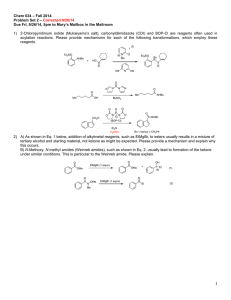
Nature's Catalysts: A Search for Synthetic Equivalents I
advertisement

R.A. Rodriguez
=K$",$2>$7+7)3")$"6.B(7A"."A$$'"('+.E""
"";M"573>(-.)($7"1-$2"7.)%-+"U$-")6+".*(&()0")$")6(78"$%)3('+")6+"*$VW"
""XM"97/02+">$,8+)"$-"1%7'.2+7).&&0"1+.3(*&+@"
Y6(&$3$>60E"*+#!",-"#(!#.$/+#-01-2(/!+$%#("$/+/+.#3"!&#!+-#!3#
######################(4-#."-$(-'(#14-&/'(#!3#$%%#(/&-Z""
""LM"[)(&(/+"7.)%-+\3"K(3'$2")$"A.(7"5]RY5I;=5^]"
""PM"=.8+")(2+")$"'+,('+"(1"(1")6+",6.&&+7A+".)"6.7'"(3"
""""""_[]`;C9]=;aab">$33(*&+""
Me
Me
H
H
OH
OH
Me
H
Me
E.C. 1.14.15.4
Me
H
H
HO
C.D$-"(33%+3E"
!"#$%&'"()"*+",-./0"$1"2+")$"3.0"45")6(78")6(3"-+.,)($7"$7")6+"9:;<="!"#$%&!'(#9:;<="3%*3)-.)+"(3">$33(*&+?@"
!"=+,67$&$A(,.&".'B.7,+2+7)3"
!"C.D$-"(33%+3E"FGH")'"HGH@"
Co(OAc)3, O2
!!"I+A($3+&+,)(B()0J")6(3"(3"K6+-+")6+"2(7'").8+3"."3).7'"
5,,-+,6&#(!#24/%!'!247E"FM"U)6(3"(3"8+0W"`^"]^="a5C5="0$%-3+&1"
*0"K6.)",.7".7'",.77$)"*+"'$7+"K()6")$'.03")+,67$&$A(+3"
O
LM"FN"O"PN""
PM"<6+2$3+&+,)(B()0""
FM"I+A($3+&+,)(B()0""
QM"R)+-(,3""
HM"R)+-+$3+&+,)(B()0""
H
H
H
OH
SLT"
OH
Me
HO
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
Cl3CO2H
3
74
5
13
2
selectivity for chlorination (%) shown
Crabtree, R. Chem. Rev., 1985, 85, 245-269
R.A. Rodriguez
Nature's Catalysts: A Search for Synthetic Equivalents I
Y6(&$3$>60E"*+#!",-"#(!#.$/+#-01-2(/!+$%#("$/+/+.#3"!&#!+-#!3#(4-#."-$(-'(#14-&/'(#!3#$%%#(/&-Z""
""LM"[)(&(/+"7.)%-+\3"K(3'$2")$"A.(7"5]RY5I;=5^]"
""PM"=.8+")(2+")$"'+,('+"(1")6+",6.&&+7A+".)"6.7'"(3"_[]`;C9]=;aab">$33(*&+"
""FM"`^"]^="a5C5="0$%-3+&1"*0"K6.)",.7".7'",.77$)"*+"'$7+"K()6")$'.03")+,67$&$A(+3"
;7".))+2>)")$"A.(7"3$2+"1%7'.2+7).&"(73(A6)E"5#'-$"14#3!"#'7+(4-(/1#-86/)$%-+('####
NSO2Ar
OH
O
O
R
O
H
O
R
CO2Et
H
OH
R
OMe
N
enediol
Me
O
OMe
O3
BzO
MeO2C
OH
OH
78 %
NSO2Ar
BzO
MeO2C
CO2Et
O
H
N
OH
Me
CO2Me
O
CO2Me
Baran GM
2010-08-21
R.A. Rodriguez
Santoro, M. et. al. Polyhedron,
2007, 26, 1, 169-177
Synthetic Equivalent: chromate ester
OH
OH
HO
BRENDA (BRaunschweig ENzyme DAtabase):
A powerful tool for the synthetic chemist
OH
HO
- Founded 1987 by former German National Research Center for Biotechnology
now: Helmholtz Center for Infection Research
- Comprehensive electronic enzyme information system: web-based user interface
- Contain molecular and biochemical information on all classified enzymes: E.C. #
- Characterized with respect to its catalyzed biochemical reaction
- Currently maintained and further developed by Department of Bioinformatics
and Biochemistry at TU Braunschweig
- Updates performed twice a year (Including improvemens to the user interface)**
- Latest update was performed July 2010
[Part I] An initial attempt to gain fundamental insight
1. Cyclic polyol oxidations
HO
E.C.1.1.1.18
OH
HO
OH
HO
OH
O
HO
HO
OH
50 °C
30 %
HO
OH
HO
OH
OH
H
HO
HO
HO
H
OH strain
release
OH
OH
H
HO
HO
HO
H
O
OH
OH
OH
O
HO
OH
94%
DHA
HO
Cameron, R. E. et. al. JACS,
1985, 107, 6116
OH
O
HO
Corma, A. et al.
Chem. Rev., 2007,
107, 2411-2502
GLYD
OH
OH
HO
HO
OH
OH
O
GLYA OH
OXALA
Synthetic Equivalent: heterogeneous catalysis
HO
O
Pt/Bi
OH
Heyns, K. et al. Methods Prep.
Org. Chem., 1963, 2, 303
OH
OH
O
O
OH
OH
O
Rules:
1. Only axial are oxidized
2. Selective in case of > 1 axial
3. Oxidation stops at monoketone
O
O
HPYA OH
OH
O
HO
glycerol
dehydrogenase
HO
HO
OH
OH
OH
O
Synthetic Equivalent: oxygen-platinum catalysis
Pt/C, O2
OH
pH 8-9
OH
O
Cr
E.C.1.1.1.6
OH
HO
OH
HO
O
O
OH
OH
O
PCT Int. Appl.,
2000075355
HO
2. Acyclic polyol oxidation
OH
HO
pH < 1
2 h, 33 °C HO
OH
OH
OH
O
Oxidations of industrial importance: An underdeveloped market due to selectivity
OH
HO
Cr
HO
VI
OH
95%
myo-Inositol
dehydrogenase
Xanthomonas sp.
OH
OH
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
OH
HO
pH = 2-3
OH
Kimura, H. et al. Appl. Catal.
A. General 1993, 96, 217
conversion: 75%;
yield: 37%
Influence of pH on product distribution during
optimization of Pt/Bi oxidation of glycerol
pH
GLYA (%)
DHA (%)
HPYA (%)
OXALA (%)
8
6
4
20
36
5.5
1
25
20
40
OH
OH
OH
E.C.1.1.1.67
HO
OH
mannitol
2-dehydrogenase
OH
OH
OH
OH
OH
HO
HO
O
OH
OH
O
O
H
!
O
R
O
HO
isomerization
1. Me2CO,
2. K2CO3, CH2O
O
SiO2, Et3N
OH
OH
OH
70 %, 3 steps
OH
OH
isomerization
OH
56 %
OH
O
O Mo
O
HO
O
Mo
O
H
O
H
MeO
OH
Me
Et
OH
OAc
N
CO2Me
Me
O
H
OH
Et
OAc
CO2Me
Application to simple #-hydroxy aldehydes/ketones
O
OH
OH
- Voight amination
O
CO2Me
N+
MeO
OH
O
OAc
OH
N
OH
OH
O
N
Me
CO2Me
OH
MoO3, H2O,
90 °C
HO
Et
O
85 %
H+
O
OH
Et
N
MeO
OAc
N
O
HO
OH
OH
Bilik RR
OH
Me
HO
OH
MeO
OH
3. TFA, H2O
D-Mannose
O
O
N
H+
OH
OH
N
Synthetic Equivalent: oxidation/isomerization
OH
NHR
Landmarks in the history of carbohydrate chemistry
or unrecognized synthetic opportunities?
H
OH
OH
HO
Amadori retron
Topics in current chemistry,
2001, vol. 215/2001, 115-152
SpringerLink
OH
R
OH
OH
NR
Strategic oxidation of natural products: Boger's asymm. synthesis of vindoline
O
HO
kinetic
driving force
Amadori
OH
- Bilik RR
- Lorby-de Bryun-van Ekenstein
(LdB-AvE)
enediol
- Heyns
- Amadori
- Voight
!
OH
OH
N-glycoside
OH
R
epimerization
NR
(ring-opened form for simplicity)
Carbohydrate isomerizations: enediol intermediates
OH
HO
Heyns
OH
OH
O
OH
OH
RNH2
Option 1 via isomerization after oxidation of the primary alcohol
R
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
OH
R1
R2
NHR
RNH2
P2O5, "
- Exploitation of additional driving
forces??
O
R1
R2
- strain release (furanoid to pyranoid)
- steric and/or electronic relief
Option 2 via selective oxidation of secondary alcohol
Selective oxidation of 1° alcohols using TEMPO:
Many commonly used oxidizing reagents oxidize 2° aliphatic alcohols at rates
slightly faster than 1° alcohols, but the difference in rates is not significant.
R
OH
H
[Cr]
R
R
O
H
[Cr]
R
complexation
B:
RDS
R
deprotonation
O
R
O
H
X
vs.
R
R
O
H
H
2°
more electron rich
R
OH
X
1°
Reasoning and considerartions behind highly selective oxidizing reagents:
- Sterics
- Electronics (electron rich or electron deficient carbon in the transition state?)
TEMPO & TEMPO-type oxidations
Angelin, M. et. al. Eur. J. Org,
Chem., 2006, 4323-4326
Basic
Me
Me
- H+
N
O
(cat.)
Me
Me
OH
Me
Me
N+
Me
Me
Me
Me
B:
H
N+
HO O
H
OMe
a. Halogen-based oxidants
O
R
X
- Bromide/chloride-based
via
H
- N-halogenated reagents:
hypohalite
R
N-bromoacetamide (NBA),
B: proton
N-bromosuccinimide NBS),
transfer
N-chlorosuccinimide (NCS),
trichloroisocyanuric acid (ICC)
Br2, HMPT,
NaHCO3,
OH
OH
R
R
R
Br
OH
?
R
O
R
X
H
hydride
anion
O
Me
Me
N
R
H
R
+ 2Br
-
H
+ H+ + H3O+
R
Br
O
H
O
(A)
R
R
H2O
O
R
H
H
O
H2O
Me
Me
Me
Me
R
O
R
OH
Acidic
HO
HO
Mechanism of bromide oxidation
(Venkatasubramanian N. et. al. Tet. Lett., 1968, 14, 1711-1714)
O
Me
Me
O
R
86%
91 %
N+
-O
O
H
DMF, 2,6 Lut.
OH
O
Selective secondry aliphatic alcohol oxidations discussed
a. Halogen-based oxidants
b. Peroxides
Arterburn, J.B. Tetrahedron
c. Dioxiranes
2001, 57, 9765-9788
d. Oppenauer variants
Br
[O]
OMe
Breton, T. Eur. J. Org. Chem
2007, 1567-1570
H2O/CH2Cl2
Proposed mechanisms: alkaline vs. acidic conditions
Naik, N. et. al. Tetrahedron, 1998, 54, 667
(Anelli-Montanari process)
O
TEMPO+ BF4-
OH
O
HO
HO
R
- Discovered by Lebedev and
Industry applications:
Kazarnowskii in 1960
Me
Me
- Stability of radical attributed to
Pagliaro, M. et. al. OPRD,
Me
Me
N
steric protection of methyls
2010, 14, 245-251
- Highly selective for 1° alcohols
O
- R = OH, NHAc reactivity steered
- Chemoselectivie: inert towards 2° alcohols but
converts aldehydes to carboxylic acids
Me
Me
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
R
Br
H2O
(B)
Br
R
Br
O
H
H
Br
H
Br
R
R'
O
H
Br
(C)
H2O
- Parallelism with chromic acid oxidation (alcohols > ethers)
- However, while 2-propanol reacts 1500X faster than diisopropyl ether, the rate
constants for the Br2 oxidation of both 2-propanol and diisopropyl ether (25 °C
pH 4.6) are identical.
R.A. Rodriguez
Linear free energy correlation: Taft plot (like Hammett but for aliphatics)
Rate mesurements on the bromine oxidation of alcohols with suitable
electronegative and electropositive substituents
C2H5 -
Et4NCl3, py.,
DABCO,
OH
- Et
- Me
OH
CH3CN, rt
100%
Me
O
R H
Electronegative
and Electropositive groups
compared to R = H
standard
OH
H
HO
O
OH
Me
R
H
Cl
H
H
H
OH
H
Me
R
H
Me
Me
O
Me
Cl
O
Cl
Me
N
N
CO2Bn
OH
BnO2C
i. Bu2SnO, MeOH, #
H
H
Me
H
H
OH
Me
Me
Et
ii. Br2, CH2Cl2, Bu3SnOMe
92%
!: 62 %
": 100 %
OH
O
O
CO2Bn
OH
O
-O
HO
O
O
OMe
Me
O
Me
OH
Me
HO
Me
N+
O
Me
Me
Me
OH
O
O
H
N
Me
Me
HO
OBn
Me
OH
Me
Me
HO
(5 eq.) py,
CHCl3
O
H
BnO
OH
H
Cl2 (1.1 eq.),
H
H
Cl
OH
O
Me
HO
OH
O
BnO
87%
OBn
Me
conclusion: It is evident that the influence of polar groups is very pronounced.
While several arguments can be advanced to explain the negative
$* value, the simplest explanation is a rate-determining loss of the
secondary hydrogen as an anion.
O
ii. Br2, CH2Cl2, Bu3SnOMe
N
BnO2C
-F
i. Bu2SnO, MeOH, #
O
HO
BnO
magnitude of $* = -2.6
HO
O
OH
BnO
OH
O
64%
- Br
H
HO
CH2Cl2, rt
- OMe
H
O
(Bu3Sn)2O, Br2,
O
Ph -
HO
O
OH
Taft plot for the Br2 oxidation of:
H-
H
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
OH
Me
(Bu3Sn)2O, Me
Me
Br2
Et
Me
CH2Cl2, rt
58 %
Me
OH
Me
N+ Me
HO
O
O
O
-O
O
OMe
O
Me
O
Me
OH
Me
Me
BzO
OH
O
MeO2C
DME/H2O
OH
NBA in excess
BzO
1.5 eq. NBS,
MeO2C
78 %
OH
Clarke. C. et. al. Tetrahedron,
1988, 44, 13, 3931-3944
Me
Me
OH + 2
H
Me
N
Me
O + 2
Br
Me
Me
Me
NH
+ Br2
[1]
O
[2]
+ 2HBr
HO
Me
2HBr + 2
Br
2
Me
NH + 2Br2
[3]
OH
acetone,
pyridine
OH
Me
- [3] is
than [2]
- NBS oxidation is composed of two stages, an initial slow reaction superceded
by a faster one involving the attack of Br2 on the alcohol moiety
Cl
(ICC)
R
O
H
H
O
slow
R
N
O
N
O
H
H
N
O
Cl
HO
HgBr2 + 2OAc-
Kinetics & Mechanism
of KBrO3 [Br(V)] see:
Srinivasan N. S. et. al.
Tetrahedron, 1974, 30, 2785
Cl
N
N
Et
OH
S+
°
2.170 A
Et
Snyder and co workers?
NCS/Diisopropylsulfide: temperature-controlled selectivity
Me
O
O
HO
AcHN
Me
O
Me
S
O
Me
Kim, K. S. et. al. J. Chem.
Soc., Chem. Commun.,
1984, 762-763
Me
NCS,
S
O
Me
CH2Cl2, 0 °C
70%
Me
Me
Me
HO
Me
O
O
AcHN
65%
Me
O
HO
Me
CH2Cl2, -78 °C
OH
Proposed mechanism: [3]/[2]
Second stage involving the fast attack by Br2
is completely supressed by addition of Hg(OAc)2
Br
Cl
Br
NCS,
Kinetics of NCS see:
Srinivasan N. S. et. al. Tet.
Lett., 1970, 24, 2039-2042
Sb
°
3.173 A
N
O
O
Cl
Cl
O
OH
O + HBr + HN
N
2Br - Hg(OAc)2
H
Cl
Cl
R
Br
OH
O
Proposed mechanism:
Initial slow stage
O
H
72 %
104 faster
R
Me
Reagent dependent results: trichloroisocyanuric acid (ICC)
Me
N
Me C(CN)CH2OH
O
90-96%
H
Me
H
H
CH3OH/H2O
py. rt, 5 h
H
H
H
O
4 eq. NBA,
H
OH + Br2
H
10 °C, 5 h
71%
H
OH
Me
Me C(CN)CH2OH
Me
Me
O
tBuOH/H O
2
H
H
Me
Me
H
O
Me
2 eq. NBA,
OH
H
OH
O
Me
Me
HO
OH
O
O
Mechanism of NBS oxidation
Venkatasubramanian N. et. al. Can. J. Chem., 1969, 47, 1969,
Venkatasubramanian N. et. al. Tet. Lett., 1967, 35, 3349-3354
Me
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
HO
N
Ac
O
O
Me
Me
R.A. Rodriguez
Nature's Catalysts: A Search for Synthetic Equivalents I
b. Peroxides
- Molybdenum-cat. with peroxides
c. Dioxiranes
- DMDO proposed mechanism (Bovicelli, P. et. al. Tetrahedron, 1996, 52, 10969)
(NH4)6Mo7O24, K2CO3,
H2O2, Bu4NCl, THF
OH
[C5H5N+(CH2)15CH3]3[PMo12O40]3-,
tBuOOH,
OH
rt, 42 h
OH
OH
77%
HO
O
OH
Tricetylpyridinium-12-tungstophosphate (CWP) = [PW12O40][C5H5N(CH2)15CH3]3
H2O2, H2O,
tBuOH
68%
Cr-PILC cat./TBHP,
O
CH2Cl2, rt, N2
H2O2, TS-1
rt, 2 days
Titanium doped zeolites (TS-1)
OH O
Me
OMe
OMe
OH
OH
rt, 12 h
> 95%
OH
n
O
DMDO (1.5 eq.),
Acetone
N3
OH
n
1, 2 diol (n = 0); 89%
1, 4 diol (n = 2); 100%
1, 7 diol (n = 5); 94%
1, 3 diol (n = 1); 84%
1, 4 diol (n = 2); 100%
1, 5 diol (n = 3); 93%
OH
O
O
OH
OH
Bovicelli, P. et. al. Tetrahedron,
1998, 54, 14301-143134.
OH
H2O2, H2O,
CHCl3,
rt, 16 h
93%
Chromiapillared montmorillonite (Cr-PILC)
n
HO
O
OH
- Linear 1,2 diol; 1,3 diol; 1,4 diol; 1,5 diol; 1,7 diol = no problem
OH
H
Me
rt, 12 h
> 90%
OH
O
CWP (1.6%)
OH
DMDO (1.5 eq.),
Acetone
OH
OH
CWP (1.6 %),
OH
heterogeneous
O
!+
DMDO superb regioselectivity for cyclic polyol systems:
Not only 2° C-H > 1° C-H, but also selective for single 2° C-H
- Tungsten-cat. with peroxides: solvent dependent cleavage
OH
O
Me
OH
PhH, 75 °C
100%
homogeneous
!- O Me
most e- rich C-H:
O
O
1. hyperconjugation
(oxygen lone pair donating into O-C bond making H hydridic) Me
OMe
2. 2° C-H more e- rich than 1° C-H due to inductive effects
OH
- Neopentyl: quantitative yield
HO
DMDO (4 eq),
acetone
O
OMe
O
88%
OH
OH
Me
rt, 24 h
Baran GM
2010-08-21
OH
N3
DMDO sensitivity to sterics
(Buxton, P. C. et. al. Tet. Lett., 1999, 40, 4729-4732)
Me
Me
H
R
DMDO (2 eq.), Acetone
HO
H
H
0-5 °C, 16 h
H
75%
OH
Me
Me
O
R
HO
H
H
R.A. Rodriguez
d. Oppenauer (OPP) variants (aka Meerwein-Ponndorf-Verley-Reduction: MPV)
- Mechanism: internal hydride transfer to accepting surrogate
OH
R1
O
R2
+
R3
R4
R3
Oi-Pr
Al(OR)3
R1
AlO+
R4
Oi-Pr
HOi-Pr
H
O
R2
Potent analogues from natural products: Semi-synthesis real world example
- provide the need for training of synthetic chemist in natural product synthesis
NH2
O
hydride
OH
transfer R1
R2
(OPP)
R3
R4
O
OH
Al2O3 (2 eq.), PhCHO
8
N
(MPV)
rt, 24 h
65%
OH
S
O
HN
8
[Nature's catalyst build complexity as well as degrade complexity]
S
Increased
complexity
O
NH
CO2H
Me
The discovery of CB-184,375 and
other semi-synthetic Thiopeptide Antibiotics.
Cubist Pharmaceuticals, 50th ICAAC National Meeting
Total selective degradation: Some current tools for synthetic analysis
Bergman degredation:
R2
Macromolecules
- Proteins
- Lipids
- Carbohydrates
HN
HO
HO
A paradigm: Cell regulation and Biomimetic synthesis
Macromolecules
- Proteins
- Lipids
- Carbohydrates
O
N
N
N
N
The Cell: The most basic and fundamental unit of life
O
H
N
R1HN
O
R2
1. curtius RR
OH
BnOH, !
R3
H
N
R1HN
O
H
N
R3
O
O
Me
2. [H]
Interesting point...
2° Metabolites
CELL CYCLE:
HOMEOSTASIS
Advanced
intermediates
for other cellular
processes
S
O
[Part II] A big lesson from nature: Achieving true biomimicry
2° Metabolites
O
N
N
O
S
N
N
H
S
NH
Antimicrobial agent:
Thiazolylpeptide (GE37468A)
O
O
+
- Oppenauer variant: 1,10 diol selective oxidation
OH
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
Reusable
starting
materials
NH2
R1
O
Advanced
intermediates
from other cellular
processes
H2N
+
O
R2
-CO2
R1
O
R2
NH2
R1HN
O
O
+
R3
H
Degradation name reactions: Edman degradation, Emde degradation,
Gallagher-Hollander degradation, Hooker degradation,
Marker degradation (Parke-Davis and Syntex), Strecker degradation,
Von Braun amide degradation.
Fragmentations and RR's: Grob fragmentation, Hofmann RR, etc.
+ NH3
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
- Another company that values marine natural product drugs: PharmaMar (Zeltia) partnership with Johnson & Johnson
Market drug: Yondelis!;
Pipeline compounds: Aplidin!, Irvalec!, Zalypsis!, and PM01183
HO
OMe
NH
MeO
O
HO
AcO
Me
O
S
H
H
N
Me
O
OH
Ecteinascidia turbinata
O
O
Me
Me
O
O
OH
O
NH
O
Me
O
Me
Me
HO
Me
O
N
O
O
Me
Me
Aplidin!
(anticancer-apoptosis inducing agent)
H O
H
H
O
H
Me
H
O
H
H
H
H
Me
Newman and Cragg. Curr. Med.
Chem, 2004, 11, 1693-1713
O
O
H
H
H
O
O
H
H
H
O
Me
Aquaculture initial results
promising but not ideal
For more examples of advanced
preclinical and clinical trials of
marine natural products:
O
H
O
O
O
HO
O
Me
H
H
O
HO
Me
N
N
H
Me
molluscs and sponges
Another class of privileged marine natural products: The Halichondrinbs
Me
N
O
NH
CF3
Zalypsis!
O
N
NH
OH
O
OMe
Me
Me
H
O
Ecteinascidin 743
Yondelis!
Me
N
H
O
H
H
Me
H
N
O
OAc
Me
N
Me
HO
OMe
O
H
Halichondrinb B
(anticancer-tubulin interactive agent)
O
O
H
R.A. Rodriguez
3. Oxidative aromatic ring fission: Synthetic strategy and selective degradation
O
HO
E.C.1.13.11.22
OH
caffeate
3,4-dioxygenase
HO
OMe
CO2H
MeO
CO2H
NSO2Ar
OMe
N
O
CO2Et
O3, AcOH, H2O
N
CO2Me
Me
OMe
MeOH, HCl, !
NSO2Ar
NH
N
H
75%
OH
CO2Et
H
CO2Me
N
H
MeO2C
O
CO2Me
N Me
Woodward R. B. et. al.
JACS, 1954, 76, 4749
reasonable
mechanism for 3?
HO
HO
N
H
OH
OEt
B
O
OH
N
O
O
H2O
O
R
H
CO2H
R = H; O2
R = substrate
H
N
H
koumine
A
Me
EtO
Liu, C.-T. et. al. JOC,
1983, 48, 44-47
H
O
CO2H
N
N
2. -H2O
Me
CO2Me
OH
1. [H]
CO2Et
N
OH
1. LAH,
2. HCl 39%
3. h" / O2, 34%
OH
isomerization
NSO2Ar
OiPr
N
N
H EtO
75%
H
N
O
2. ClCO2iPr,
Na2CO3,
CHCl3/EtOH
NSO2Ar
CO2Et
O
1. CH2O,
3d, rt, 95%
CO2Me
O
OMs
CO2Me
Application to natural products: Liu progress towards koumine
H
29%
47%
O
N
MeO
-78 °C !
OMs
CO2Me
O
MeO2C
MeO2C
O3, BF3-Et2O
CH2Cl2
O
N
MeO
NSO2Ar
CO2Et
H
Biomimetic Synthesis of (±)-8-Oxoerymelanthine: BF3-Et2O as an additive
(Yoshida, Y. et. al. JOC, 2009, 74, 6010-6015)
O
OH
Application to natural products: Woodward fission (Strychnine)
Me
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
OH
CO2H
CO2H
O
OH
ROOH
e- transfer
type
O
hv
O2
CO2H
CO2H
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
Oxidative aromatic ring fission: Oxidation with ozone
Oxidation with ozone: BF3-Et2O as an additive, a proposed explanation
Unstable at high concentrations, decaying to O2
(half life of 30 min. at atmospheric conditions)
Combustion of ozone at > 10 wt%
Explosive nature attributed to reduced ozonide
O
O+
O-
-O
O+
Additional increase in electrophilicity:
O
O
- Generally accepted mechanism of ozonolysis of alkene (Rudolf Criegee-1953)
-O
O+
[1,3]
O
O
O
retro
O
dipolar
R1
[1,3]
R1
R2
R2
O+
O-
R1
O carbonyl
R2
O+
O-
[1,3]
General selectivities for alkenes:
- electron rich > neutral > electron poor
Schreiber work-up conditions:
- reductive or oxidative work-up yields different products
O
Pschorr (1907)
Wieland (1928)
B
O
C
MeO
O
R2
O
OH
O-
O
ozonide
R1
O
O3
Path A
O
OH
BF3
O
O3
O
Path B
O
O
OH
H2O
NMe
standard
ozonolysis??
60% (Na salt)
-H2O
O
MeO2C
OHC
- O3 xs.
A
40%
B
OH
RO2C
O
75%
O
NMe
HO
OH
O3
H2O
O-
O
O
OOH
OH
O
-H2O
OH
O
O
Rapoport & Payne (1950)
0 °C, 1 mol O3/5 h
0.5 M AcOH (4.5 N)
H
O
O
NMe
OH
NMe
- HCO2H (aq.)
RO
O
Bailey, P. S.,Ozonation in organic
chemistry: Nonolefinic Compounds,
Academic, New York, 1982.
-O2
Speyer (1926)
R = Me, Et
O+
OH
OH
O
HO
-O
MeO
A
C
BF3
Why these conclusions?
Studies of dry ozonations on silica gel (examples on next slide)
Oxidative aromatic ring fission: Ozonolysis on the morphine related alkaloids
(Mander, L. N. and Williams, C. M. Tetrahedron, 2003, 59, 1105-1136)
O
O
Proposed mechanism for ozonolysis of phenols (vide infra)
- for kinetic study on !,!,!-trifluorotoluene and 1,3,5- trifluorobenzene see:
Karpel Vel Leitner, N. et. al. New. J. Chem., 1997, 21, 187-194
O
R1
Criegee
intermediate
O+
R2
flip
MeO
Baran GM
2010-08-21
-H2O2
O
O
-O2
O
O
O
OOH
O
Oxidative degradation of benzene rings: carboxylic acid equvalents
- The carboxylic acid group is one of the most common functional groups
- It's not always an easy task to carry this functionality through a sequence
Expansion of the benzenoid synthon: An alternative to the bis acid
MeO
Me
h!
Me
MeO2C
CO2Me
MeO2C
O
O
76%
mechanism ?
Me
1. Ph2CuLi
Na+
CO2Me
CO2Me
CO2Me
O
Me
i. O3, HOAc
ii. H2O2
iii. CH2N2
88%
O
H
O
Me
Me
Yates et. al. J.C.S.
Chem.Comm. 1980, 990
H
Et
HO
MeO2C
O
CO2H
Me
Me
Me
Me
B
1. O3, rt, 3.5 h
Me
O
Me
O
BV
O
H
Me Me
B, C
CO2H
CO2H
H
Me Me
Conditions (A-D)
Me
[O]
Wenkert, E, et. al.
JOC, 1964, 86, 2044-2050
NO2
CO2H
CO2H
Me
H
Me Me
OH
NO2
NH
H
Me Me
O
1. hydrolysis
2. O3
40%
B: 95% conversion;
90% yield
D: 25% yield
Me
HN3
1. O3, HOAc
0 °C, 1 h
2. H2O2 (aq.)
A: 85-90% conversion;
60-80% yield
A, B, D
Me
H
3. NaOH (aq)
4. CH2N2
H
O
Dry ozonation on silica gel (see: Cohen, E. K. et. al. JOC., 1975, 40, 2141)
Reactivity vs Selectivity: A unique reactivity of ozone
(Klein, H. et. al. Tet. Lett., 1975, 4249-4250)
Expansion of the benzenoid synthon with ozone: Possible selectivity?
Schaffner et. al. Helv. Chim. Acta,
1956, 39, 174-183
Schmidt
H
H
O
O
Jung, M. E. et. al. JACS,
1980, 102, 2463-2464
Et
steps
H
steps
Me
2. NaCl
65%
DMSO/H2O
125-127 °C (2 steps)
CO2Me
46%
Me
O
", 1h,
THF
OMe
OMe
H
OH
Me
Me
MeO2C
OMe
-O
Application to synthetic strategy: Yates synthesis of sesquiterpene skeleton
MeO2C
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
A: 10 mmol O3/100g SiO2, -75 °C
B: 10 mmol O3/200g-300g SiO2, -75 °C
C: 10 mmol O3/200g-300g SiO2, 25% H2O, -75 °C
D: RuO4 [O] (previously reported result)
B: 20-25% conversion;
20% yield
B: 95% conversion;
50% yield
C: 95% conversion;
75% yield
Oxidative degradation of benzene rings: Oxidation with RuO4
Readily decompose explosively at elevated temperatures
Safer anionic salt of TPAP (Pr4N+ RuO4-) is commonly used
Mechanism for its use in benzene degradation is unknown
Catalytic from RuCl3 or RuO2 in presence strong re-oxidant:
NaIO4, NaOCl, NaBrO3, oxone, O3, Ce(SO4)2, and K2S2O8.
Expansion of the benzenoid synthon: One carbon dehomologation technique
O
O
RuO4, NaIO4
60 °C 10 d
25%
Ru
O
O
Me
57%
O
CO2Me
F3C
OAc
O
NaIO4
rt, 90 h
RuCl3, NaIO4,
CCl4, MeCN, H2O
rt, 24 h
Org. Lett., 2009,
11, 20, 4668-4670
O
OAc
ii. K2CO3
i. RuCl3-H2O
N
Me
N
F3 C
CO2H MeOH
O
72%
N
O
OEt
O
Me
2X
45 °C
2X
35 °C
Me
RuCl3, HIO4,
CCl4, MeCN, H2O,
50 °C, 2 h
PCT Int. Appl.
# 2009037719
2X
25 °C
RuCl3, NaIO4,
CCl4, MeCN, H2O,
70 °C, 3 h
Tet. Asymm., 2007,
18, 12, 1434-1442
40%
H
70%
D
O
N
H
CO2H
Me
O
N
H
NBoc
OAc
HO2C
O
Me
Me
RuO4: Chemoselectivity can be achieved
(Haddad, M. et. al. JOC, 2010, 75, 6, 2077-2080)
No [O]
H
Clayden, et. al. Tetrahedron, Chem. Comm., 2009, 29, 4396-4398
2002, 58, 4727-4739
!
H
?
H
55 °C " 4 h
NBoc
O
H
Reactivity vs Selectivity: An attempt to realize relative rate
71%
2. CH2N2
H
H
HO2C
O
Clayden, J. et. al. Tetrahedron,
2002, 58, 4727-4739
H
Me O
H
O
O
OH
1. RuCl3, NaIO4,
EtOAc/MeCN/H2O
NBoc
H
Me
O
O
!
H
OtBu
RuO4: Stereochemical information adjacent to reactive site is conserved
O
O
HO
OH
rt, several days, 12%
H
HO2C
H
H
Caputo, J. A. et. al.
Tet. Lett, 1967, 4729-4731
RuO4, NaIO4
OH
Me O
Me O
O
Oxidative degradation of benzene rings: carboxylic acid equivalents
- Caputo: first to apply RuO4 to cyclobutanols (30% vs 78%) and aromatic rings
Me
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
O
MeO
RuCl3, HIO4,
CCl3 CCl4, MeCN, H2O
rt, 2 h
> 54% (4 step)
H
R
1. RuCl3, NaIO4,
EtOAc, MeCN, H2O,
rt, 4 h
2. CH2N2, Et2O
R = H; > 57% (2 steps)
R = OMe; > 67% (2 steps)
Catalysis Comm., 2007, 9, 3, 416-420
RuCl3, NaIO4,
CH2Cl2, CH3CN, H2O,
rt, 2h
82%
CO2H
RuO4 degradation of steroids: An opportunity to gain mechanistic insight
(Piatak, D. M. et. al. JOC., 1969, 34, 116-120)
Conditions: RuO2, NaIO4, Acetone/H2O
Me
Me OAc
RuO2,
NaIO4,
Me2CO/H2O
H
H
H
OH
rt, 4.5 h
H
OAc
HO2C
H
AcO
H
RuO2,
NaIO4,
HO2C
H
Me2CO/H2O
H
H
H
rt, 4.5 h
AcO
H
Me OAc
H
Me
OH
HO
51%
H
O
minor (5%)
O
H
HO2C
HO2C
H
H
H
Expected:
O
H
H
Me
H
H
R
MeOH
O
H
H
R = CO2Me; 30% O
R = OMe; NOT observed
(decarboxylation)
O
Conclusions: At least two competing pathways at play involving benzylic [O]
A: Slow when e- density is taken from oxygen; C-H [O] at 3° benzylic carbon
B: Additional e- donating groups may overcome the slower benzylic C-H [O]
C: Presence of tertiary hydroxyl retards further decarboxylation
Anhydride intermediates via decarboxylation?
Decarboxylation process is sensitive to sterics?
Destabilizing effect
OH
Me OAc
Me OAc
MeOH
Me O
Me
Me OH
H
OH
MeO
AcO
Me OH
H
MeO2C
O
MeO
H
H
H
2. CH2N2
3. MeOH
H
H
Me OAc
1. RuO2, NaIO4,
Acetone/H2O
rt, 4.5 h
H
H
OH
H
HO2C
RuO2, NaIO4,
Acetone/H2O
rt, 4 h
HO2C
+
major (77%)
H
71%
Me OAc
minor
Me OAc
H
H
HO2C
HO
H
2X benzylic [O]: major (40%)
Me OAc
H
Me O
RuO2, NaIO4,
Acetone/H2O
rt, 4.5 h
H
HO2C
O
Destabilizing effect
Me O
Me OAc
+
AcO
Me
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
RuO2, NaIO4,
Acetone/H2O
rt, 4 h
99%
Me O
H
HO2C
HO2C
H
Me O
Me O
HO2C
O
H
H
H
O
HO
O
H
O
H
Me O
O
H
O
O
H
O
Ru
H
-CO2
O
H
O
Me O
H2O
H
HO2C
H
H
HO2C
H
Expansion of the benzenoid synthon: Reactivity vs Selectivity
Me
CO2H
O3 vs RuO4
Me
Me O
Me
CO2H
H
Me Me
RuO4 catalyzed oxidative polyenecyclization:
The next generation of the cyclase/oxidase phase approach
RuO2, NaIO4,
rt, 4 h
H
H
H
Me
O
head
HO2C
O
H
H
e.g. tail to head
HO2C
R
OH
H
O
H
RuO4: Reactivity
H
OH
Me
R
R
R
(1,5 diene) R
O
O
Me H
HO2C
HO
R
O
R
H
Me
H
OH
R
Me
Me
Me O
4
O
5
Me
H
HO
O
H
R
H
Me
OH
neodolabelline
H
H Me
O
H
Me
O
Me
OH
H
yields: 40-85%
0 °C, 10 min
48%, dr >95:5
HO2C
C-C bond
cleavage
O
RuCl3 5 mol%,
NaIO4/wet silica (4 eq.)
EtOAc/MeCN (1:1)
Me O
O
R
R
Me H
(All threo)
H
R
O
H
Achieving true
biomimecry
C-O bond
formation
H
H
Degredation
chemistry
OH
R
Me
HO
Efficient oxidative cyclization of 1,6-Dienes:
Roth and Stark. Angew. Chem. Int. Ed., 2006, 45, 6218-6221.
H
R
Increased
complexity
Me
Me
Me O
Achieving true biomimicry: RuO4, a privileged reagnt
R
RuO2-2H2O (20 mol5)
NaIO4 (8 eq.)
EtOAc/MeCN/H2O (3:3:1), 0 °C, 30 min
50%
Conclusions: Both ozone and RuO4 have their advantages. Ozone seems to be
a more selective reagent. The use in catechol-type substrates shows promise for
its use in complex systems. RuO4 on the other hand, is one of a kind in its ability
to degrade much less electron rich substrates and is a somewhat
underappreciated reagent but may prove to be selective, especially in cases of
m-methoxybenzene or p-methoxybenzene substrate types.
R
tail
e.g. tail to tail
H
NH
H
Me Me
oxidative polycyclization of squalene with cat. RuO4:
(Caserta, T. et. al. Tetrahedron, 2005, 61, 927-939)
Me O
Me
O3, rt,
3.5 h
Baran GM
2010-08-21
Nature's Catalysts: A Search for Synthetic Equivalents I
R.A. Rodriguez
O
OH
O
H
OH
H
?
O
H
O
Me
Me
R
H
Me
OH
O
*only one synthesis of reported of 4,5 deoxy:
longest linear 16 steps, 4.5% overall
Williams and Heidebrecht, W. JACS, 2003, 125, 1843.