (auristatine) Kadcyla approved Immunogen - 2 ADCs in Phase I (DM1 and DM4) Pfizer - 1 ADC in Phase III - non-Hodgkin's lymphoma Progenics - 1 ADC in Phase I (auristatine) - prostate cancer 1913 1910 1958 1920 1930 1940 1950 1960 1988 1993 1972 1975 1970 1980 Covalent linked ADC tested in animal models Drug Discov. Today, 2013 1990 Roche - 1 ADC in Phase III and 2 ADCs in Phase I (DM1) - breast cancer 2000 ADC with calicheamicin 2013 2010 2011 Sanofi - 2 ADCs in Phase II (DM4) - lymphoma and leukemia Seattle Genetics - 1 ADC in Phase III and 1 ADC in Phase I (auristatine) - non-Hosdgkin's lymphoma and carcinoma Adcetris approved 1 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Mode of Action Baran Lab Group Meeting General Linkers - Use of highly potent drugs (Especially negative-charged drugs) - Ab can be also anti-cancer - Drug is stable and usually inactive until it reaches the cancer cells ADC with a specific drug is usually more potent than the drug alone O O O NH2 N O R N H NHS O R amide S NH2 S C N R N H isothiocyanate O SH X N R H thiourea O S R R haloacetyl thioether ImmunoGen Inc. O Antibody-Drug Conjugates - Components & Demands N O Attachment Site Linker O SH S R N maleimide O -Stable -Slow hydrolysis Drug R thioether Simple Conjugation Antibody: 1. Targets a well-characterized antigen with high tumor expression 2. Maintain all of it's properties upon conjugation to the drug 3. Minimal non specific binding Attechment site: 1. Typically through cysteine or lysine residues on the Ab 2. Variable drug:Ab ratio or site-selective conjugation O O O N H O S N O N H H N Drug maleimidocaproyl H N Drug mercaptoacetamidocaproyl O O S Drug O O Linker: 1. Cleavable or non cleavable 2. Stable with selective release of the drug Drug: 1. Highly potent (usually 2-4 drugs per mAb) 2. Non-immunogenic 3. Could be linked to the Ab 4. Defined mechanism of action H N O O Current Opinion in Chemical Biology, 2010, 14, 529 2 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Canadian J. Org. Chem., 1984, 62(3), 586 Chem. Abstr., 1968, 68 Thiolation Reagents Chem. Commun., 2013, 49, 8187 O S R NH2 R N H SH NH S R NH2 S O Br Br Toluene reflux Cl S S S N3 -N2 1g = 3.5 $ SH CN K2CO3 quant. 1g = 7.4 $ CN S 67% S NH2 Cl PhS PhS O O N O SAc R O NH2 R N H SAc R N H O PNPCl Pyridine O O H2N O O O Drug NO2 O O HN O PhS NH2OH Drug O N R PhS TCEP O O Br O N PhS OMe OH O N HCl/MeOH S S O S O N R S HS SH O SH S O pH=7.4 S O OH N R S OH N O N PhS PhS O N O Br PABA O PhS NH O O Br PhSH NEt3 S O Br MeOCOCl NMM O 35% Br Drug O NH NaN3 O SH N H 2-iminothiolane "Traut's Reagent" NCS O O N R = mAb or Drug NH R Baran Lab Group Meeting O S S pH=4.5 O O NHR S O H2N Drug O PABA 3 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Bifunctional Linkers O O O O O N O NH2 HS HO S O N H O N H B Drug H O S O HO O N H H N N H S N H Can deconjugate (retro-michael) H Drug S N H S Drug Hydrazone (Rapid hydrolysis in the lysosome) Disulfide (Reduction in lysosome) Mainly by Glutathione O O N H HO O OH O NH H N N OH O S SH H N NH H2N S Drug Linking by Click N H O N H H N O Drug Val-Cit dipeptide (site-specific proteolysis) Sometimes also Phe-Lys copper-free click - developed by Bertozzi (2007) PNAS, 104(43), 16793 bosutinib Drug O Can't deconjugate Drug NH2 N H O N O O N H J. Controlled Release, 2012, 161, 422 O S Drug O S O -TolSO2- SH O O O pH=7.4 O Piperidine O Drug O O Oxone Misc. Drug Linking with Specific Release Mechanism Bioconjugate Chem., 2014, 25, 460 O STol N O NH Drug O Drug O HO O Drug HS O HO O N O O O O N H N O CH2O Piperidine/HCl O Baran Lab Group Meeting N3 Drug N N N N JACS, 2013, 135, 12994 4 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Traceless Linkers NH2 Precise Controll on Conjugation to Synthesize homogeneous ADCs Advantages: 1. Known mAb:Drug ratio. 2. mAb stability remains H N CHO Drug Drug SH Baran Lab Group Meeting O (Hydrolysis) S O O NH2 Drug Drug Drug O O N N thiazolidine Oxime Ligation SH Drug S S SH (Reduction) Drug Genetically encoded unnatural amino acid with ortogonal chemical reactivity HOOC Schultz, PNAS, 2012, 92(3), 13 O2N S COOH O 2 SH S S Ellman Reagent NO2 N N OH = auristatine Drug Ph N Ph S S N N S O N N H OH O N3 O H2N O NH2 3 N H O 3 N3 1. PNPCl, NEt3 2. EtOCONHNH2 H2N 3. K2CO3 / MeOH 2,2'-dithiopyridine O O COOH S S P HOOC H N EtO TCEP SH O N H O N H O SH HS OH S S HO HO N H O N HN HN O N3 3 O O COOH N H O 3 N3 N N S S K2CO3 / MeOH N H NBS, Pyridine OH N3 3 O DTT (dithiothreitol) (Cleland's Reagent) N 1. TFAA 2. EDC, HOBt OH N O OH Herceptin O S NH2 RGD NH O Nature Protocols, 2013, 8, 2079 O O Click-like reaction for Tyrosine O O N H Drug O PBS, RT, 30 min N H SH N H N O Drug Drug N O Barbas, JACS, 2010, 132, 1523 5 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Spacers Baran Lab Group Meeting Self-Immolative Spacers (Cont.) Drug n X O Drug O O O Drug n X O Self-Immolative Spacers X -CO2 Drug Drug R O X Examples O N H O X O N H OH X -CO2 O Drug H2N O O Drug O Drug HO Drug HO Drug Drug O H2O OH O O X O Drug O OH O X Y O Drug O S S O O N N S O O Drug O O X Y O O O Drug Y X HO Drug -CO2 S O O N N HO Drug 6 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting Adcetris (Brentuximab Vedotin) mAb - Brentuximab, directs to protein CD30 (expressed in Hodgkin's lymphoma and large-cell lymphoma) Attachment Site - thiomaleimido caproyl Linker - Cathepsin (protease) cleavable linker (Val-Cit) Spacer - PABA Drug - Monomethyl auristatine E (MMAE) O O O N S O N H H N O O N O H N N N O N H O H N O O O OH O NH H2N NH MMAE - antimitotic drug (inhibits mitosis, cell division), from the marine shell less Dolabella Auricularia - Blocking the polymerisation of tubulin - 200 times more potent than vinblastine H N N O O H N N N O O O N S O Dolastatine 7 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Adcetris (Brentuximab Vedotin) O H N N O O O O Pettit, JACS, 1989, 111(14), 5463 N H N N N Baran Lab Group Meeting S O Dolastatine O NaH, CH3I THF OH NHCbz O O BH3, THF OH NCbz 83% OH NCbz 95% SO3-Pyridine, DMSO H 78% NCbz O O N Cbz LDA, THF -78o 87% total 33% separated H N Boc CH3N2, BF3 etherate O OH N Cbz 67% O H2, Pd/C O N H 63% OMe O O OMe O (3R,4S, 5S) 1. BH3 - THF 2. modified Swern H OH N Boc 75% (2 steps) O O H N Boc Ph O O Ph H LDA, MgBr2 Ph O OH O H Me3O BF4 Ph N Boc DCM Ph OMe O Ph O Ph Ph Ph 47% after separation -20o 57% Ph H KOtBu N Boc O OMe O Ph Ph H 1. H2/Pd 2. TFA TFA N H2 OMe O 1. O OH NHBoc O H NHBoc OH H2N 2. MnO2 SH N S NHBoc 8 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Adcetris (Brentuximab Vedotin) O H N N H N N N O O O O Baran Lab Group Meeting Tetrahedron, 2007, 63, 6115 N S O Dolastatine 1. CDI 2. Mg(O2CCH2COOEt)2 BocHN 62% COOH RuBr2[(S)-SYNPHOS) H2, 12bar, 50o, 24h OEt BocHN O (S)-Boc-Isoleucine O 1. LHMDS. HMPA, MeOTf 2. aq. NaOH / EtOH 100% conversion 92:8 OEt BocHN 60% (2 steps) OH O O OH BocHN OMe O PPh2 PPh2 O O O H N Boc O OH N Boc O OEt N Boc OMe O O OH (S)-Boc-Proline HN OMe Cl O HCl Pyridine 77% O O PhO P N3 PhO PPh3, DEAD OMe N S S N N 56% O 1. Ipc2BCl 2. NaOH 3. H2O2 60% 99% ee S N OH S N N3 S Li 1. PPh3, NH4OH 2. DIBOC 44% >99%ee N NHBoc 9 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting O Kadcyla (Trastuzumab emtansine) HN O MeO mAb - Trastuzumab, targets HER2 receptor, also stop cell groth alone H OH Attachment Site - SMCC (succinimidyl trans-4-(maleimidylmethyl)cyclohexane-1-carboxylate) Drug - Mertansine (3.5 units/mAb in avarage) O Mertansine (DM1) - Thiol derivative of Maytansine - Antimitotic O O N O N S N O Cl H N O O O O Cl Cl LiAlH4 THF NHMe MeO Cl NHMe MeO 1. MeOCOCl, K2CO3 2. NaOH/MeOH COOEt OH OH OH LDA 1.DHP, pTsOH 2. Buli 50% 3. Br I Li OMe Cu OTHP Cu N MeO OTHP OMe Cl Cl Cu Cl N O 94% OMe OH OMe Li OMe MeO N MeO 1. MsCl, NEt3 2. NaI OH Br Br OMe O 90% 88% Br2, CCl4 Cl N MeO O 90% O 1. pTsOH/MeOH 2. MnO2 OMe N MeO OMe O 95% O OTHP I O NH2 Cl N N LDA, TMSCl 73% TMS MeO 1. sec-BuLi 2. OMe O Cl MeO N N OMe O O Corey JACS, 1978, 100(9), 2916 Tel. Lett. 1978, 12, 1051 JACS, 1980, 102(4), 1439 O 3. AcOH/NaOAc 10 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting O Kadcyla (Trastuzumab emtansine) HN O MeO AcO 1. NaOMe/MeOH 2. HgOAc O 75% AcO AcOHg O H HO HO O H HO HO 99% OTrt O H MeLi, CuI OH HO SH OEt OH CHCl3/HCl OMe 96% 75% O O HO SH S O O H N OMe Cl O O O N S O N H N O O O O O O O MEMCl OH BF3 Et2O 86% S H OTrt OMe OMe OAc OH 1. TrtCl, Pyridine 2. NaH (4 eq.) 3. isopropylbenzene sulfonyl imidazole OH 1. NaCl 2. NaBH4 3. HCl OH S 99% OMEM S S S O O MeO O N 1. nBuLi 2. NaH, MeI OMe O OMe N MeO S S O S Cl 1. CH3SLi 2. HClO4 3. Pb(OAc)4 O O OMEM S Cl Cl OMEM O MeO P MeO NH OMe OMEM then Pyridinium chloride 82% O O Cl OMe MeO Li S S OMEM N OMe 1. Cl MeO TMS S S OMe O Li O NH MeO 1. PNPCl, Pyridine 2. NH4OH / t-butanol 3. HgCl2, CaCO3 N 2. Bu4NOH 3. MesSO2Cl 4. 10% H2SO4 S S OH 76% OMe O Cl MeO N (-)-N-Methylmeysenine Corey JACS, 1978, 100(9), 2916 Tel. Lett. 1978, 12, 1051 JACS, 1980, 102(4), 1439 O N H OMe OH O 11 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting Mylotarg (Gentuzumab ozogamicin) mAb - Gentuzumab (antibody to CD33 expressed in leukemia cells) Linker - includes Hydrazone and stable S-S linkage Drug - Calicheamicin Calicheamicin - from Micromonospora echinospora, Texas, 1980 - Causes DNA strand scission O initiation HO S S O NHCO2Me O Bergman Cycloaromatization HO S Sugar O O NHCO2Me O O Sugar NHCO2Me HO DNA diradical S DNA HO S Sugar NHCO2Me O O2 DNA scission Sugar 12 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting O O NHCO Me 2 Et3Si O Pd C-C OH acetyilide-aldehyde condensation BzO O O O ethylene glycol pTsOH O 60% HO O O DIBAL O 84% O OH O tetronic acid MEMO 1. TBSCl, Im 2. PhCOCl, Pyr H2O2, NaOH Ipc2BOMe MEMO Li OMEM TBSO OMEM BIpc2 s-BuLi O OMEM HO (Z) O OMEM 1. TBAF 2. Swern 3. NH2OH HO O OBz N O OH O O NaOCl O N O OBz BzO O OMEM O N O BzO O 1. NaOMe 2. CrO3, H2SO4 O H OMEM N AcO 3. TMS 4. Ac2O 5. ZnBr2 O Li TMS O H OH 1. Swern 2. PPh3=CHCO2Me O O N AcO TMS O CO2Me isoxazoline 51% after separation Nicolaou, JACS, 1992, 114, 10082 13 The Chemistry Behind Antibody-Drug Conjugation Eran Sella Baran Lab Group Meeting O O NHCO Me 2 Et3Si O Pd C-C OH acetyilide-aldehyde condensation O BzO O O O 1. K2CO3/MeOH 2. TES triflate N AcO TMS O 3. O O N Et3Si O O 1. Mo(CO)6 2. K2CO3 Et3Si NH2 O CHO 82% Cl O O Et3Si O O N O O Pyridine MeNO2 CO2Me CO2Me CO2Me Cl Cl O O CO2Me TMS Pd(PPh3)4, CuI H TMS H 91% O O NPhth O O NPhth 1. SiO2 2. Ac2O KHMDS -90o Et3Si O 1. MsCl 2. SiO2, Pyr Et3Si O Et3Si O 48% CHO CO2Me 1. MeNHNH2 (99%) 2. Triphosgene, Pyr/MeOH (82%) 3. DIBAL, NaBH4 (82%) 4. PhCOCl, Pyr (84%) O O NPhth CO2Me H OH O O O O NHCO Me 2 Et3Si O OH BzO Nicolaou, JACS, 1992, 114, 10082 14