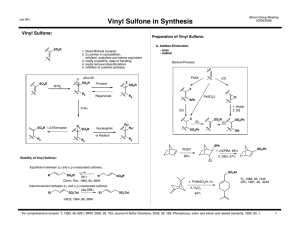
Chem 331 Exam 3 2011 Prof. Fox
advertisement

Chem 331 Exam 3 2011 Prof. Fox 50 minutes The exam is open book, Open notes. Models are permitted Show your work in detail WRITE YOUR NAME ON EVERY PAGE NAME Chem 331, Exam 3. 2011 ! ! NAME 1. The reactions shown below would not proceed as shown. Explain why (be concise), and indicate which product would be formed instead. a. H H O OEt O OEt O O O (5 pts each) OH HO H+ Aldehydes react with alcohols or diols to form acetals. Esters do not. The product would be: O H OEt O b Me 1) MeLi (large excess) O O H3C O O O 2) H3 CH3 HO O+ (workup) The lactone (a cyclic ester) would also react with MeLi. The product would be HO H3C CH3 OH HO c H O Me Me 1) excess EtMgBr O O 2) H3O+ (workup) OH both the aldehyde and epoxide would react with EtMgBr. The product would be: HO OH O O Chem 331, Exam 3. 2011 ! ! NAME 2. Propose a synthesis for 1 starting with any materials that contain 4 carbons or less. You may also use reagents that contain more than 4 carbons (e.g. Ph3P, LDA, MCPBA, DIBAL, DCC, pyridine, TsCl, Et3N). (25 points) retrosynthesis N O H2N N O OH 1 O N N BuLi forward synthesis N mCPBA Li N OH O H3O+ workup tBuOH, H+ H2N O LiAlH4 N O Li Chem 331, Exam 3. 2011 ! ! NAME (20 pts ) 3. Propose the structure of 2. Provide a mechanism for it's formation. 2 OH O NMR 110.2, s 69.6, t, 2 carbons 29.8, t, 2 carbons 20.8, t, 2 carbons catalytic H+ HO 13C C7H12O2 1H NMR 3.90–3.80 (m, 4H) 2.06–1.81 (m, 4H) 1.90–1.80 (m, 4H) H OH HO OH O OH H+ +O H O +O OH OH OH + 2 OH H+ O O O O O H + + HO + O HO resonance stabilized Chem 331, Exam 3. 2011 ! ! NAME 4. Propose a detailed arrow pushing mechanism (20 pts ) Cl MeO HCl OMe MeO H + OMe resonance stabilized Cl– H+ MeO OMe H MeO + OMe + : OMe + MeO H Cl MeO H