CHEM 334: Spring 2014 Recitation Section 3/20/2014 BF OH
advertisement
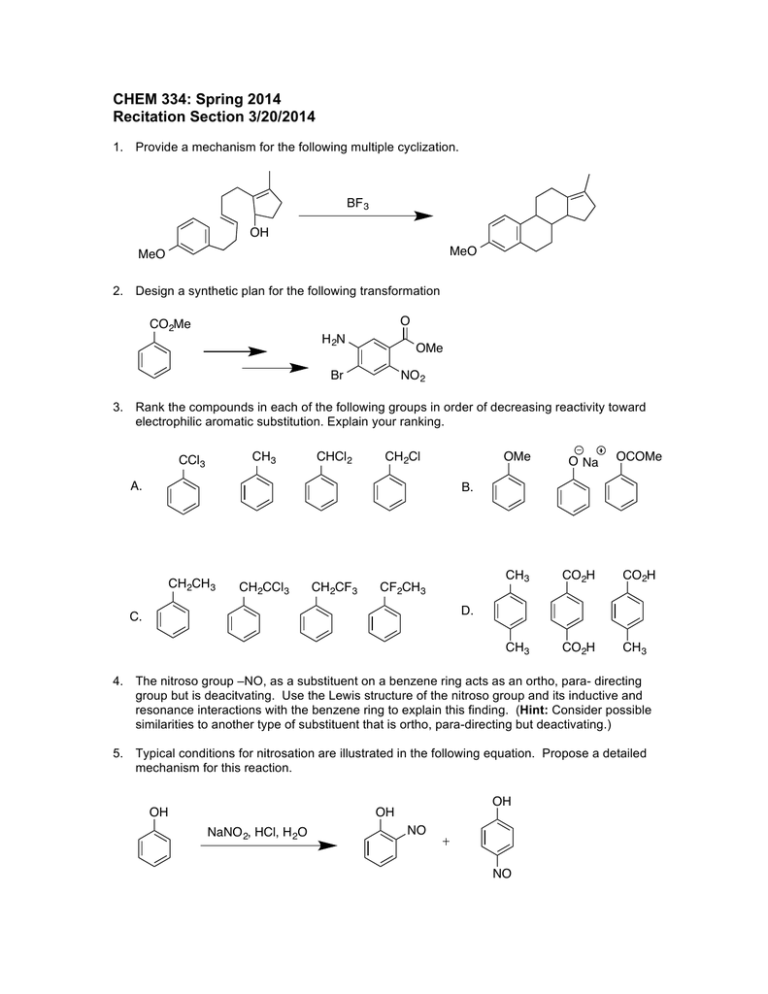
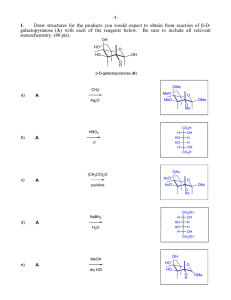
CHEM 334: Spring 2014 Recitation Section 3/20/2014 1. Provide a mechanism for the following multiple cyclization. BF 3 OH MeO MeO 2. Design a synthetic plan for the following transformation O CO2Me H 2N OMe Br NO 2 3. Rank the compounds in each of the following groups in order of decreasing reactivity toward electrophilic aromatic substitution. Explain your ranking. CH 3 CCl 3 CHCl2 CH2Cl A. OMe O Na OCOMe CH 3 CO2H CO2H CH 3 CO2H CH 3 B. CH2CH 3 CH2CCl 3 CH2CF 3 CF2CH 3 D. C. 4. The nitroso group –NO, as a substituent on a benzene ring acts as an ortho, para- directing group but is deacitvating. Use the Lewis structure of the nitroso group and its inductive and resonance interactions with the benzene ring to explain this finding. (Hint: Consider possible similarities to another type of substituent that is ortho, para-directing but deactivating.) 5. Typical conditions for nitrosation are illustrated in the following equation. Propose a detailed mechanism for this reaction. OH OH OH NaNO 2, HCl, H 2O NO NO