Chemistry of High Energy Materials R.A. Rodriguez
advertisement

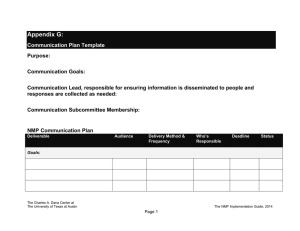
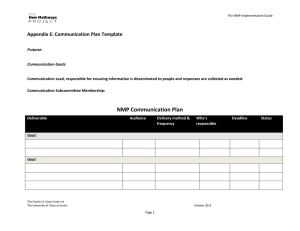
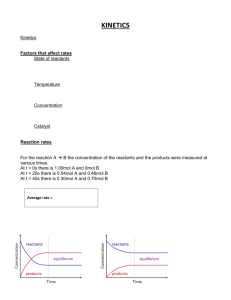
Chemistry of High Energy Materials R.A. Rodriguez Baran GM 2012-08-18 History of Explosives 7th century A.D.- "Greek Fire" petroleum distillate used by Byzantines of Constantinopole High Energy Materials 13th century - black powder (aka gunpowder) Chinese alchemists Non-explosive materials Explosives 18th century - black powder composition became standardized: KNO3/charcoal/sulfur (75/15/10 w/w) 19th century - NH4NO3 replacement for KNO3 in black powder 1846 - Italian Chemist Ascanio Sobrero invented nitroglycerin (NG) High Explosives Low Explosives [detonate] 1° Explosives 2° Explosives [detonate by ignition] - Lead azide - Tetrazene [need detonator] - TNT - RDX [deflagrate-burn rapidly] Propellants - Black powder - liquid/solid Pyrotechnics - Fireworks - Color/flash/sound Organic Chemistry of Explosives by J.P. Agrawal and R.D. Hodgson Prof. Thomas Klapotke - Ludwig-Maximilians-Universität München - Germany Chair of inorganic chemistry Dr. Michael A. Hiskey - Los Alamos National Laboratory What makes an explosion? 1863 - German chemist Julius Wilbrand invented 2,4,6-trinitrotoluene (TNT) originally used as a yellow dye. Potential as an explosive not appreciated due to difficulty to detonate (insensative). 1866 - Mixing of NG with silica (PBX) to make malleable paste (dynamite) late 19th century - nitration chemistry on common materials (resins,cotton, etc.) 1910 - military use of TNT for artillery shells and armour-piercing shells 1939-1945 - World War II - Research on explosives intesified (nitration chemistry) Developlment of cyclotrimethylenetrinitramine (RDX) and cyclotetramethylenetetranitramine (HMX). Introduction to high energy materials terminology Brisance: Shattering capabiliy of explosive. Measure of rate an explosive develops its maximum pressure. Relative effectiveness factor (R.E. factor): Measurement of an explosive's power for military purposese. It is used to compare an explosive's effectiveness relative to TNT by weight (TNT equivalent/kg or TNTe/kg). Exothermic chemical reaction comprised of an oxidant and fuel, which releases energy (gas and heat) in a given time interval. Detonation velocity (VoD): The velocity at which the shock wave front travels through a detonated explosive. Difficult to measure in practice so use gas theory to make prediction. Whats the difference between explosive, propellants and pyrotechnics? Specific impulse (Isp): The force with respect to the amount of propellant used per unit time. Used to calculate propulsion performance. Rate at which it burns (Flamming gummy bear vs. rocket booster vs. TNT) Speed of reaction will determine subsonic vs. supersonic blast pressure waves Oxygen balance (OB%): Expression used to indicate degree to which explosive can be oxidized. If explosive contains just enough oxygen to form carbon dioxide from carbon, water from hydrogen molecules and all metal oxides from metals with no excess, the explosive is said to have zero oxygen balance. Chemistry of High Energy Materials R.A. Rodriguez Chemical types/class of organic explosives Aromatic C-nitro compounds Aliphatic C-nitro compounds NO2 R1 1° nitroalkane R2 2° nitroalkane NO2 high explosive with high thermal and chemical stability NO2 Internal gem-dinitroalkane Trinitromethyl N H O2NN NO2 O2N S O2N O2N N H N 4-amino-3,5-dinitropyrazole (LLM-116) O2NN NO2 NNO2 H2N NO2 N NH2 O pyridine N-oxide O2N H 2N N N O NH2 NO2 NH2 N N N O O2N NO2 N NH2 pyrazine N-oxide tetrazine N-oxide NO2 Ar F2 N benzotriazoles tetrazoles N H2N N H N NO2 1,2,4 triazoles NH2 nitroguanadine O2N NNO2 O2N NF2 3,3-bis(difluoroamino) octahydro-1,5,7,7tetranitro-1,5-diazocine (TNFX) N Ar N O NO2 NO2 O2NN ONO2 N N NO2 O2N H2N N H N N ONO2 ONO2 O2NO N N NO2 O2N NO2 N H O2N Si H2N NO2 N,N'-dinitrourea (DNU) Si-PETN N N H ONO2 NO2 O2NO N N O O2N novel energetic nitrate eseter furazans/benzofurazans furoxans/benzofuroxans N N O ONO2 O2NO N N N RDX (VOD=8440 m/s) HMX (VOD=9110 m/s) O NO2 O2N Pentaerythritol tetranitrate (PETN) (VOD=8310 m/s) O2NO O2N NO2 ONO2 NNO2 N NH 2,4-dinitroimidazole (2,4-DNI) Aliphatic N-nitro compounds Nitroglycerin (VOD=7750 m/s) O O NH2 x≥4 poor chemical stability N O2NO O2N N NO2 O2N ONO2 O2NO NO2 (NO2)x 1,3,5-trinitrobenzene 2,4,6-trinitrophenol (TNB) (picric acid) O2NO O H2N O2N TNT NO2 O2N NO2 Aliphatic O-nitro compounds Nitro derivatives of pyrroles, thiopenes, and furans are not practical explosives: 1. heat of formation offers no benefits over standard arylene hydrocarbons 2. during nitration, these heterocycles are much more prone to oxidation and acid cat. ring opening compared to arenes H-bonding reduction in sensativity HO Me ONO2 NO2 heterocycles NO2 NO2 R1 NO2 NO2 3° nitroalkane NO2 R2 NO2 R1 acidic protons (condensation chemistry) Terminal gem-dinitroalkane R2 R3 R1 R NO2 NO2 NO2 NO2 R2 R Baran GM 2012-08-18 1,3,4-oxadiazoles N N NO2 NO2 N N NO2 Hexanitrohexaazaisowurtzitane (HNIW or CL-20) (VOD=9380 m/s) H N O2N Ar N N N O NH 3-nitro-1,2,4-triazol-5-one (NTO) Chemistry of High Energy Materials R.A. Rodriguez Nitration chemistry TMS _ OAc TMS Routes to C-Nitro functionality NO2 Me HNO3 25% Me Me N2O4 Me O Me Me NO2 H Me NO2 Me O2N O N O O2N N Me Me Me Me O O2N ONO Me Me Me Me dinitro O2N + ONO2 nitro-nitrite 1. NaHMDS 2. N2O4 O2N NO2 Ph OH DCE/ rt Ph + NO2 Ph 76% 74% O2N NO2 2. N2O4 -78 °C 77% NItration selectivity on arene/heteroarene Kakiuchi, et al. Synlett 1999. 901 H H NaNO3 xs, CAN 2eq R R AcOH/CHCl3 80 - 90% H R NO2 R H H R H R NO2 R R R R Hwu et al. J Chem Soc Chem Commun 1994. 1425 Taniguchi et al. JOC 2010. 75, 8126 Ph NO2BF4 MeCN 84% TBAN CO2R O NO2 NO2 NO2 O2N NO2 Cl N N CO2R Cl NO2 R O2N N NHAc Ph O2N Cl 80 - 90% NO2 H MeCN, reflx NO2 H2SO4 traditional [NO2+] 44% N Fe(NO3)3 9H2O R FeCl3 O2N KNO3 Cl NO2 92% NO2 S 23% Ph OAc NO2 Al2O3 Mukaiyama et al. Chem Lett 1995. 505 O O2N 1. nBuLi S NO2 O N NO2 O2N Br - colorless liquid 1 atm NO NO2 O N NO2 nitro-nitrate Radical methods Nef AcONO2 OAc O Me Me Me Me NO2 NO2+ ONO2 alkaline nitration O2N + _ O Nitration with HNO3 is difficult NO2 O N Direct nitration of aliphatic and alicyclic hydrocarbons possible in the vapor phase using HNO3 or NO2 (toxic redish-brown gas) at elevated temperatures. H O ONO2 [2+2] Borgardt et al. Chem Rev 1964. 64, 19 (polynitro functionality) NO2 NO2 NO2 AcONO2 The nitro gorup whether attached to aromatic or aliphatic carbon, is probably the most widely studied of the functional groups and this is in part attributed to its use as an 'explosophore' in many energetic materials. Baran GM 2012-08-18 F3 C O O O TBAN CF3 F3C O NO2 TFAA 76% N NO2 N CO2R * No rxn in presence of TEMPO Chemistry of High Energy Materials R.A. Rodriguez 1° and 2° nitro compounds Victor Meyer rxn - alkyl chlorides too slow - only good for 1° (2° alkyl halide gives nitrate ester) - nitrate ester arises from desproportionation of silver nitrate acc. by heat/light R + X N O NOH O ether R OAg NO2 + AgX R O NO 1.KOtBu amyl nitrate 2. H+ 1. NBS 2. [O] 3. [H] O NaNO2 DMSO fast X R R R N NO2 + O R nitrite ester R H+ OH NO2 HO OH phloroglucinol O O N NO R CO2H R HNO3 F 20 - 30% R NO2 CO2H O NO2 MeO2C NH3+Cl- O Ag+ N R2 O O Acetone 91% NH3+C C+H3N NH DMDO Acetone/H2O 85% O2N Eaton et al. JOC 1988. 5353 Ag0 Ag+ Ag O O N O O O -Ag0 N O R1 R2 O R2 O N N O R1 retro aldol O N O NH O Me via amine thus H2O essential R2 ONO2 R2 -CH2O O Me O NO2 Me O OH N Fe NO2 NO2 R1 Me N O2N NCO 2. AgNO3 NO2 ONO2 NO2 NO2 Me N O2N NO2 O2N N O NO2 O NO2 Original Target/Route via modified Kaplan Shechter Rxn O oxidation of isocyanates OCN R1 O Na DMDO C+H3N N O2N NO2+ O2N Kaplan Shechter Rxn 1. NaNO2 O O Me NO2 O R2 R1 nitronate N Me N R2 NO2 oxidation of amines O NaOH NO2 NO2 HNO3 60 % N O2NO R1 MeO2C H+ O Chavez, D.E. et al. Angew. 2008, 47, 8307 NO2 NO2 O O2NO R1 NO2 R NO2 S2O8 2- Synthesis of an energetic nitrate ester Kornblum et al. JACS 1956. 78, 1497 gem-dinitros from acids R only useful oxidant OH R OR NO2 NO2 NO2 (or) NO2 O2N NO2 F NO2 N (low yields) O Fluorotrinitromethane NO2 OH R NO2 slow O R R R O NaNO 2 O NO2+ O2N NO2 NO2H modified VM (alkali metal nitrites e.g. NaNO2) time and solubility is VIMP R CF3CO3H Baran GM 2012-08-18 O Me N O activation NH O Me O Me O NH N N N N O [O] O R N O NH NH O NH N N N R NH N N N Chemistry of High Energy Materials R.A. Rodriguez Initial Results: homocoupled product HO 1. 2-methoxypropene Me cat. H+ NO2 HO OH Me 2. NaOH Me O N O Me O O N O O O Me Me O O Me O N O NC CN +CN- CN CN O2NO m.p. 86 °C Det.Temp 140 °C O2NO - Use of mixed acids (esterification) and nitrogen oxides described for C-Nitraion Key points: H N 1. fuming (anhydrous) HNO3 prep: dry air bubbled through anhydrous HNO3 to O O N X H2N remove any oxides of nitrogen present, followed by addition of trace urea N N NO2 to remove any nitrous acid present. AKA "white nitric acid" N O N N 2. Urea destruction of nitrous acid important to avoid violent fume-off Me NO2 NH 3. O-nitrations with mixed acids of "white nitric acid" above ambient temperatures Me O O Me is dangerous and has increase risk of explosion NO2 NH Me 4. anhydrous HNO3/Ac2O- Acetyl nitrate is generally a weak nitrating agent but 12% O in the presence of a strong acid like HNO3, ionization to nitronium ion occurs O O Me O -NaSO4NO2 N NC CN NC Fe NC O O2NO O NaO3S O OSO3Na O ONO2 1.HCl, MeOH ONO2 2. Ac2O/HNO3 N O Me 72% (2 steps) O2NO O HO OH O NO2 OH O Me 65% optimized ONO2 OH NO2 OH NO2 O2NO ONO2 comparable stability to PETN N N N N NO2 Me Me N Me 2 eq NO2 Olah G.A. et al JOC, 1965. 30, 3373 ONO2 + MeCN quant yield ONO2 Me N H BF4Me in situ halide displacement with AgNO3 R O2N O2NO Me Me O2N 70% ONO2 Transfer nitration (neutral conditions - good for acid sensative alcohols) NO2 O HO Ac2O shock sensative Fe O2NO 20,164 MPH!! 90% ONO2 OH 90% HNO3 BF4Me NO2 anh HNO3 HO Ac2O NO2 O Me NO2 ONO2 SO3Na CN NO2 O Me O O Me O N O Me Me O NC Fe O N Me CNNC Routes to O-Nitro functionality 1.cat. K3[Fe(CN)6] Na2S2O8 NO2 Me activation - O Me O O Baran GM 2012-08-18 OH PPh3, I2 R I AgNO3 R CHEETAH calculates as powerful as HMX Low yields for 2° HgNO3 can be used for 2° and 3° alkyl halide displacements ONO2 decomposition of nitrocarbonates (very mild, rt or reflux MeCN) O RO O AgNO3 Py Cl RO -AgCl ONO2 -CO2 ring opening of strained oxygen heterocycles NO2 works for 1°, 2°, 3° alcohols N2O4 O (or) CH2Cl2 O ONO ONO2 R ONO2 80% H2O [O] N2O5 HO O2NO ONO2 ONO2 Chemistry of High Energy Materials R.A. Rodriguez selective O-nitrations 1 eq SOCl(NO3) 65% ONO2 OH nitrodesilylation anhydrous ZnCl2, hydrochloride salt of amine, or dissolved HCl(g) can serve as a source of electropositive chloride under the oxidizing conditions of nitration ONO2 OH 3 eq SOCl(NO3) O2NO 100% RO SiR3 -chloride ion catalysis OH 2 eq SOCl(NO3) O2NO 70% OH HO HO 2 HCl ONO2 RO CH2Cl2 ONO2 + O2NO SiR3 R NH2 R2NCl NO2F R MeCN HN N R N O N R NC N O CN Me 93% 22% How to get around this problem?? - synthesis via condensation chemistry (Mannich, 1,4 addition, etc) N R NH2 R NH2 Ar NH2 nBuLi -78 °C R NHLi 1. Na 2. EtONO2 Et ONO2 R N N - O Ar N N ONa HNO3 Ac2O R2NNO2 + + + + 4AcOH AcOH + AcOCl + AcOH N HNO3/Ac2O R = Cl- (N+) CN R N NC HNO3/Ac2O R=H CN NO2 N NC 70% CN Me 2 HOCl R NCl2 HNO3/Ac2O R N NO2 NaHSO (aq) 3 R NHNO2 Cl 6% A Path A O2N O R1 N R2 N + R1 CO2H R2 R2 O Path B R2 B R1 NO2 N + R2 OH R2 Nitramine formation via nitrolysis possible from: NO2 R NH R1 OLi Ar NH2 Na+ EtONO2 R2NCl N2O3 - Nitrolysis of fully substituted nitrogen rupture of C−N bond leading to formation on N−NO2 What about if need more direct method?? -non-acidic nitrating reagents (nucleophilic nitration) H+ R2NH + X analines - must contain one or more nitro groups on the aromatic ring O 2AcOCl AcOCl + 93% HNO3/Ac2O (w/o chloride) NO2 N NO2 R OH + N2O 3Ac2O NO2 NC - Direct nitration of a 1° amine to a nitramine using HNO3/mixed acids is not possible due to instability of the tautomeric isonitramine in strongly acidic conditions. 2° amines are more stable and can undergo electrophilic nitration using HNO3/Ac2O NO2 2° w/ HNO3/Ac2O H+ + Wright et al. Can. J. Res. 1948. 26B, 294 - Compounds resulting from nitration of nitrogen are of far less use for mainstream organic synthesis. However the N-NO2 group is an important 'explosophore' and is present in many enrgetic materials OH 2HNO3 ONO2 Routes to N-Nitro functionality O + ONO2 deamination N2O5 Baran GM 2012-08-18 H+ NO2 Ar NH R1 N R1 NO2+ R1 Ph N NO2 Ph O R2N NR2 R2N SiR3 R2N NO R1 - carbamate - urea R2 - formamide N - acetamide R2 - sulfonamide Ease of alkyl nitrolysis depends on stability of the resulting cation: benzyl, tertiary (t-Bu), etc... Chemistry of High Energy Materials R.A. Rodriguez Synthesis of Hexanitrohexaazaisowurtzitane (HNIW) Syntheses of some nitramine explosives - Many nitramines are more powerful than aromatic C-nitro compounds and have high brisance and high chemical stability and low sensativity to impact and friction compared to nitrate ester explosives. This is why they are of interest to military applications. O NH2 + Ph cat. H+ N O Ph [O] N Bn Ph O N CrO3 O Bn N N N N O Me Ac2O N Bn Bn Bn Bn Bn N Ph Bn N MeCN/H2O H H 2 eq. Bn Bn N N N N O2N O2N N N N Bn N N NO2 N NO2 NNs NsCl, K2CO3 (aq) 95% OH Bn 1. CrO3, AcOH 2. HOCH2CH2OH TsOH NNs 82% (2 steps) OH Br Bn X NO2+ O2N 1. HNO3/NH4NO3 urea, 33% NNs 2. H2SO4 92% NNs 1. O3, CH2Cl2 2. DMS NNs O O NNs NNs O O 76% Br K2CO3 NOH NO2 NNs decomp. messy. Nitration of aromatic rings NNs 3. NH2OH/NaOAc 86% (3 steps) O NNs O O Ph Ac O2N 1. N2O4 N O Bn Bn H , Pd(OAc) Ac Ac 2. HNO3/H2SO4 2N N 2 2 N N via nitroso Ac2O, PhBr cat. N N N N N 93% 65% Bn O2N Bn Bn Bn DANGER! H2, 99% HNO3 NO2 [Pd] O2N N NH2 N N H2, [Pd] X NH2 Bn N N N Baran GM 2012-08-18 Ac Ac Ac HN N N N Ac N N N N Ac NH 1. HCO2H 2. Ph, Δ -H2O Ac Ac OHCN HNIW aka CL-20 - explosive/propellant (low smoke-emission) - most powerful to date (better oxidizer-to-fuel than RDX/HMX) - first prep by Nielsen 1987 Naval Air Warefare - pilot plant in 1990 for 200 kg in China Lake facility - unmatched performance in specific impulse, burn rate, detonation velocity (9.38 km/s = 21,000 MPH!!) - highest density than any other explosive (d = 2.044 g/cm3) - thermally stable (250 -260 °C) but sensative to mechanical stress still greater stability than nitrocellulose, PETN and others. - 4 different polymprphs with different densities/properties NO2 N N NO2 F2NSO3H, 90% HNF2, H2SO4, CFCl3 Ns N NO2 O2N NO2 N N F2 N O2N HNO3/SbF6 Ns CF3SO3H N N N NO2 NF2 TNFX (3,3 bis(difluoroamino)octahydro 1,5,7,7 tetranitro 1,5 diazocine) Ac NCHO N F2N NF2 Ac N N O2N NO2 NH2 OH HBr-AcOH Br HO 160 °C 72% OH O2N NO2 N NO2 TNAZ NH3+BrBr Br NaOH, 80 °C 60 mmHg Br 1. NaHCO3, NaI DMSO, 100 °C N O2N 2. NaNO2, NaOH K3Fe(CN)6, K2S2O8 29% (2 steps) CH2Br 1. NaNO2 (aq) 2. HCl (aq) 10% O2N CH2Br HNO3/TFAA N NO2 81% N NO Chemistry of High Energy Materials R.A. Rodriguez N2O5 No single nitrating agent is as diverse and versatile It is considered as the future for energetic materials synthesis Preparation of N2O5 [Deville 1849] O AgNO3 non-acidic nitrating reagents (neutral) 1° amines/nitramines leads to deamination and formation of nitrate ester by-product but analines are successful. N2O5 O O O nitronium nitrate salt N N O [NO2+][NO3-] O condition: chlorinated solvents - clean and selective - non-oxidizing &n non-acidic HNO3 - adopts two structures depending on condition condition: anhyd. HNO3 - powerful but acidic and non-selective - 1st prepared over 150 years ago but due to difficult prep and low thermal stability (require -60 °C long term storage) received little attention. - Environmental restrictions and push for green chemistry sparked interest Advantage: Rxns are very clean - faster and less exothermic (due to absence of oxidation byproducts) - high yields - simple isolation - non-acidic conditions possible with this reagent (compared to mixed acids) rt Stable for 2 weeks at -20 °C N2O5 2 N2O4 + O2 Stable for up to 1 yr at -60 °C synthesis of TNT under mild conditions Me N2O5/HNO3 O2N NO2 H N O O N H O2N HNO3 H2SO4 N O O-nitrations of polyols OH OH OH HO OH OH O2N H N N NO2 P2O5 N N N N O O O2N NO2 ONO2 ONO2 N2O5, CCl4 0 °C quant yield HNO3 NO2 ONO2 ONO2 AgNO3 N2O5 H3PO4 N2O4 O3 N2O5 Process chemist at Defense and Evaluation Research Agency (DERA) in the UK Development of a flow process: Using commercial ozonizer to generate 5 - 10% mix of ozone in oxygen and mixed in flow with N2O4. N2O5 is trapped in solid condenser tubes (cooled by dry ice/acetone). Explosives in JACS: Sila explosives O2NO O2NO ONO2 PETN - Det. Velocity 8,400 m/s - d= 1.7 g/cm3 VERY high edens Si Klapotke T.M. JACS 2007. 129, 6908 - Used in WW I - One of most high energy explosives known - more shock sensative than TNT. used as booster mix - Europe marketed as lentonitrat (vasodilator) like NG ONO2 ONO2 ONO2 "The crystalline compound exploded on every occasion upon contact with Teflon spatula... Solutions in diethyl ether exploded upon the slighest evaporation of the solvent." Si(CH2OAc)4 Si(CH2Cl)4 Why does Si-PETN have drastically increased sensativity? Theoretical: electrostatic potential: 1. Surface electrostatic potential, in general, is related to the sensitivity of the bulk 2. The more evenly distributed the electrostatic potential is over the surface of a molecule, the more stable it is to impact. O2NO O2NO ONO2 O2NO + + - isolation by sublimation and collection trap at -78 °C - stream of ozone needed to avoid collection on N2O4 - if don't care about acidity, can use HNO3/P2O5 mixture directly (no ozone stream) VERY low e- dens O N H N2O5 Cl Δ O2NO NO2 N O P2O5 O2NO No explosion hazard N-nitrations of ureas Cl2 (g) Silicon analogue of PETN 32 °C, quant yield NO2 N H + Me NO2 H N + Dehydration of nitric acid polar sublime slightly above rt Baran GM 2012-08-18 O2NO O Si N O O