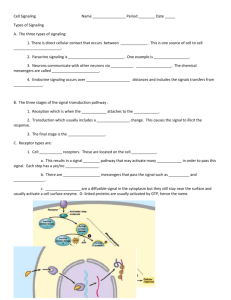
Immunology
advertisement

Immunology Nascent Drosophila Toll-8 crystals. Toll receptors are critical signaling receptors of development and of innate immunity conserved in evolution from flies to humans. Recombinant forms of these receptors are used to grow crystals and determine structures in order to establish a structure-function relationship. Work done in the laboratory of Luc Teyton, M.D., Ph.D. He Zhou, M.D., Ph.D., Research Associate Ralph A. Reisfeld, Ph.D., Professor Yunping Luo, M.D., Ph.D., Research Associate Department of Immunology IMMUNOLOGY DEPAR TMENT OF IMMUNOLOGY S TA F F Richard J. Ulevitch, Ph.D. Professor and Chairman Bruce A. Beutler, M.D. Professor Roberto Baccala, Ph.D. Assistant Professor Gary M. Bokoch, Ph.D. * Professor Dennis R. Burton, Ph.D. ** Professor Tsung-Hsien Chuang, Ph.D. Assistant Professor Charles G. Cochrane, M.D. Professor Emeritus Neil R. Cooper, M.D. Professor Emeritus Linda K. Curtiss, Ph.D. Professor Edward A. Dennis, Ph.D. Adjunct Professor Henrik Ditzel, M.D., Ph.D. Adjunct Professor Frank J. Dixon, M.D. Professor Emeritus Director Emeritus, Scripps Research Thomas S. Edgington, M.D. Professor 2006 THE SCRIPPS RESEARCH INSTITUTE 99 Peter Ghazal, Ph.D. Adjunct Associate Professor David Nemazee, Ph.D. Professor R. Anthony Williamson, Ph.D. Associate Professor Jiahuai Han, Ph.D. Professor Glen R. Nemerow, Ph.D. Professor Curtis B. Wilson, M.D. Professor Emeritus Wendy L. Havran, Ph.D. Professor Per A. Peterson, M.D., Ph.D. Adjunct Professor Rong Xiang, M.D., Ph.D. Assistant Professor Shuang Huang, Ph.D. Assistant Professor Pascal Poignard, M.D. Adjunct Assistant Professor Michael Zwick, Ph.D. Assistant Professor Julie Jameson, Ph.D. Assistant Professor Ralph A. Reisfeld, Ph.D. Professor S TA F F S C I E N T I S T S Jonathan G. Kaye, Ph.D. Associate Professor Matthias Riewald, M.D. Assistant Professor Richard Klemke, Ph.D.*** Associate Professor Moores Cancer Center San Diego, California Hugh Rosen, M.D., Ph.D. Professor Norman R. Klinman, M.D., Ph.D. Professor Ulla Gissi Knaus, Ph.D. Associate Professor Dwight Kono, M.D. Associate Professor Jiing-Dwan Lee, Ph.D. Associate Professor Erguang Li, Ph.D. Assistant Professor Cheng Liu, M.D., Ph.D. Assistant Professor Nigel Mackman, Ph.D. Associate Professor Steven Brown, Ph.D. Udayan Chatterji, Ph.D. Wolfram Ruf, M.D. Professor Xin Du, Ph.D. Colleen Fearns, Ph.D. Vladimir Kravchenko, Ph.D. Daniel R. Salomon, M.D. Adjunct Associate Professor Erica Ollmann Saphire, Ph.D. Assistant Professor John Mathison, Ph.D. Anil Munshi, Ph.D. Rafal Pawlinski, Ph.D. Nora Sarvetnick, Ph.D. Professor David Schlaepfer, Ph.D. Associate Professor Ramona Petrovan, Ph.D. M. Germana Sanna, Ph.D. Laura Solforosi, Ph.D. Linda A. Sherman, Ph.D. Professor Jonathan Sprent, M.D., Ph.D. Adjunct Professor Deborah Witherden, Ph.D. SENIOR RESEARCH A S S O C I AT E S Charles D. Surh, Ph.D. Associate Professor Ann J. Feeney, Ph.D. Associate Professor Michael McHeyzer-Williams, Ph.D. Associate Professor Luc Teyton, M.D., Ph.D. Associate Professor Philippe Gallay, Ph.D. Associate Professor Dianne McKay, M.D. Assistant Professor Argyrios N. Theofilopoulos, M.D. Professor Nicholas R.J. Gascoigne, Ph.D. Professor Donald E. Mosier, M.D., Ph.D. Professor Peter S. Tobias, Ph.D. Associate Professor Amanda Gavin, Ph.D. Assistant Professor Kerri A. Mowen, Ph.D. Assistant Professor Susan R. Webb, Ph.D. Associate Professor Jasimuddin Ahamed, Ph.D. Gourab Bhattarcharjee, Ph.D. Joao da Silva Correia, Ph.D. Amr Abdelhamid El Sheikh, Ph.D Kasper Hoebe, Ph.D. Eujing Jo, Ph.D. Hyun-ku Lee, Ph.D. 100 IMMUNOLOGY Rui Lin, Ph.D. Qilin Pan, Ph.D. Andrew Saphire, Ph.D. 2006 Eleuterio De La Camara, Ph.D. *** Centro Nacional de Investigaciones Cardiovasculares Madrid, Spain Yan Wu, Ph.D.**** THE SCRIPPS RESEARCH INSTITUTE Emma Hamilton-Williams, Ph.D. Lars Hangartner, Ph.D. Carsten Kreig, Ph.D. Joerge Krueger, M.D.*** Charité Children’s Hospital Berlin, Germany Masaaki Hayashi, M.D., Ph.D. Toru Kurokawa, Ph.D. Natasha Hill, Ph.D. Yumi Kurokawa, Ph.D. Neil John Hime, Ph.D. Young Back Kwon, Ph.D.**** Shihe Hou, Ph.D.*** Abraxis BioScience Inc. Los Angeles, California Cheng Yu Lai, Ph.D. Hong Hua, Ph.D. Elise Landais, Ph.D. Timothy Huang, Ph.D. Mansun Law, Ph.D. Christoph Huber, Ph.D. Jeff Lee, Ph.D. Milena Iacobelli, Ph.D. Sang-Un Lee, Ph.D.*** Cummings School of Veterinary Medicine at Tufts University North Grafton, Massachusetts Violane Delorme, Ph.D. R E S E A R C H A S S O C I AT E S Djemel Ait-Azzouzene, Ph.D. Christopher Alfonso, Ph.D.*** BD PharMingen San Diego, California Sandrine Arnaud-Dabernat, Ph.D. Celine Der Mardirossian, Ph.D. Benoit Besnues, Ph.D. Anthony Don, Ph.D. Helen Donners, Ph.D.*** Institute of Tropical Medicine Antwerp, Belgium Caroline Aylott, Ph.D.**** Celine Eidenschenk, Ph.D. Ann Bellon, Ph.D. Celia Espinoza, Ph.D.**** Michael Berger, Ph.D. Nicolas Fazilleau, Ph.D. Dafang Bian, Ph.D.**** Clemens Feistritzer, M.D. Joerge Birkenfeld, Ph.D. Christofer Flood, Ph.D. Onur Boyman, Ph.D.*** Centre Hospitalier Universitaire Vaudois Immunology and Allergology Lausanne, Switzerland Linda Frederick, Ph.D.*** Favrille, Inc. San Diego, California Carlos Cantu, Ph.D.*** Digital Gene Technologies La Jolla, California Guo Fu, Ph.D. Stefan Freigang, Ph.D. Hassan Issafras, Ph.D. Zhengfan Jiang, Ph.D. Jennifer Lamoureux, Ph.D. Wong Soon Justin, Ph.D. Young Jun Kang, Ph.D. Sung-Hyung Lee, Ph.D.*** Baylor College of Medicine Houston, Texas Yu-Ya Kao, Ph.D. Cheng Li, Ph.D.**** Charles Kaplan, Ph.D.*** Genentech Corporation San Francisco, California Jiali Li, Ph.D. Xiang Li, Ph.D. Linda Kidd, Ph.D. Michelle Fung, Ph.D. Yang Mi Li, Ph.D. Chang-Hoon Kim, Ph.D. Marie Cherrier, Ph.D. Philippe Georgel, Ph.D.*** Laboratoire d’Immunogénétique Moléculaire Humaine Strasbourg, France Roshni Chintalapati, Ph.D. Davide Gianni, Ph.D. Jae Ho Cho, Ph.D.**** Cristina Gil-Lamaignere, Ph.D.*** Department of Cell Biology Scripps Research John B. Carey, Ph.D. Jianming Chen, Ph.D. Hee Ok Kim, Ph.D.**** Jun-Sub Kim, Ph.D. Sungwoo Kim, Ph.D.*** Assistant Professor University of Calgary Calgary, Alberta, Canada Yilei Li, Ph.D.*** South China Medical University Guangzhou, China Ssang-Taek Lim, Ph.D. Yang Mi Lim, Ph.D. Edguardo Kolkowski, Ph. D. Ting-Kun Lin, M.D., Ph.D.*** Kaiser Permanente Medical Center San Francisco, California Aimee de Catherlineu, Ph.D. Pedro Gonzalez-Cabrera, Ph.D. Marek Kovar, Ph.D.**** Guoxun Liu, Ph.D.**** Shrimati Datta, Ph.D. Fang Guo, Ph.D. Jirina Kovarova, Ph.D.**** Yuan Liu, Ph.D. David Chodniewicz, Ph.D.**** Ben Croker, Ph.D. Rachel Kohler, Ph.D. IMMUNOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE 101 Carina Lotz, Ph.D.*** Netherlands Cancer Institute Amsterdam, the Netherlands Jared Purton, Ph.D. Joie Trifilo, Ph.D.**** Sun-Hee Yoon, Ph.D.**** Christopher Ramsey, Ph.D. Marina Tsatmali, Ph.D. Kenji Yoshida, Ph.D. Christine Louis dit Sully, Ph.D. M. Rachel Richards, Ph.D. David Valenta, Ph.D. Jiangiang Yu, Ph.D. Elise Romeo, Ph.D. Sebastian Vallee, Ph.D. Hui Zhang, Ph.D. Mark Rubenstein, Ph.D.*** University of California San Diego, California Ester Van Leeuwen, Ph.D. You Qing Zhang, Ph.D. Tieming Zhao, Ph.D. Monica Ruse, Ph.D. Laurent Verkoczy, Ph.D.*** Duke University Durham, North Carolina Sophie Rutschmann, Ph.D. Hendrik Versteeg, Ph.D. Janelle Salkowitz-Bokal, Ph.D. Nicole Von Allmen-Zurcher, Ph.D. Huamin Zhou, Ph.D.*** Xiamen University Xiamen, China Prabhakar Salunkhe, Ph.D.*** Burnham Institute La Jolla, California Katharina Von Lohneysen, Ph.D. Jorge Luis Schettini, Ph.D. Yingchun Wang, Ph.D.*** Moores Cancer Center San Diego, California Yunping Luo, M.D., Ph.D. James P. Luyendyk, Ph.D. Michael Lyman, Ph.D. Chitladda Mahanivong, Ph.D. Laurent Malherbe, Ph.D. Maria Manukyan, Ph.D. He Zhou, Ph.D. Annette Marleau, Ph.D. Beatriz Maroto, Ph.D.*** Centro Nacional de Investigaciones Oncológicas Madrid, Spain David Marsolais, Ph.D. Nicolas Schrantz, Ph.D. A S S O C I AT E S Meng Wang, M.D. Javier Martinez, Ph.D. Terrence Meehan, Ph.D. Satyajit Mitra, Ph.D. Johann Mols, Ph.D. Adam Mullick, Ph.D. Perihan Nalbant, Ph.D. Reto Andreas Schupbach, Ph.D. Zhao Wang, Ph.D. Megumi Watanabe, Ph.D. Alim Seit-Nebi, Ph.D. *** Department of Molecular Biology Scripps Research Suganya Selvarajah, Ph.D. Chenghong Wei, Ph.D. Christopher Wiethoff, Ph.D.*** Loyola University Chicago, Illinois Ron Nepomuceno, Ph.D. Shigeki Shimada, Ph.D. Frank Karl Niessen, Ph.D. Jason Smith, Ph.D. Miyo Ota, Ph.D. Michelle Solomon, Ph.D.**** Chia Cheng Wu, Ph.D. Takayuki Ota, Ph.D. Gabriel Sternik, Ph.D. Wenyuan Wu, Ph.D. Motoyuki Otsuka, Ph.D. Konstantin Stoletov, Ph.D.*** Moores Cancer Center San Diego, California Chengran Xu, Ph.D. Joyce Tan, Ph.D.*** Anadys Pharmceuticals San Diego, California Pia Yachi, Ph.D. Rachel Tilley, Ph.D. Michael Ye, Ph.D. Antoine Toulon, Ph.D. Sook Wah Yee, Ph.D. Sandrine Pacquelet, Ph.D. Nadige Pelletier, Ph.D. Olivier Pertz, Ph.D.*** University of California San Diego, California Helle Petersen, Ph.D. SCIENTIFIC Justin Soon Boon Wong, Ph.D. Rosana Gonzales-Quintal, Ph.D. Marcie Rose Kritzik, Ph.D. Nora Leaf Ralph Pantophlet, Ph.D. Dongyuan Xia, Ph.D. * Joint appointment in the Department of Cell Biology ** Joint appointment in the Department of Molecular Biology *** Appointment completed, new location shown **** Appointment complete Yue Xu, Ph.D. Deepak Yadav, Ph.D. 102 IMMUNOLOGY 2006 Richard Ulevitch, Ph.D. Chairman’s Overview rogress in biomedical research demands innovation and depends on our ability to identify the leading edges of science. The approach to science that looks backward instead of forward stifles originality and perpetuates conventional thinking. I am always impressed by the ability of our department members to develop new paradigms to advance scientific knowledge. The environment at Scripps Research encourages our scientists to apply the very latest approaches to solving core problems of immunology. This often involves taking risks, especially in today’s world of peer-reviewed funding where out-ofthe-box thinking is not always rewarded. However, I am pleased to report that our investigators are continuing to take appropriate risks with their science and, as a result, are being widely recognized for their accomplishments. This is evidenced by the numerous scientific papers published by our department members and by the prestigious awards and other forms of recognition bestowed upon them. Some of these are highlighted below. But first I would like to acknowledge some key transitions within our faculty. During the past year, Norman Klinman retired after an incredibly productive career of more than 30 years in the field of immunology research. P THE SCRIPPS RESEARCH INSTITUTE In 2006, he received the Excellence in Mentoring Award from the American Association of Immunologists for “exemplary career contributions to a future generation of scientists.” Many of Dr. Klinman’s more than 50 former trainees attended the award ceremony, which was held in Boston in May. We wish Dr. Klinman all the best in his future activities. We will always welcome his presence at Scripps Research. The end of 2005 also marked the departure of Jonathan Sprent, who returned to his native Australia after 30 years in the United States—first at the University of Pennsylvania and then at Scripps Research. Dr. Sprent is currently professor at the Garvan Institute of Medical Research in Sydney, where he will continue his seminal work on T-cell function combining his unique knowledge of the immune response in whole animals with molecular models using cell-based systems. We look forward to reading about Dr. Sprent’s new findings as they impact immunologic memory and tolerance, transplantation immunity, and cancer immunotherapy. His longstanding collaborations with Charlie Surh at Scripps Research will undoubtedly continue, and we will welcome his visits to our campus. Coinciding with these departures is the recent recruitment of Karsten Sauer, who joined us after a number of years at the Genomics Institute of the Novartis Research Foundation. Dr. Sauer specializes in studies of lymphocyte signal transduction and will bring additional strength to our department in this important field. Defects in lymphocyte development or function underlie various immune disorders, including immunodeficiencies, inflammatory and autoimmune diseases, and allergy. Dr. Sauer’s studies promise to significantly expand our understanding of these key processes in health and disease. Specifically, his laboratory will combine state-of-the-art genomic and proteomic profiling techniques, functional genomics, high-throughput screening, and imaging technologies as well as classical biochemistry, molecular biology, and cell biology to explore the functions of novel signaling modules. This effort will result in a new understanding of how antigen-receptor signaling directs diverse cellular responses and how its malfunction leads to immunologic disease. We look forward to Dr. Sauer ’s contributions as an active faculty member in our department. Highlights of scientific publications provide clear evidence of the important place our department holds within the national and international community of scientists interested in immunology. Bruce Beutler has provided Scripps Research with a unique resource in the form of IMMUNOLOGY 2006 a program of random germline mutagenesis. During the past year, he and his colleagues have provided remarkable new insights into mechanisms of innate and adaptive immunity. Key findings include those of Koichi Tabeta and his colleagues, published in Nature Immunology, in which an unanticipated function has been assigned to the Unc93b1 gene product. These investigators showed that a mutation in Unc93b1, known as the 3d mutation, disrupts signaling via Toll-like receptors 3,7, and 9, and also alters antigen presentation. Building on these data are findings from Kasper Hoebe and colleagues, published this year in Immunity, have identified a novel pathway of T-cell activation that appears to be independent of Toll-like receptor signaling. Taken together, these two publications promise to shape our thinking about mechanisms involved in promoting adaptive immunity through the innate immune networks. In addition to uncovering new biology, the germline mutagenesis approach provides new insights into protein structure as revealed in Proceedings of the National Academy of Sciences by Zhengfan Jiang and his colleagues, who describe molecular details of interactions between MyD88 and Toll-like receptors. We also look forward to continued productivity and seminal advances from Bruce Beutler and his group during the coming year. Adding to the department’s strengths in innate immunity is the work done in the laboratory of Jiahuai Han. DaSilva Correia and his group provided an unexpected insight into a mechanism whereby Nod1 regulates the function of the estrogen receptor. Nod1 provides a brake on the estrogen receptor, and the absence of Nod1 results in enhanced sensitivity to estrogen and promotes tumor growth in a xenograft model. This work was published in Proceedings of the National Academy of Sciences. Members of Luc Teyton’s laboratory combine structure-function studies with both cell-based and animal models to probe the function of the natural killer T-cell receptor and its ligand. This work, which was published in Nature and Nature Immunology, includes a longstanding collaboration with Albert Bendelac at the University of Chicago. Following on studies of immune regulatory pathways is work done by Charlie Surh and his colleagues, which was published in Science. This report documents an unexpected finding showing how an immune complex of anti-IL2 and IL2 act to stimulate T-cell subsets. These data not only provide new insights into uses of therapeutic antibodies but also alert us to concerns about unanticipated side effects. Another advance in understanding adaptive immunity comes from Michael McHeyzer- THE SCRIPPS RESEARCH INSTITUTE 103 Williams and members of his laboratory, whose work on B-cell function was recently described in an article published in Immunity. In both the United States and worldwide, HIV and hepatitis C infection remain significant problems for which there are still no appropriate therapies. Dennis Burton and his group continue their important research in these two areas. This past year, they published articles in Science, Nature Immunology, and Proceedings of the National Academy of Sciences that provided insights into key issues for advancement of new treatments. These recent publications represent the important collaborations established by Dennis Burton and his colleagues both inside and outside of Scripps Research. Work from the laboratory of Hugh Rosen combines chemical and genetic approaches to proof-of-concept studies that will allow a better understanding of human disease mechanisms. This work paves the way for the development of new small-molecule therapeutics for use in immunologic and inflammatory diseases in humans. Specifically, publications in Nature Immunology, Nature Chemical Biology, and other top journals illustrate the importance of the approaches implemented by Hugh Rosen and his group. Further illustration of the power of genetics is found in work published in Nature Medicine by members of the J-D Lee’s laboratory. Here they used conditional knock-out of the HSP40 gene to prove a role for this protein in a serious human syndrome, cardiomyopathy. This work may well lead to the development of drugs that can be used to selectively intervene in this serious medical problem. These examples illustrate the productivity of the members of our department. More details and a complete list of accomplishments can found in the individual reports. I apologize in advance for not including comments about each publication from the 2005-2006 period and urge readers to examine the reports for more details. As always, it is a great pleasure for me to read the material from each of our faculty members and to reflect on the progress of the past year. 104 IMMUNOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE INVESTIGATORS’ R EPORTS Analyzing Host Resistance: Phenomenon to Phenotype to Gene M. Barnes, K. Benson, M. Berger, B. Croker, K. Crozat, X. Du, C. Eidenschenk, K. Hoebe, Z. Jiang, B. Layton, N. Nelson, B. Ortiz, T. Palmer, S. Rutschmann, S. Sovath, H. Uy, K. Whitley, Y. Xia, B. Beutler ecause microbes are an enormous threat to most vertebrate species, mammalian evolution has been shaped by the microbial environment, and many examples of protein modification were driven by the need to evade infection. On an evolutionary timescale, a fairly large fraction of the genome in mammals has been appropriated chiefly to create resistance to infection. Those proteins with nonredundant function in resistance to a particular organism are components of the host “resistome.” To identify key resistance proteins, we use random germ-line mutagenesis. In this process, a chemical mutagen, N-ethyl-N-nitrosourea, is used to induce a distinctive phenotype (e.g., inadequate defense against infection). The phenotype is then genetically mapped, and the causal mutation is found by using DNA sequencing. Once cause and effect have been established, we can determine precisely how a given protein contributes to defense against infection. To date, we have created scores of mutations that impair resistance to various pathogens; we have mapped a total of 32 of these mutations to chromosomal intervals. Most of the mapped mutations have been solved at a molecular level (Fig. 1). Many of the proteins identified in this way are previously unknown components of the resistome. Some of the proteins serve signaling by the Toll-like receptors, which we previously showed are key sensors by which mammals detect infection. Other identified proteins are important components of the cytokine response apparatus, required for the production or activity of TNF or type I interferons. Remarkably, many of the proteins do not yet fit into a cohesive picture. Rather, they are puzzles waiting to be solved. Germ-line mutations can disclose the function of proteins where no function was previously assigned. The mutations can also reveal new functions of a protein, if some functions were previously known. And the mutations can shed light on the func- B F i g . 1 . Map locations of mutations with immunologic effects produced to date. Shaded ovals indicate that the mutation has been identified by using positional cloning. tion of proteins, which in the final analysis often act as minute “machines.” We have seen each of these outcomes in the course of our research. A phenotype called 3d (to connote a “triple defect” of nucleic acid sensing) was tracked to a multispanning membrane protein with no previously known function. This protein, known as UNC-93B, is required for the integrity of a specific class of endosomes, small bodies within cells within which sensing of nucleic acids and processing of antigens for activation of the immune system occur. The 3d mutation causes susceptibility to a broad range of infections because microbial nucleic acids are an important clue to the presence of infection, and when the host remains unaware of these nucleic acids, infections may grow out of control. In addition, the 3d mutation prevents antigen-presenting cells from delivering foreign proteins to T lymphocytes, particularly CD8+ cells, which are required to directly attack and eliminate infection within tissues of the host. Some mutations create subtle changes within proteins, revealing functions that were previously hidden because total disruption of the protein is lethal. One such mutation, called woodrat, causes graying of the fur and a moderate, generalized immunodeficiency (e.g., failure to contain infection caused by mouse cytomegalovirus). This mutation affects an enzyme that is of central importance in cholesterol metabolism. Known as the site 1 protease, the enzyme cleaves a latent transcription factor required for activation of genes that encode other enzymes required to synthesize cholesterol. Although mice lacking the site 1 protease cannot survive to term, woodrat mutants are fully viable, have exceptionally low blood levels of cho- IMMUNOLOGY 2006 lesterol, and have a diminished ability to cope with infection. Hence, a new function of the site 1 protease has come to light. The syndrome of shock that occurs during serious infections of all kinds is mediated by signaling via Tolllike receptors, and for this reason, the exact structure of the transduction apparatus that alerts the host to the presence of lipopolysaccharide, bacterial lipopeptides, unmethylated DNA, and other signature molecules has been the object of intense interest. The Toll-like receptors recruit specific adapter proteins, which in turn activate protein kinases that ultimately promote the transcription of hundreds of genes that shape the inflammatory response. A mutation in one such adapter protein, MyD88, has helped us understand precisely how the initial signal is transmitted. Called Pococurante, this mutation has revealed the location of the receptor-adapter interface, and molecular docking studies, performed in collaboration with I.A. Wilson and A.J. Olson, Department of Molecular Biology, have suggested exactly how the receptor and adapter subunits fit together (Fig. 2). THE SCRIPPS RESEARCH INSTITUTE 105 Beutler, B. Microbial pathogenesis and the discovery of Toll-like receptor function. In: Vaccine Adjuvants: Immunological and Clinical Principles. Hackett, C.J., Harn, D.A., Jr. (Eds.). Humana Press, Totowa, NJ, 2005, p. 1. Beutler, B. The Toll-like receptors. In: Genetic Susceptibility to Infection. Kaslow, R.L., McNicholl, J., Hill, A.V.S. (Eds.). Oxford University Press, New York, in press. Beutler, B. The Toll-like receptors: analysis by forward genetic methods. Immunogenetics 57:385, 2005. Beutler, B., Casanova, J.-L. New frontiers in immunology. EMBO Rep. 6:620, 2005. Beutler, B., Georgel, P., Rutschmann, S., Jiang, Z., Croker, B., Crozat, K. Genetic analysis of innate resistance to mouse cytomegalovirus (MCMV). Brief. Funct. Genomic Proteomic 4:203, 2005. Beutler, B., Hoebe, K., Georgel, P., Tabeta, K., Du, X. Genetic analysis of innate immunity: identification and function of the TIR adapter proteins. Adv. Exp. Med. Biol. 560:29, 2005. Beutler, B., Jiang, Z., Georgel, P., Crozat, K., Croker, B., Rutschmann, S., Du, X., Hoebe, K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353, 2006. Crozat, K., Georgel, P., Rutschmann, S., Mann, N., Du, X., Hoebe, K., Beutler, B. Analysis of the MCMV resistome by ENU mutagenesis. Mamm. Genome 17:398, 2006. Du, X., Tabeta, K., Mann, N., Crozat, K., Mudd, S., Beutler, B. An essential role for Rxrα in development of Th2 responses. Eur. J. Immunol. 35:3414, 2005. Fischer, H., Yamamoto, M., Akira, S., Beutler, B., Svanborg, C. Mechanism of pathogen-specific TLR4 activation in the mucosa: fimbriae, recognition receptors and adaptor protein selection. Eur. J. Immunol. 36:267, 2006. Hoebe, K., Beutler, B. TLRs as bacterial sensors. In: Toll-like Receptors in Inflammation. O’Neil, L.A.J., Brint, E. (Eds.). Birkhauser, New York, 2006, p. 000. Progress in Inflammation Research, Parnham, M.J. (Series Ed.). Hoebe, K., Beutler, B. TRAF3: a new component of the TLR-signaling apparatus. Trends Mol. Med. 12:187, 2006. Hoebe, K., Jiang, Z., Georgel, P., Tabeta, K., Janssen, E., Du, X., Beutler, B. TLR signaling pathways: opportunities for activation and blockade in pursuit of therapy. Curr. Pharm. Des., in press. Hoebe, K., Jiang, Z., Tabeta, K., Du, X., Georgel, P., Crozat, K., Beutler, B. Genetic analysis of innate immunity. Adv. Immunol. 91:175, 2006. Huber, M., Kalis, C., Keck, S., Jiang, Z., Georgel, P., Du, X., Shamel, L., Sovath, S., Mudd, S., Beutler, B., Galanos, C., Freudenberg, M.A. R-form LPS, the master key to the activation of TLR4/MD2 positive cells. Eur. J. Immunol. 36:701, 2006. F i g . 2 . The junction between a Toll-like receptor and its cytoplasmic adapter protein. Only the so-called TIR domains, which are involved in binding, are shown. These few examples illustrate the power of the classical genetic approach—which proceeds from phenomenon to phenotype to gene—in analyzing immunity. However, the same approach also sheds light on many other aspects of biology. Some of the mutations that we have produced answer questions in the realm of behavioral neuroscience, metabolism, and development and raise new questions in turn. PUBLICATIONS Beutler, B. Innate Immunity. In: Williams Hematology, 7th ed. Lichtman, M.A., et al. (Eds.). McGraw-Hill, New York, 2005, p. 231. Janssen, E., Tabeta, K., Barnes, M.J., Rutschmann, S., McBride, S., Bahjat, K.S., Schoenberger, S.P., Theofilopoulos, A.N., Beutler, B., Hoebe, K. Efficient T cell activation via a Toll-Interleukin 1 receptor-independent pathway. Immunity. 24:787, 2006. Jiang, Z., Georgel, P., Li, C., Choe, J., Crozat, K., Rutschmann, S., Du, X., Bigby, T., Mudd, S., Sovath, S., Wilson, I.A., Olson, A., Beutler, B. Details of Toll-like receptor:adapter interaction revealed by germ-line mutagenesis. Proc. Natl. Acad. Sci. U. S. A. 103:10961, 2006. Kim, K.I., Malakhova, O., Hoebe, K., Yan, M., Beutler, B., Zhang, D.-E. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J. Immunol. 175:847, 2005. Nemazee, D., Gavin, A.L., Hoebe, K., Beutler, B. Immunology: Toll-like receptors and antibody responses. Nature 441:E4, 2006. Rutschmann, S., Hoebe, K., Zalevsky, J., Du, X., Mann, N., Dahiyat, B.I., Steed, P., Beutler, B. PanR1, a dominant negative missense allele of the gene encoding TNFα (Tnf), does not impair lymphoid development. J. Immunol. 176:7525, 2006. Tabeta, K., Hoebe, K., Janssen, E.M., Du, X., Georgel, P., Crozat, K., Mudd, S., Mann, N., Sovath, S., Goode, J., Shamel, L., Herskovits, A.A., Portnoy, D.A., Cooke, M., Tarantino, L.M., Wiltshire, T., Steinberg, B.E., Grinstein, S., Beutler, B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156, 2006. 106 IMMUNOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE Wieland, C.W., Florquin, S., Maris, N.A., Hoebe, K., Beutler, B., Takeda, K., Akira, S., van der Poll, T. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable Haemophilus influenzae from the mouse lung. J. Immunol. 175:6042, 2005. Xia, C.H., Liu, H., Cheung, D., Cheng, C., Wang, E., Du, X., Beutler, B., Lo, W.K. Gong, X. Diverse gap junctions modulate distinct mechanisms for fiber cell formation during lens development and cataractogenesis. Development 133:2033, 2006. Regulation of Cell Function by Rho GTPases G.M. Bokoch, J. Birkenfeld, V. Delorme, C. DerMardirossian, A.M. DeCathelineau, D. Gianni, T. Huang, Y.-Y. Kao, J.-S. Kim, P. Nalbant, K. Pestonjamasp, S.-H. Yoon, H. Zhang, T. Zhao, B.P. Bohl, M. Crawford, B. Fowler, J.-Y. Seo, Z.-F. Chang* F i g . 1 . Two-step activation mechanism for Rac GTPase–mediated regulation of oxidant formation by the phagocyte NADPH oxidase. * National Taiwan University, Taipei, Taiwan ho GTPases control the assembly of the actin and microtubule cytoskeletons, the production of reactive oxygen species (ROS), and the activity of kinase cascades that mediate cell growth, death, and motility. This spectrum of activities makes Rho GTPases key components of such physiologic and pathologic processes as tumor growth and metastasis, wound healing, neuronal connectivity, inflammatory responses, and development. We use cellular, molecular, biophysical, and biochemical approaches to understand how the activities of Rho GTPases are regulated, to identify the proteins they interact with to control cell function, and to ascertain how these regulatory processes are abnormal in various disease states. R RHO GTPases AND HUMAN LEUKOCYTES We previously established that the GTPase Rac2 regulates the formation of ROS that are used by human phagocytic leukocytes for microbial killing and that result in inflammatory responses. Our discovery of a functional interaction between Rac2 and cytochrome b, a component of the membrane-bound NADPH oxidase, independent of p67 phox , led us to propose a 2-step mechanism for regulation of electron transfer to form superoxide (Fig. 1). We are mapping the binding site for Rac2 on cytochrome b to investigate the molecular basis for regulation of ROS production by Rac2. In addition to their role in innate immunity, NADPH oxidases participate in intracellular signaling. Regulation of nonphagocytic NADPH oxidases is largely not understood, but we are investigating their modulation by kinase pathways that phosphorylate regulatory components of the oxidases. We are using live-cell imaging in combination with fluorescent methods to determine the spatial and tem- poral localization of Rho GTPase activation. We are beginning to determine the molecular signals that govern the chemotactic responses of human leukocytes. Recently, we described the ability of Rac1 signaling in neutrophils to stimulate RhoA activation at the rear of cells. Such Rho GTPase cross talk promotes the development of the stable cell polarity necessary to maintain directionality of chemotaxis during inflammatory responses. Studies of the dynamics of Cdc42 activation during neutrophil chemotaxis are ongoing. R E G U L AT I O N O F I N N AT E I M M U N I T Y B Y A N T H R A X TOXINS Bacillis anthracis inhibits the function of immune cells by generating lethal toxin and edema toxin. As part of a program grant funded by the Centers for Disease Control and Prevention, we are investigating the molecular basis for the suppressive effects of the anthrax toxins on the function of human leukocytes. We have established that anthrax edema toxin and lethal toxin effectively block the ability of chemoattractant receptors to stimulate the production of ROS by human neutrophils. The molecular basis for such inhibition is currently under investigation. A requirement for Rho GTPases in the uptake and action of anthrax toxins in macrophages is also under study (Fig. 2). C Y T O S K E L E TA L R E G U L AT I O N B Y R H O G T P a s e s The p21-activated kinases (PAKs) are Rac and Cdc42 effectors that serve as important mediators of chemotaxis, wound healing, tumor metastasis, neurite outgrowth, antigen presentation, and other processes dependent on cytoskeletal polarization. In collaborative studies with G. Danuser and C. Waterman-Storer, Department of Cell Biology, we are using quantitative fluorescent speckle microscopy to investigate the regu- IMMUNOLOGY 2006 F i g . 2 . Rho GTPase regulation of the action of anthrax lethal toxin. Anthrax toxin is a ternary complex consisting of 1 binding subunit, protective antigen (PA), and 2 enzymatic subunits, edema factor (EF) or lethal factor (LF). Full-length PA (PA83) binds to receptors on the cell surface and is cleaved by a furinlike protease to its active form (PA63). Active PA oligimerizes, driving receptor aggregation and internalization by endocytosis. During normal maturation and acidification of the endosomes, PA forms a channel through which EF and LF are transported from the endosomal compartment and into the cytoplasm to act on their respective effectors. Rho GTPases may act to regulate endocytosis, endosomal maturation, and toxin escape or activity. Figure courtesy of Aimee DeCathelineau. lation of leading-edge actin dynamics by PAK1 downstream of Rac GTPase. We found that PAK1 plays an important role in coupling cell-edge protrusion mechanics to upstream signaling events and downstream motility. The phosphorylation of cofilin, which depolymerizes and severs actin, by PAK1 acting through LIM kinase is an important regulatory point in cell motility. Using a biochemical screen, we identified a unique cofilin phosphatase, termed chronophin, that regulates stimulus-dependent activation of cofilin. Using small interfering RNA to reduce the expression of chronophin, we discovered that this phosphatase is involved in the control of cytokinesis during cell division. Chronophin is implicated in the formation of aneuploid cancers; it is overexpressed in such tumors and is an autoantigen in patients with cancer. Our recent data indicate that this unique regulatory phosphatase orchestrates actin dynamics at the leading edge by modulating cofilin activity, thereby increasing cancer cell motility stimulated by epidermal growth factor. We have also linked chronophin to cytoskeletal changes initiated during cellular energy (ATP) depletion induced by processes such as ischemia. THE SCRIPPS RESEARCH INSTITUTE 107 GDP dissociation inhibitors are critical regulators of Rho GTPase function. They have been linked to kidney disease and to the ability of cancer cells to metastasize. We found that the interaction of GDP dissociation inhibitors with Rho GTPases is regulated by phosphorylations initiated through various signaling pathways. Indeed, tyrosine phosphorylation may disrupt the regulatory capability of the inhibitors to promote cell transformation and metastasis. Cell division also requires highly regulated actinmyosin-microtubule dynamics. We established that cross talk between the actin and microtubule cytoskeletons involving Rho regulation occurs via physical sequestration of the Rho guanine nucleotide exchange factor H1 (GEF-H1) by microtubules. GEF-H1 serves as a link between mitotic spindle microtubules and the initiation of Rho-dependent formation of cleavage furrows in dividing cells (Fig. 3). GEF-H1 activity is also controlled by F i g . 3 . Immunofluorescent images show colocalization of endog- enous GEF-H1 with microtubules (tubulin) in the mitotic spindle. cell cycle–dependent kinases. Detailed analysis of the function of GEF-H1 in cell division and motility is under way. Of interest, GEF-H1 is abundant in blood cells and is downregulated by recently developed drugs that inhibit chronic leukemias. PUBLICATIONS Belvindrah, R., Nalbant, P., Ding, S., Wu, C., Bokoch, G.M., Müller, U. Integrinlinked kinase regulates Bergmann glial differentiation during cerebellar development. Mol. Cell. Neurosci. 33:109, 2006. Birukova, A.A., Adyshev, D., Gorshkov, B., Bokoch, G.M., Birukov, K.G., Verin, A.D. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 290:L540, 2006. Bokoch, G.M., Zhao, T. Regulation of the phagocyte NADPH oxidase by Rac GTPase. Antioxid. Redox. Signal. 8:1533, 2006. Chang, Y.-C., Lee, H.-H., Chen, Y.-J., Bokoch, G.M., Chang, Z.-F. Contribution of guanine exchange factor H1 in phorbol ester-induced apoptosis. Cell Death Differ., in press. Crawford, M., Aylott, C., Bourdeau, R.W., Bokoch, G.M. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J. Immunol. 176:7557, 2006. DeCathelineau, A.M., Bokoch, G.M. Peptide inhibitors MAP the way towards fighting anthrax. Biochem. J. 395:e1, 2006. DerMardirossian, C., Bokoch, G.M. Phosphorylation of RhoGDI by p21-activated kinase 1. Methods Enzymol. 406:80, 2006. 108 IMMUNOLOGY 2006 DerMardirossian, C., Rocklin, G., Seo, J.Y., Bokoch, G.M. Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell, in press. Dong, X., Mo, Z., Bokoch, G.M., Guo, C., Li, Z., Wu, D. P-Rex1 is a primary Rac2 guanine nucleotide exchange factor in mouse neutrophils. Curr. Biol. 15:1874, 2005. Huang, T.Y., DerMardirossian, C., Bokoch, G.M. Cofilin phosphatases and regulation of actin dynamics. Curr. Opin. Cell Biol. 18:26, 2006. Pestonjamasp, K.N., Forster, C., Sun, C., Gardiner, E.M., Bohl, B., Weiner, O., Bokoch, G.M., Glogauer, M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 108:2814, 2006. Stofega, M., DerMardirossian, C., Bokoch, G.M. Affinity-based assay of Rho GTPase activation. Methods Mol. Biol. 332:269, 2006. Human Antibodies and Design of a Vaccine to HIV Type 1 M.B. Zwick, R.A. Pantophlet, R.O. Aguilar-Sino, R.D. Astronomo, D.R. Bowley, H. Donners, A.K. Gakhal, E. Giang, L. Hangartner, A.J. Hessell, R.C. Jensen, M. Law, J.D. Nelson, S. Pollock, E.M. Scherer, M. Wang, R.A. Dwek,* D. Calarese, R.M. Cardoso, R.L. Stanfield, I.A. Wilson, D.R. Burton * Oxford Glycobiology Institute, Oxford, England IV type 1 (HIV-1) is a scourge on humanity. Nearly 40 million persons are infected with the virus, and about 20 million have died of AIDS. It is widely recognized that a vaccine most likely is the best way to control HIV infection worldwide. All current antiviral vaccines elicit antibody responses that are thought to be crucial to the efficacy of the vaccines. We wish to understand antibody responses to HIV in humans and to design vaccines that will elicit protective responses to the virus. We used phage display technology to generate panels of human monoclonal antibodies to HIV. We are examining human antibody responses to the virus and the antiviral activities of these antibodies. In particular, we generated a human monoclonal antibody, b12, that neutralizes a broad array of different strains of HIV. The existence of this antibody indicates that some features of HIV are conserved and are attractive targets for vaccines. Further, b12 and a few monoclonal antibodies with similar qualities are powerful tools for exploring antibody activity against HIV-1. Among the first questions we have tackled were the following: Can antibodies protect against HIV-1 infection, and, if so, under what conditions? On the basis H THE SCRIPPS RESEARCH INSTITUTE of passive transfer studies in a number of animal model systems, the answer is clearly yes. Complete protection is possible at serum titers of neutralizing antibody greater than about 1:100, although lower titers can provide benefit in terms of lowered or delayed viremia. We also showed that topically applied antibody can protect monkeys against vaginal challenge with virus. In addition, passive transfer studies with engineered antibodies in macaques suggest that antibody effector functions, as well as classical neutralization, may be important in protection against HIV. Another major issue is the best method for eliciting protective neutralizing antibodies. Accumulated evidence suggests that protective neutralizing antibodies are those antibodies that bind avidly to the envelope trimer on the surface of HIV-1 virions. However, such antibodies, particularly those to conserved regions of the envelope that are most important for vaccines, are difficult to elicit. Apparently the envelope trimer, which is composed of 2 glycoproteins, gp120 and gp41, has low antigenicity and immunogenicity. Several strategies to circumvent these problems are being investigated. One strategy is to study the interaction of the neutralizing antibodies with envelope glycoprotein at the molecular level and then use the knowledge gained to design antigens capable of eliciting the relevant antibodies. In these studies, we are collaborating with I.A. Wilson, Department of Molecular Biology. We are also working closely with P. Dawson, Department of Cell Biology, to design peptide immunogens and with C.-H. Wong, Department of Chemistry, and R. Dwek, Oxford Glycobiology Institute, to design and select carbohydrate immunogens. Finally, we are exploring the specificities of antibodies from those rare humans who make antibodies that neutralize a broad array of different strains of HIV. We have evidence that a number of specificities are involved, and we are attempting to describe these. We are generating human monoclonal antibodies by using not only phage display but also yeast display and the rescue of memory B cells. PUBLICATIONS Braciale, T.J., Hahn, Y.S., Burton, D.R. Adaptive immune responses to viral infection. In: Fields Virology, 5th ed. Knipe, D.M., et al. (Eds.). Lippincott Williams & Wilkins, Philadelphia, in press. Brunel, F.M., Zwick, M.B., Cardoso, R.M.F., Nelson, J.D., Wilson, I.A., Burton, D.R., Dawson, P.E. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J. Virol. 80:1680, 2006. Burton, D.R., Stanfield, R.L.. Wilson, I.A. Antibody versus HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. U. S. A. 102:14943, 2005. IMMUNOLOGY 2006 Calarese, D.A., Lee, H.-K., Huang, C.-Y, Best, M.D., Astronomo, R.D., Stanfield, R.L., Katinger, H., Burton, D.R., Wong, C.-H, Wilson, I.A. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc. Natl. Acad. Sci. U. S. A. 102:13372, 2005. Calarese, D., Scanlan, C., Lee, H.-K., Rudd, P., Wong, C.-H., Dwek, R.A., Burton, D.R., Wilson, I.A. Towards a carbohydrate-based HIV-1 vaccine. In: Carbohydrate Drug Design. Klyosov, A.A., Witczak, Z.J., Platt, D. (Eds.). Oxford University Press, New York, 2006, p. 161. American Chemical Society Symposium Series. Delves, P.J., Martin, S.J., Burton, D.R., Roitt, I.M. Roitt’s Essential Immunology, 11th ed. Blackwell Publishing, Oxford, England, in press. Koff, W.C., Johnson, P.R., Watkins, D.I., Burton, D.R., Lifson, J.D., Hasenkrug, K.J., McDermott, A.B., Schultz, A., Zamb, T.J., Boyle, R., Desrosiers, R.C. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 7:19, 2006. Law, M., Sanna, P.P., Burton, D.R. Viral subversion of humoral immune responses. In: Microbial Subversion of Immunity: Current Topics. Lachmann, P.J., Oldstone, M.B.A. (Eds.). Caister Academic Press, Norfolk, England, 2006, p. 177. Lindenbach, B.D., Evans, M.J., Syder, A.J., Wolk, B., Tellinghuisen, T.L., Liu, C.C., Maruyama, T., Hynes, R.O., Burton, D.R., McKeating, J.A., Rice, C.M. Complete replication of hepatitis C virus in cell culture. Science 309:623, 2005. Moore, P.L., Crooks, E.T., Porter, L., Zhu, P., Cayanan, C.S., Grise, H., Corcoran, P., Zwick, M.B., Franti, M., Morris, L., Roux, K.H., Burton, D.R., Binley, J.M. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80:2515, 2006. O’Connor, D.H., Burton, D.R. Immune responses and HIV: a little order from the chaos. J. Exp. Med. 203:501, 2006. THE SCRIPPS RESEARCH INSTITUTE 109 deadliest human pathogens. Results indicate strong cross-reactivity and immunogenicity of the different glycoproteins. This finding supports the hypothesis that some of the soluble forms of the glycoproteins act as decoys. We have also been investigating the effects of a human monoclonal antibody that neutralizes Ebola virus; the antibody was isolated from a patient who was infected with the virus in the Democratic Republic of Congo and who recovered. Previously, we showed that the antibody protects guinea pigs against challenge with Ebola virus. However, studies done in collaboration with scientists at the National Institute of Allergy and Infectious Diseases, indicate that the antibody does not protect monkeys and indeed appears to offer little benefit even when given at high doses. Surprisingly, viral replication apparently can proceed unhindered in the tissues of the monkeys even in the presence of high serum concentrations of antibody. We are attempting to understand this phenomenon and reconcile it with the ability of certain vaccines to protect against challenge with Ebola virus. Pantophlet, R., Burton, D.R. GP120: target for neutralizing HIV-1 antibodies. Annu. Rev. Immunol. 24:739, 2006. Selvarajah, S., Puffer, B., Pantophlet, R., Law, M., Doms, R.W., Burton, D.R. Comparing antigenicity and immunogenicity of engineered gp120. J. Virol. 79:12148, 2005. Venturini, S., Allicotti, G., Zhao, Y., Simon, R., Burton, D.R., Pinilla. C., Poignard, P. Identification of peptides from human pathogens able to cross-activate an HIV-1gag-specific CD4+ T cell clone. Eur. J. Immunol. 36:27, 2006. Yuste, E., Sanford, H.B., Carmondy, J., Bixby, J., Little, S., Zwick, M.B., Greenough, T., Burton, D.R., Richman, D.D., Desrosiers, R.C., Johnson, W.E. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 80:3030, 2006. Zhang, M.X., Bohlman, M.C., Itatani, C., Burton, D.R., Parren, P.W.H.I., St. Jeor, S.C., Kozel, T.R. Human recombinant antimannan immunoglobulin G1 antibody confers resistance to hematogenously disseminated candidiasis in mice. Infect. Immun. 74:362, 2006. Zhong, J., Gastaminza, P., Cheng, G., Kapadia, S., Kato, T., Burton, D.R., Wieland, S.F., Uprichard, S.L., Wakita, T., Chisari, F.V. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 102:9294, 2005. Antibodies and Emerging Viruses W.B. Oswald, E.O. Saphire, N.L. Sullivan,* P.B. Jahrling,* P.W.H.I. Parren,** D.R. Burton * National Institute of Allergy and Infectious Diseases, Bethesda, Maryland ** Genmab, B. V., Utrecht, the Netherlands W e are interested in determining the immunogenicity of soluble vs surface glycoproteins of Ebola virus, a filovirus that is one of the Toll-like Receptors and Inflammation in Atherosclerosis A.E. Mullick, C. Flood, N.J. Hime, M.R. Richards, D.T. Valenta, R.J. Petrovan, P.S. Tobias, L.K. Curtiss he ability of Toll-like receptors (TLRs) to detect a spectrum of pathogen-derived molecules defines the importance of the receptors in innate immunity and provides a mechanistic link between infection and disease. Atherosclerosis is a chronic inflammatory disease in which immune and metabolic factors interact to initiate and propagate arterial lesions. An understanding of TLRs in atherosclerosis could clarify the etiology of this complex process. Furthermore, the existence of host-derived endogenous ligands for TLRs may implicate involvement of the receptors in disease mechanisms beyond innate immunity, such as homeostatic mechanisms to resolve injury. Our studies of atherosclerosissusceptible mouse models highlight TLR involvement in the process of this vascular disease. Distinguishing between local and systemic factors that contribute to the development of atherosclerotic lesions via activation of TLRs induced by endogenous TLR ligands is formidable. However, bone marrow trans- T 110 IMMUNOLOGY 2006 plantation allows alteration of gene expression for specific types of cells found in atherosclerotic lesions. Such chimeric mice can be produced to express or lack a single gene in key effector cells, including macrophagederived foam cells, involved in the development of atherosclerosis. Furthermore, by using reverse bone marrow transplantation, chimeric mice can be produced that express the gene solely in bone marrow–derived cells. These procedures enable us to study the effects of gene expression specific to bone marrow cells during disease progression. Using bone marrow transplantation, we generated atherosclerosis-susceptible chimeric mice with bone marrow cells that lacked the gene for either TLR2 or TLR4. Lesions containing macrophage-derived foam cells lacking TLR2 or TLR4 developed in these animals. Surprisingly, when chimeric mice that lacked TLR2 or TLR4 solely in bone marrow–derived cells and that also lacked receptors for low-density lipoprotein (LDLr–/–) were fed an atherogenic diet for 4 months, no changes occurred in the size of lesions in either the aortic sinus or the aorta. To rule out the possibility of a role for TLR2 expression by bone marrow–derived cells, we used reverse bone marrow transplantation. Chimeras with TLR2 expression confined to bone marrow–derived cells were produced by using female LDLr–/–TLR2–/– mice as recipients and wild-type or TLR2–/– mice as donors. Again, no differences in the severity of atherosclerotic lesions were observed. All LDLr–/–TLR2–/– mice that received bone marrow transplants had smaller lesions than did LDLr–/– recipient mice, regardless of the donor TLR2 genotype. Thus, a TLR2 deficiency in cells not derived from bone marrow led to less disease. Collectively, these data suggest that endogenous ligand signaling via TLR2 and/or TLR4 affected disease outcome and that TLR2 and/or TLR4 expression on cells not derived from bone marrow mediated the effects of TLR2 and/or TLR4 activated by endogenous TLR ligands. To determine the atherosclerotic effect of systemic activation of TLR2, we administered the exogenous TLR2 ligand synthetic N-palmitoyl-S-[2,3-bis(palmitoyloxy)-(2RS)-propyl]-(R)-cysteine (Pam3), a lipopeptide that induces signaling in cells of the immune system through TLR2, to hypercholesterolemic LDLr –/– mice. After 10 weeks of high-fat feeding and weekly intraperitoneal injections of Pam3, the mice had a striking dose-dependent increase in lesion severity. Compared with lesions in control mice not given Pam3, the areas THE SCRIPPS RESEARCH INSTITUTE of aortic sinus and en face aortic lesions were increased 82% and more than 400%, respectively. In similarly treated LDL–/– mice that also lacked the gene for TLR2, no increase in lesion severity occurred. To dissect the proatherosclerotic mechanism of TLR2 activation, we used bone marrow transplantation to produce TLR2-deficient chimeras. LDLr–/– mice were reconstituted with bone marrow cells from wildtype or TLR2-deficient mice. Using the same method of TLR2 activation as before, we found a proatherosclerotic effect of Pam3 only in mice reconstituted with wild-type bone marrow. These mice had increases in lesion severity of 89% and 240% in the aortic sinus and the aorta, respectively. Hence, although both groups of mice had intact TLR2 signaling in cells not derived from bone marrow, only those mice that expressed TLR2 in bone marrow– derived cells, such as monocyte-derived macrophages, had increased atherosclerosis when activated with the exogenous agonist. Additionally, the TLR2-induced acceleration of lesion development occurred with an unusual pattern of atherosclerosis, with profuse abdominal lesions. Subsequent experiments with intravenous administration of Pam3 did not reproduce this peculiar development of abdominal lesions, thereby implicating a role of peritoneal inflammatory mediators in the effects of Pam3 injected intraperitoneally. PUBLICATIONS Boisvert, W.A., Rose, D.M., Boullier, A., Quehenberger, Q., Sydlaske, A., Johnson, K.A., Curtiss, L.K., Terkeltaub, R. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler. Thromb. Vasc. Biol. 26:563, 2006. Boisvert, W.A., Rose, D.M., Johnson, K.A., Fuentes, M.E., Lira, S.A., Curtiss, L.K., Terkeltaub, R.A. Up-regulated expression of the CXCR2 ligand KC/GROα in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am. J. Pathol. 168:1385 2006. Curtiss, L.K. Is two out of three enough for ABCG1? Arterioscler. Thromb. Vasc. Biol. 26:2175, 2006. Curtiss, L.K., Valenta, D.T., Hime, N.J., Rye, K.-A. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler. Thromb. Vasc. Biol. 26:12, 2006. Mullick, A.E., Tobias, P.S., Curtiss, L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115:3149, 2005. Mullick, A.E., Tobias, P.S., Curtiss, L.K. Toll-like receptors and atherosclerosis: key contributors in disease and health? Immunol. Res. 34:193, 2006. Tilley, R.E., Pedersen, B., Pawlinski, R., Sato, Y., Erlich, J.H., Shen, Y., Day, S., Huang, Y., Eitzman D.T., Boisvert, W.A., Curtiss, L.K., Fay, W.P., Mackman, N. Atherosclerosis in mice is not affected by a reduction in tissue factor expression. Arterioscler. Thromb. Vasc. Biol. 26:555, 2006. Valenta, D.T., Bulgrien, J.J., Banka, C.L., Curtiss, L.K. Overexpression of human apoAI transgene provides long-term atheroprotection in LDL receptor-deficient mice. Atherosclerosis, in press. IMMUNOLOGY 2006 Valenta, D.T., Ogier, N., Bradshaw, G., Black, A.S., Bonnet, D.J., Lagrost, L., Curtiss, L.K., Desrumaux, C.M. Atheroprotective potential of macrophage-derived phospholipid transfer protein in low-density lipoprotein receptor-deficient mice is overcome by apolipoprotein AI overexpression. Arterioscler. Thromb. Vasc. Biol. 26:1572, 2006. Enhanced Tissue Factor Initiation of the Coagulation and Thrombogenic Cascades by α2-Macroglobulin G. Bhattacharjee, S. Arandjelovic, N. Mackman, W. Ruf, S.L. Gonias, T.S. Edgington he broad spectrum poteinase inhibitor α2-Macroglobulin differs from all 4 mechanistic classes of proteinase inhibitors. It inhibits coagulation proteinases such as thrombin and factor Xa and fibrinolytic proteinases such as plasmin. a2-Macroglobulin is also a carrier of growth factors and may regulate their function. Receptors for a2-macroglobulin include the low-density lipoprotein receptor-related protein (LRP) and 78-kD glucose-regulated protein (Grp78), a highaffinity receptor. In its native conformation, a2-macroglobulin is present in plasma at high concentrations. Upon binding to serum proteinases or small primary amines, a2-macroglobulin is converted to the receptorrecognized form, which binds to LRP. Complexes composed of LRP, a2-macroglobulin, and proteinases are internalized by cells. Previously, we found that Grp78 binds tissue factor and inhibits its function. Recently, we discovered that the receptor-recognized form of α2-macroglobulin enhances initiation of the coagulation and thrombotic cascades by tissue factor, generation of factor Xa, and expression of the gene for tissue factor. This enhancement depends on binding of the receptor-recognized form of α2-macroglobulin to LRP, but not to Grp78, because receptor-associated protein blocks the response. The effects of α2-macroglobulin on tissue factor–dependent procoagulant activity are most pronounced in RAW 264.7 macrophage-like cells, but the effects also occur in THP-1 monocytic cells. A mutant of the receptor-recognized form of α2-macroglobulin that does not bind LRP has no effect on tissue factor activity or gene expression. T THE SCRIPPS RESEARCH INSTITUTE 111 Infarctive Eradication of Tumors by Selective Tumor Vascular Thrombosis A. EL-Sheikh, P. Borgstrom, G. Bhattacharjee, M. Belting, T.S. Edgington hen fused to the extracellular domain of tissue factor (TFt), the exon-encoded polypeptide of the heparin-binding domain (HBDt) of vascular endothelial cell growth factor localizes selectively to endothelial surfaces of intratumoral microvasculature. Tissue factor is the initiating receptor and requisite cofactor for initiation of the thrombogenic cascade. In tumor-bearing mice, intravenous infusion of the fused domains, HBDt-TFt, results in selective rapid occlusive thrombosis of tumor microvasculature. We found that infusion of an optimal combination of HBDt-TFt and its ligand factor VIIa in tumor-bearing animals results in infarctive eradication of tumors and often is curative. Binding studies and confocal microscopy indicated that the target for HBDt-TFt is a trimolecular complex of chondroitin C sulfate proteoglycan, neuropilin-1, and vascular endothelial cell growth factor receptor 2, which is overexpressed in highly angiogenic sites of the tumor microenvironment. HBDt-TFt also colocalized with the trimolecular receptor complex in endothelial sprouts from tumor tissues, and binding inhibited growth of sprouts. In vitro, HBDt had the highest affinity for chondroitin 6 sulfate. We are evaluating the potential of HBDt-TFt as a therapeutic agent. W Legumain Expression and Cell-Surface Translocation as a Catalytic Target for Prodrug Delivery and Therapy W. Wu, Y. Luo, C. Sun, Y. Liu, P. Kuo, J. Varga, R. Xiang, R.A. Reisfeld, K.D. Janda, T.S. Edgington, C. Liu egumain, a novel and highly specific asparaginyl endopeptidase of the cysteine protease family, is conserved as the only proteinase of this specificity from plant to humans. It is highly expressed by endo- L 112 IMMUNOLOGY 2006 thelial, neoplastic, and stromal cells in most tumors, in humans and animals. We developed a legumain-activated, cell-impermeable preprodrug that incorporates doxorubicin as the cytotoxic element, although a wide variety of cytotoxic molecules can be incorporated to make the molecules nontoxic. Designated LEG3, the preprodrug targets extracellular legumain on tumor microvasculature, tumor stroma, and neoplastic cells. Upon binding, it is catalytically converted to a cell-permeable prodrug, which is then converted to doxorubicin inside the cells, where it results in tumor-specific killing. Pharmacokinetics and tissue distribution of LEG3 compared with doxorubicin alone confirmed selective local prodrug activation leading to 30- to 100-fold increases of doxorubicin in tumor cell nuclei but with 100-fold reductions of doxorubicin in normal tissues. Importantly, this prodrug completely inhibited growth of a variety of multidrug-resistant human tumors in mice, produced tumor eradication, and significantly extended survival without any evidence of myelosuppression or toxic cardiac effects. THE SCRIPPS RESEARCH INSTITUTE PUBLICATIONS Bhattacharjee, G., Ahamed, J., Pedersen, B., EL-Sheikh, A., Mackman, N., Ruf, W., Liu, C., Edgington, T.S. Regulation of tissue factor-mediated initiation of the coagulation cascade by cell surface Grp78. Arterioscler. Thromb. Vasc. Biol. 25:1737, 2005. EL-Sheikh, A., Borgstrom, P., Bhattacharjee, G., Belting, M., Edgington, T.S. A selective tumor microvasculature thrombogen that targets a novel receptor complex in the tumor angiogenic microenvironment. Cancer Res. 65:11109, 2005. Lin, R., Maeda, S., Liu, C., Karin, M., Edgington, T.S. A large noncoding RNA is a marker for murine hepatocellular carcinoma and a spectrum of human carcinomas. Oncogene, in press. Wu, W., Luo, Y., Sun, C., Liu, Y., Kuo, P., Varga, J., Xiang, R., Reisfeld, R., Janda, K.D., Edgington, T.S., Liu, C. Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Cancer Res. 66:970, 2005. Control of V(D)J Recombination and Formation of the Antibody Repertoire in Normal and Autoimmune Mice A.J. Feeney, C.R. Espinoza, J. Lamoureux, M. Cherrier, C.R. Xu, J. Carey, S. Salerno, L. Watson Association of a Large Noncoding RNA Riboregulator With Neoplasia R. Lin, S. Maeda, C. Liu, M. Karin, T.S. Edgington arge noncoding RNAs account for an unexpectedly large proportion of the transcribed genome but have been sparsely analyzed. We found a novel murine gene encoding a 7000-base mRNA-like transcript. This gene and mammalian counterparts lack credible or conserved open reading frames, characteristics of large noncoding RNAs. In murine hepatocellular carcinomas induced with a procarcinogen and in all samples of human hepatocellular carcinomas analyzed, expression of this gene was enhanced. Compared with normal liver, hepatocellular carcinomas had a 6- to 7-fold increase of this noncoding RNA. Thus, this large noncoding RNA is a new marker for hepatocellular carcinoma. The RNA was also significantly overexpressed in all 5 nonhepatocellular human carcinomas analyzed. Expression was enhanced in cells enriched at the mitotic stage, advancing this large noncoding RNA as a generic marker for carcinomas and suggesting a role for it as a riboregulator (regulatory RNA) in the molecular cell biology of neoplasia. L main focus of our laboratory is the molecular analysis of factors that influence the composition of the antibody repertoire and elucidation of the mechanisms that control the V(D)J rearrangement process. In each precursor B lymphocyte, a different set of V, D, and J genes recombine to form exons for the light and heavy chains of the antibody molecule. Each locus has many V, D, and J genes, but the gene segments are not used equally. One of our goals is to understand the basis of this nonrandom use of gene segments. We previously showed that much of this bias occurs because V genes undergo recombination with different intrinsic frequencies due to differences in the recombinase signal sequence, the binding site for the recombinase, flanking each gene segment. The recombinase signal sequence is composed of a relatively conserved heptamer and nonamer flanking a “spacer” of conserved length but only modestly conserved sequence. Few genes have consensus sequences, however, and changes in this natural variation in the recombinase signal sequence can greatly affect recombination frequency in vitro and in vivo. In addition, other factors clearly influence recombination frequencies; currently we are focusing on the role of transcription factors and chromatin modifications A IMMUNOLOGY 2006 in controlling accessibility to V(D)J recombination and recombination frequency. Genes in loci that are undergoing V(D)J recombination are often associated with histones that are acetylated. We hypothesized that the extent of histone modification affects the frequency of recombination of individual genes, and indeed we observed a positive correlation between the relative rearrangement frequency of several individual genes in vivo and the extent of acetylation of histones H3 and H4 associated with those genes, as assessed by chromatin immunoprecipitation. Other modifications associated with repressed or activated genes are now being investigated. We have also uncovered a novel role for the transcription factor Pax5 in promoting V(D)J rearrangement. Pax5 is essential for B-cell development. In the absence of Pax5, VH to DJH rearrangement is severely impaired. We found Pax5 binding sites in the coding regions of many V H genes. Furthermore in collaboration with Z. Zhang and M. Cooper, University of Alabama, Birmingham, we showed that Pax5 binds to the recombinase proteins RAG1 and RAG2. Hence, we propose that Pax5 may recruit RAG1 or RAG2 to the recombinase signal sequence or may stabilize the interaction of the RAG complex with its binding site. In other studies, we are examining the breakdown of B-cell tolerance in autoimmunity. When precursor B cells successfully recombine both heavy- and lightchain gene segments, they express a B-cell receptor for the first time. If the receptor is autoreactive, then the immature B cell normally continues to undergo lightchain V-J rearrangement until an innocuous receptor is made. This process is termed receptor editing and is an important checkpoint in B-cell tolerance. We have evidence that this process is not functioning as efficiently in lupus-prone mice as in nonautoimmune mice, and we are investigating why this difference occurs. Such misregulation of this key checkpoint could lead to the release of autoreactive B cells into the periphery, where they can become activated to secrete autoantibodies and cause autoimmune disease. PUBLICATIONS Espinoza, C.R., Feeney, A.J. The extent of histone acetylation correlates with differential rearrangement frequency of individual VH genes in pro-B cells. J. Immunol. 175:6668, 2005. Espinoza, C.R., Feeney, A.J. Quantifying chromatin accessibility of individual gene family members by combining ligation-mediated PCR with real-time PCR. Biotechniques 41:404, 2006. Watson, L.C., Moffatt-Blue, C.S., MacDonald, R.Z., Kompfner, E., Aït-Azzouzene, D., Nemazee, D., Theofilopoulos, A.N., Kono, D.H., Feeney, A.J. Paucity of V-DD-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT–/– and TdT+/+ mice. J. Immunol. 172:1120, 2006. THE SCRIPPS RESEARCH INSTITUTE 113 Zhang, Z., Espinoza, C.R., Yu, Z., Stephan, R., He, T., Williams, G.S., Burrows, P.D., Hagman, J., Feeney, A.J., Cooper, M.D. Transcription factor Pax5 (BSAP) transactivates the RAG-mediated VH-to-DJH rearrangement of immunoglobulin genes. Nat. Immunol. 7:616, 2006. Syndecans and HIV Type 1 Pathogenesis M. Bobardt, U. Chatterji, S. Selvarajah, A. de Parseval,* J.H. Elder,* P.A. Gallay * Department of Molecular Biology, Scripps Research he syndecans belong to the heparan sulfate family of proteoglycans. In syndecans, the sulfation pattern of the heparan sulfate chains dictates the ligand specificity. HIV type 1 (HIV-1) has maximized its use of syndecans. It uses them as receptors to facilitate infection of macrophages. It uses them as receptors on the endothelium to enhance endurance of the virus in the hostile environment and to trans infect circulating T cells. HIV-1 also exploits syndecans to facilitate transport of the virus through the blood-brain barrier and the genital epithelium. Thus, the interplay between HIV-1 and syndecans may profoundly affect HIV-1 pathogenesis. Sexual transmission is the most common mode of infection in the global HIV-1 epidemic. In the absence of an effective vaccine, additional strategies to prevent new HIV-1 infections are urgently needed. Mucosal dendritic and Langerhans cells are the first cells that HIV-1 encounters. These cells may thus play a crucial role in HIV-1 transmission. Interactions between HIV-1 and dendritic or Langerhans cells are poorly understood. Although researchers initially proposed that C-type lectin receptors such as DC-SIGN, the mannose receptor, and langerin mediated contact between HIV-1 and dendritic or Langerhans cells, blocking of CD4, CCR5, and C-type lectin receptors only partly prevents HIV-1 capture by dendritic cells. This finding led researchers to postulate the existence of unidentified HIV-1 receptors on dendritic cells. We found that syndecan-3, which is normally poorly expressed or even absent on many cell types and tissues, is highly expressed on both dendritic cells and Langerhans cells. More importantly, we obtained evidence that syndecan-3 plays a key role in HIV-1 capture and trans infection of T cells by dendritic cells. Because dendritic and Langerhans cells may be the first Trojan horses that HIV-1 exploits to colonize T 114 IMMUNOLOGY 2006 humans, understanding the contribution of syndecan-3 in the capture and transfer of HIV-1 by dendritic cells and Langerhans cells to T cells is imperative to develop new therapies to prevent HIV-1 infections. PUBLICATIONS Binley, J.M., Ngo-Abdalla, S., Moore, P., Bobardt, M., Chatterji, U., Gallay, P., Burton, D.R., Wilson, I.A., Elder, J.H., de Parseval, A. Inhibition of HIV Env binding to cellular receptors by monoclonal antibody 2G12 as probed by Fc-tagged gp120. Retrovirology. 3:39, 2006. Bobardt, M.D., Chatterji, U., Selvarajah, S., Van der Schueren, B., David, G., Kahn, B., Gallay, P.A. Cell-free HIV-1 transcytosis through primary genital epithelial cells. J. Virol., in press. de Parseval, A., Bobardt, M.D., Chatterji, A., Chatterji, U., Elder, J.H., David, G., Zolla-Pazner, S., Farzan, M., Lee, T.H., Gallay, P.A. A highly conserved arginine in gp120 governs HIV-1 binding to both syndecans and CCR5 via sulfated motifs. J. Biol. Chem. 280:39493, 2005. Saphire, A.C., Gallay, P.A., Bark, S.J. Proteomic analysis of human immunodeficiency virus using liquid chromatography/tandem mass spectrometry effectively distinguishes specific incorporated host proteins. J. Proteome Res. 5:530, 2006. Innate Intracellular Immunity and Infection With HIV Type 1 U. Chatterji, M. Bobardt, S. Selvarajah, P.A. Gallay onhuman cells contain intracellular innate factors that inhibit infection by HIV type 1 (HIV-1) by targeting the incoming viral capsid core, which makes up the shell that surrounds the viral genome. The first intracellular primate restriction factor identified, TRIM5α, is a member of the tripartite motif (TRIM) family of proteins. However, the mechanism by which TRIM5α blocks HIV-1 infection is unknown. We asked if TRIM5α blocks HIV-1 by diverting the incoming capsid core into an abortive degradation pathway. We compared the degradation status of incoming cores in restrictive and nonrestrictive cells. We found that monkey, but not human, TRIM5α provokes destruction of capsids immediately after HIV-1 entry. The capsid degradation is specific because the integrity of other abundant viral proteins is preserved. TRIM5α directs capsids to a nonproteasome degradation pathway. Capsids delivered into the vesicular compartment undergo similar degradation in restrictive and nonrestrictive cells. In contrast, capsids delivered into the cytosol of nonrestrictive cells remain intact, whereas capsids delivered into the cytosol of restrictive cells are destroyed. Our finding that TRIM5α specifically degrades cytosolic capsids suggests that cytosolic capsids represent authentic infectious events, whereas vesicular N THE SCRIPPS RESEARCH INSTITUTE capsids represent abortive infectious events. Further work is required to determine whether TRIM5α-mediated capsid degradation and capsid core uncoating represent linked or distinct events. An understanding of the nature of restrictions to HIV-1 infection after the virus enters cells is critical for several reasons. First, information on the viral and cellular factors that modulate these processes will shed light on the poorly understood series of events that govern the fate of capsids after entry. Second, speciesspecific barriers to HIV-1 infection present obstacles to the development of animal models for the study of HIV-1 pathogenesis and treatment. Finally, an understanding of this critical part of the HIV-1 life cycle may suggest approaches to intervene in transmission of the virus or its spread within the host. PUBLICATIONS Barry, S.M., Melar, M., Gallay, P., Hope, T.J. Review of the twelfth West Coast Retrovirus Meeting. Retrovirology 2:72, 2005. Chatterji, U., Bobardt, M.D., Gaskill, P., Sheeter, D., Fox, H., Gallay, P.A. TRIM5α accelerates degradation of cytosolic capsid associated with productive HIV-1 entry. J. Biol. Chem., in press. Chatterji, U., Bobardt, M.D., Stanfield, R., Ptak, R.G., Pallansch, L.A., Ward, P.A., Jones, M.J., Stoddart, C.A., Scalfaro, P., Dumont, J.M., Besseghir, K., Rosenwirth, B., Gallay, P.A. Naturally occurring capsid substitutions render HIV-1 cyclophilin A independent in human cells and TRIM-cyclophilin-resistant in Owl monkey cells. J. Biol. Chem. 280:40293, 2005. Imaging Molecular Interactions in T-Cell Development and Activation N.R.J. Gascoigne, J. Ampudia, G. Fu, C. Goergen, K. Holmberg, H.-C. Hung, H.-O. Kim, C. Lotz, A. Munshi, G. Sternik, S. Vallee, P. Yachi, M.A. Zal. T. Zal, N. Bosco,* R. Ceredig,* M. Daniels,** E. Palmer** * U548 INSERM, Grenoble, France ** University Hospital, Basel, Switzerland IMAGING OF INTERACTIONS BETWEEN T-CELL RECEPTORS AND CORECEPTORS IN T-CELL A C T I VAT I O N luorescence resonance energy transfer (FRET) is a physical phenomenon that occurs at distances less than 10 nm. Thus, FRET between fluorescent proteins, for example, between cyan and yellow fluorescent proteins, attached to proteins of interest can be used to investigate interactions between proteins in living cells. F IMMUNOLOGY 2006 Using FRET, we showed that the coreceptor CD8 and the T-cell receptor (TCR) signal-transducing protein CD3ζ are recruited to the “immunologic synapse,” where they interact when antigenic MHC-peptide complexes are presented to T cells. No FRET occurs when weaker (e.g., TCR antagonist) ligands are used. We showed previously that endogenous MHC-peptides aid in recognizing antigenic MHC-peptide complexes. The interaction between CD8 and endogenous MHC-peptides improves TCR recognition of antigenic MHC-peptides, including the ability to associate with CD8. This surprising finding suggests how T cells can respond to small amounts of antigens in a “sea” of nonstimulatory MHC-peptides. We are now testing the relative importance of TCR and CD8 interactions with the endogenous MHC-peptides in helping stimulation by antigenic MHC-peptide complexes. We also compared the formation of immunologic synapses and the TCR-CD8 interaction in a system in which the affinity of the interaction between TCRs and MHC-peptides is known. The strength of weak agonists is more closely related to the speed at which they recruit TCRs to the synapse and start to induce FRET than it is to the affinity of the interaction between TCRs and MHC-peptides. In collaboration with E. Palmer, University Hospital, Basel, Switzerland, we investigated new signaling induced through the TCR that differs between positive and negative selection. The induction of FRET appears to explain why some agonists are stronger or weaker than would be predicted on the basis of their affinities, and the kinetics of FRET differ between positiveand negative-selecting stimuli. We are directly testing the potentially different roles of CD8αα and CD8αβ in formation of the immunologic synapse and the dynamics of association of the kinase Lck with CD8 before and during antigenic stimulation. ROLE OF THE PROTEIN KINASE C η ISOFORM IN THE IMMUNOLOGIC SYNAPSE We previously showed that the η isoform of protein kinase C (PKC) is upregulated during positive selection of developing thymocytes. Of the PKC isoforms, only PKCθ is known to have a special role in T cells, where it is recruited to the immunologic synapse during antigen recognition. The finding that mice deficient in PKCθ have normal thymic selection suggested that PKCη could be replacing PKCθ in the developing thymocytes. We found that PKCη is also naturally recruited to the synapse in mature thymocytes and T cells. In the THE SCRIPPS RESEARCH INSTITUTE 115 absence of PKCθ, PKCη is expressed at an earlier stage of thymocyte development, where it functions in place of PKCθ. Inhibition of PKCη expression during thymocyte development by short hairpin RNAs results in inhibition of T-cell development. We are now using short hairpin RNA and mice lacking the gene for PKCη to determine the roles of PKCη in T-cell activation and development. I D E N T I F I C AT I O N O F A N O V E L P R O T E I N I M P O R TA N T I N T - C E L L D I F F E R E N T I AT I O N We identified a novel protein that is expressed primarily during thymocyte differentiation. It is expressed during the stages at which TCR genes undergo rearrangement, and it interacts with the cell-cycle and DNA damage-repair enzyme ATM. Transgenic expression of the gene that encodes the protein provides some protection against DNA double-strand breaks induced by ionizing radiation, suggesting that the novel protein acts in synergy with ATM. The protein also interacts with phospholipase C γ1, important in T-cell signaling. We have produced a strain of mice that lack the gene for this novel protein. Mice that lack the gene have defects in thymic positive selection and reduced phosphorylation of phospholipase C γ1 in response to TCR stimulation. We intend to identify the role of this protein in T-cell signaling and development. TCR ENDOCYTOSIS, RECYCLING, AND U B I Q U I T I N AT I O N Because allelic exclusion of the TCR α-chain is maintained after translation, many mature T cells express 2 α-chain proteins. However, expression of 2 α-chains on the cell surface is rare. We previously showed that functional allelic exclusion is attained in the thymus through TCR signaling involving the kinase Lck and the ubiquitin ligase Cbl, which controls degradation of endocytosed TCRs. We are developing a transgenic minigene system to analyze the effects of expression of 2 α-chains on the cell surface. We are using FRET between ubiquitin monomers and TCR subunits labeled with fluorescent proteins to analyze ubiquitination of TCRs after endocytosis. In collaboration with R. Ceredig, INSERM, Grenoble, France, we examined the TCR α-chain repertoire of specialized CD25+CD4+ regulatory T cells. We found that the repertoire is as diverse as that of mainstream CD4+ T cells. We discovered a new property of the dye FM4-64 that enables us to selectively image the nuclear membrane in living cells. This discovery should be useful to cell biologists studying nuclear trafficking of proteins and diseases of the nuclear envelope. 116 IMMUNOLOGY 2006 PUBLICATIONS Bosco, N., Hung, H.-C., Pasqual, N., Jouvin-Marche, E., Marche, P.N., Gascoigne, N.R.J., Ceredig, R. Role of the T cell receptor α-chain in the development and phenotype of naturally arising CD4+CD25+ T cells. Mol. Immunol. 43:246, 2006. Daniels, M.A., Teixeiro, E., Gill, J., Hausmann, B., Roubaty, D., Holmberg, K., Werlen, G., Holländer, G., Gascoigne, N.R.J., Palmer, E. Thymic selection threshold defined by compartmentalization of Ras/MAK signalling. Nature, in press. Yachi, P.P., Ampudia, J., Zal, T., Gascoigne, N.R. Altered peptide ligands induce delayed CD8-T cell receptor interaction: a role for CD8 in distinguishing antigen quality. Immunity. 25:203, 2006. Zal, T., Zal, M.A., Lotz, C., Goergen, C.J., Gascoigne, N.R. Spectral shift of fluorescent dye FM4-64 reveals distinct microenvironment of nuclear envelope in living cells. Traffic, in press. Zambricki, E., Zal, T., Yachi, P., Shigeoka, A., Sprent, J., Gascoigne, N., McKay, D. In vivo anergized T cells form altered immunological synapses in vitro. Am. J. Transplant. 6:2572. 2006. Signaling Pathways in the Innate Immune System J. Chen, R.T. Cook, Y. Kang, S. Lee, J. Mols, M. Otsuka, S. Shimada, C.C. Wu, C. Xie, Y. Xu, J. Han he p38 MAP kinase pathway plays a crucial function in the cellular response after infection by pathogens or inflammatory stimulation. Our interests are the functions of p38 in innate immunity in fruit flies, in Toll-like receptor (TLR) signaling, and in control of the cell cycle. We are also interested in the involvement of microRNAs in the innate immune system. T A N E W PAT H WAY I N T H E I M M U N E R E S P O N S E T O BACTERIAL INFECTION IN DROSOPHILA Using the P-element insertion-excision technique, we generated mutant fruit flies that lacked individual p38 isoforms and a double mutant that lacked both p38 isoforms. As widely described previously, the Toll and Imd pathways are essential in Drosophila for protection against microbial infection. In contrast, we showed that the p38 pathway mediates another defense mechanism independent of those 2 pathways and that the deficiency of either Toll or Imd does not impair p38 activation after infection. In the p38 double mutants, melanization of the hindgut, which is related to bacterial infection, develops during the larval stage. Furthermore, p38 regulates some of the functions of heat-shock factor, and expression of heat-shock proteins mediated by heatshock factor is, at least in part, responsible for the p38 anti-infectious functions, revealing a new innate immune system in Drosophila. THE SCRIPPS RESEARCH INSTITUTE M E D I AT I O N O F T L R 4 S I G N A L I N G B Y T H E 4 - 1 B B LIGAND TLRs are an important first contact between host and microorganisms after infection. Once activated, the receptors generate downstream signaling pathways leading to gene expression. The 4-1BB ligand is highly induced and expressed at the cell surface of macrophages after inflammatory stimulation. We showed that once expressed, the ligand can interact with TLRs at the cell surface of macrophages, but mostly activation of the TLR4 signaling pathway leads to p38 activation. These results revealed a new mechanism in which macrophages undergo a 2-step activation process, involving expression and translocation of 4-1BB ligand to the cell surface and afterward interaction with TLRs, sustaining the inflammatory responses. A N T I V I R A L R E S P O N S E S M E D I AT E D B Y M I C R O R N A We found that some microRNAs interfere with propagation of vesicular stomatitis virus (VSV) and VSVinduced cell death. MicroRNAs are single-stranded RNA molecules of 19–23 nucleotides that originate from longer and imperfectly matching hairpin precursors processed by several ribonucleoprotein complexes. The cytosolic enzyme Dicer converts hairpin precursors into 19- to 23-nucleotide RNA duplexes and therefore is of key importance for the maturation of microRNAs. We generated Dicer-deficient mice and investigated macrophage sensitivity to VSV infection. Macrophages from Dicer-deficient mice had greater VSV propagation and VSV-induced cell death than did control macrophages from wild-type mice. Furthermore, we found that the anti-VSV functions of Dicer are at least partly mediated by endogenous microRNAs that target viral RNA genes encoding the RNA-directed RNA polymerase complex. Therefore, microRNAs may be a broadly important element of the innate immune response to viral infection in mammals. R E G U L AT I O N O F R N A I N T E R F E R E N C E B Y T N F S T I M U L AT I O N Regulation of the mRNA stability of cytokines by inflammatory stimuli is widely documented and is linked to p38 activity. Previously, we showed that adenine-uridine–rich elements (AREs) in the 3′ untranslated region of cytokine mRNAs dictate mRNA degradation in a microRNA-dependent manner. Recently, we found that exogenously added short interfering RNAs and the endogenous ARE-microRNA systems act as regulators of mRNA stability and that both systems are regulated IMMUNOLOGY 2006 by inflammatory stimulation. Indeed, TNF-α stimulation blocks both short interfering RNA– and ARE-dependent mRNA degradation. The mechanism of action involves lengthening of the poly (A) tail and a partial decrease of the activity of the RNA-induced silencing complex that does not impair the synthesis and maturation of short interfering RNAs and microRNAs. We conclude that RNA interference is based on mechanisms regulated by inflammatory stimulation, linking the ARE-dependant mRNA stabilization to the function of short interfering RNAs. PUBLICATIONS Al Sarraj, J., Vinson, C., Han, J., Thiel, C. Regulation of GTP cyclohydrolase I gene transcription by basic region leucine zipper transcription factors. J. Cell. Biochem. 96:1003, 2005. da Silva Correia, J., Miranda, Y., Austin-Brown, N., Hsu, J., Mathison, J., Xiang, R., Zhou, H., Li, Q., Han, J., Ulevitch, R.J. Nod1-dependent control of tumor growth. Proc. Natl. Acad. Sci. U. S. A. 103:1840, 2006. Fu, J., Yang, Z., Wei, J., Gu, J. Nuclear protein NP60 regulates p38 MAPK activity. J. Cell Sci. 119(Pt. 1):115, 2006 Han, J. MyD88 beyond Toll. Nat. Immunol. 7:370, 2006. Han, J., Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 6:1198, 2005. THE SCRIPPS RESEARCH INSTITUTE 117 Zhuang, S., Yan, Y., Han, J., Schnellmann, R.G. p38 Kinase-mediated transactivation of the epidermal growth factor receptor is required for dedifferentiation of renal epithelial cells after oxidant injury. J. Biol. Chem. 280:21036, 2005. Specificity and Function of Intraepithelial γδ T Cells W.L. Havran, M. Haynes, J.M. Jameson, C.H. Kim, E. Kolkowski, H.K. Komori, T. Meehan, R. Mills, A. Toulon, M. Watanabe, J. Whitelock, D. Witherden e have a long-term interest in interactions between intraepithelial γδ T cells and their neighboring epithelial cells. We focus on interactions in the thymus, skin, and intestine. We are investigating the development, specificity, and function of these γδ T cells. Our results have defined unique properties of these cells and support a specialized role for intraepithelial γδ T cells in immune surveillance, wound repair, inflammation, and protection from malignant tumors. W MOLECULES REQUIRED FOR γδ T CELL A C T I VAT I O N Liu, W., Rui, H., Wang, J., Lin, S., He, Y., Chen, M., Li, Q., Ye, Z., Zhang, S., Chan, S.C., Chen, Y.G., Han, J., Lin, S.C. Axin is a scaffold protein in TGF-β signaling that promotes degradation of Smad7 by Arkadia. EMBO J. 25:1646, 2006. Lu, G., Kang, Y.K., Han, J., Herschman, H.R., Stefani, E., Wang, Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J. Biol. Chem. 281:6087, 2006. Meng, F., Yamagiwa, Y., Taffetani, S., Han, J., Patel, T. IL-6 activates serum and glucocorticoid kinase via a p38α mitogen-activated protein kinase pathway. Am. J. Physiol. Cell. Physiol. 289:C971, 2005. Shi, G.X., Han, J., Andres, D.A. Rin GTPase couples nerve growth factor signaling to p38 and b-Raf/ERK pathways to promote neuronal differentiation. J. Biol. Chem. 280:37599, 2005. Tang, J., Qi, X., Mercola, D., Han, J., Chen, G. Essential role of p38β in K-Ras transformation independent of phosphorylation. J. Biol. Chem. 280:23910, 2005. Xie, C., Zhang, N., Zhou, H., Li, J., Li, Q., Zarubin, T., Lin, S., Han, J. Distinct roles of basal steady-state and induced H-ferritin in tumor necrosis factor-induced death in L929 cells. Mol. Cell. Biol. 25:6673, 2005. Xu, Y., Huang, S., Liu, Z.G., Han, J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 281:8788, 2006. Zarubin, T., Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15:11, 2005. Zhou, H., Zarubin, T., Ji, Z., Min, Z., Zhu, W., Downey, J.S., Lin, S., Han, J. Frequency and distribution of AP-1 sites in the human genome. DNA Res. 12:139, 2005. Zhou H., Zheng, M., Chen, J., Xie, C., Kolatkar, A.R., Zarubin, T., Ye, Z., Akella, R., Lin, S., Goldsmith, E.J., Han, J. Determinants that control the specific interactions between TAB1 and p38β. Mol. Cell. Biol. 26:3824, 2006. Zhu, X., Mei, M., Lee, H.G., Wang, Y., Han, J., Perry, G., Smith, M.A. p38 Activation mediates amyloid-β cytotoxicity. Neurochem. Res. 30:791, 2005. In murine skin, γδ T cells express an invariant γδ T cell receptor that recognizes an unknown antigen expressed by damaged or malignant neighboring keratinocytes. We have now produced soluble skin γδ T cell receptor molecules for use as a tool to detect expression and facilitate isolation and characterization of this unidentified antigen. Future structural studies will determine how these T-cell receptors interact with antigen. We propose that in addition to antigen, damaged keratinocytes express molecules that participate in activation of skin γδ T cells by binding to coreceptors or costimulatory molecules on the T-cell surface. Skin γδ T cells do not express classical molecules, including CD4, CD8, and CD28, known to affect activation of αβ T cells. We recently identified several molecules expressed by the skin γδ T cells and keratinocytes that provide important costimulatory signals for activation of γδ T cells. One such molecule, JAML, is uniquely costimulatory for intraepithelial γδ T cells. We have identified another JAM family member, the coxsackievirus-adenovirus receptor, as a ligand for JAML that is expressed on epithelial cells in the skin and intestine. Interactions between JAML and the coxsackievirus-adenovirus receptor may play important roles in γδ T cell responses during wound repair and other epithelial challenges. 118 IMMUNOLOGY 2006 We also found that the semaphorin Sema4D (CD100) is expressed by skin and intestinal γδ T cells upon activation. CD100 binds to a new member of the plexin superfamily of semaphorin receptors, plexin-B2, expressed on epithelial cells. We found that interactions between CD100 and plexin-B2 deliver signals to both the intraepithelial γδ T cells and epithelial cells in the skin and intestine. THE SCRIPPS RESEARCH INSTITUTE inflammatory disorders and may be useful in designing or testing new therapies. PUBLICATIONS Havran, W.L., Jameson, J.M., Witherden, D.A. Epithelial cells and their neighbors, III: interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 289:G627, 2005. Komori, H.K., Meehan, T.F., Havran, W.L. Epithelial and mucosal γδ T cells. Curr. Opin. Immunol. 18:534, 2006. A ROLE FOR INTRAEPITHELIAL γδ T CELLS IN EPITHELIAL TISSUE REPAIR We recently showed a role for skin γδ T cells in the reepithelialization stage of wound repair. The γδ T cells are activated at wound sites and produce cytokines, including the epithelial growth factors KGF-1 and KGF-2. In the absence of skin γδ T cells, keratinocyte proliferation and tissue reepithelialization after wounding are defective. Recent results indicated that a keratinocyteresponsive γδ T-cell receptor is necessary for activation of the T cells by damaged keratinocytes during wound healing and is also required for the maintenance of T cells in the epidermis. In addition, we found that the skin γδ T cells are necessary for the recruitment of inflammatory cells into the wound site. In a novel mechanism, γδ T cell–produced KGFs stimulate production of hyaluronan by epidermal cells, which then controls migration of macrophages into wounds. Skin γδ T cells play roles not only in the repair of damaged tissue but also in the normal maintenance of the epidermis. Insulin-like growth factor 1 is required by keratinocytes in the skin for maintenance and during wound healing. We determined that after activation skin γδ T cells produce this growth factor that affects wound healing and apoptosis in the skin. Together these results indicate a role for skin γδ T cells in multiple aspects of wound repair and for homeostasis of the epithelium. In previous studies, we showed that intestinal intraepithelial γδ T cells play a similar role in responding to tissue damage in a model of colitis. Our recent results indicate that in the absence of CD100-mediated signals, increased damage and delayed repair occur, indicating an important role for costimulation through these molecules in γδ T-cell functions in the gut. Results in both models support our hypothesis that intraepithelial γδ T cells respond to epithelial damage or disease and play important roles in tissue repair and epithelial homeostasis. Future studies should provide information that will further define the role of γδ T cells in epithelial Mechanisms of γδ T-Cell Dysfunction in Nonhealing Wounds J.M. Jameson, R. Mills, K. Taylor kin γδ T cells are activated by stressed or damaged keratinocytes to produce molecules such as insulin-like growth factor 1 (IGF-1) and keratinocyte growth factors that are important for the maintenance of skin homeostasis and wound repair. We are examining skin γδ T cells in nonhealing wounds to investigate the mechanisms that inhibit normal wound healing. S C R O S S TA L K O F γδ T C E L L S A N D K E R AT I N O C Y T E S IN THE SKIN IN DIABETES Treatment of nonhealing wounds is a considerable problem for patients and healthcare services. In murine models of diabetes, levels of IGF-1 and keratinocyte growth factors in wounds contribute to defective wound repair. When these growth factors are replenished, the markedly delayed wound repair is reversed. Previously, we showed that skin γδ T cells play roles in wound reepithelialization via the production of growth factors. Now we are investigating whether decreased levels of growth factors and impaired proliferation/migration of keratinocytes in nonhealing wounds in mice with diabetes are due to dysregulation of skin γδ T cells. Initially, we are focusing on how the cross talk between the key growth factors produced by skin γδ T cells and the factors’ receptors expressed on keratinocytes is defective in mice with diabetes. In addition, we are examining mechanisms that may contribute to skin γδ T-cell dysfunction in diabetes, including changes in insulin or IGF-1 receptor signaling and inhibition by glucocorticoids. The decreased levels of IGF-1 in the epidermal compartment may result in reduced IGF-1 receptor signaling in skin γδ T cells, a IMMUNOLOGY 2006 situation that may exacerbate dysfunction of skin γδ T cells. γδ T-CELL FUNCTION IN RESPONSE TO RAPAMYCIN Rapamycin is approved by the Food and Drug Administration for prophylaxis of acute rejection of transplanted organs. Patients who receive rapamycin have an increased incidence of complications of wound healing. Because these patients have difficulties with wound healing, rapamycin may not only be targeting allograftspecific αβ T lymphocytes but also be suppressing skin γδ T cells. In preliminary experiments, we observed defects in the proliferation of skin γδ T cells and the production of IGF-1 in the presence of rapamycin. We are investigating the mechanism of this suppression. Once we better understand the role of T cells in tissue repair, it may be possible to design therapies that enhance the ability of these immune cells to heal ulcers and chronic wounds. Our objective is to determine the mechanisms by which skin γδ T cells, normally important in wound repair of healthy wild-type mice, are not functioning properly in chronic wounds. Regulators of T-Lymphocyte Development and Function J. Kaye, P. Aliahmad, M. Fung, O. Goularte, C. Krieg, N. Sanathara recursor cells in the thymus undergo a complex developmental program before seeding peripheral lymphoid organs as mature T lymphocytes. Developmental checkpoints in the thymus, termed β-selection, positive selection, and negative selection, narrow the repertoire of T-cell antigen specificities to those that are not overtly autoreactive but maintain weak reactivity against self-MHC-peptide complexes. We are interested in the mechanisms that determine the fate of developing T cells and the control of gene expression during these processes. Our identification of a cell-surface protein that is first expressed in the T-cell lineage during positive selection led to studies on regulation of the immune response and the potential of this protein as a novel therapeutic target. P R E G U L AT I O N O F T H Y M O C Y T E S E L E C T I O N B Y A NUCLEAR ARCHITECTURAL PROTEIN We identified thymocyte selection–associated high mobility group (HMG) box protein (TOX) several years THE SCRIPPS RESEARCH INSTITUTE 119 ago. Members of the HMG box protein superfamily share one or more copies of a sequence-related and structurally related DNA-binding domain that can recognize distorted DNA structures and modify chromatin by bending DNA. In general, HMG box proteins function as architectural factors that regulate gene expression by promoting formation of transcriptional complexes or by acting as components of chromatin remodeling complexes. We found that TOX belongs to a small subfamily of evolutionarily conserved proteins whose members share almost identical HMG box sequences. The HMG box sequence in TOX can recognize distorted DNA but is a relatively poor bender of DNA, because of the lack of a critical internal wedge residue. Expression of TOX in the thymus is tightly regulated. Signaling through the serine/threonine phosphatase calcineurin is required for positive selection of thymocytes, and the gene for TOX is a target of this signaling pathway. TOX is expressed in early thymocyte progenitors and then is transiently upregulated during β-selection and positive selection. To analyze the function of this nuclear factor, we produced transgenic mice that express either wild-type or a mutant TOX and genetargeted mice that lack TOX. Our data indicate that expression of TOX is sufficient to initiate the differentiation of immature thymocytes to the CD8+ T-cell lineage, even in the absence of signals mediated by T-cell antigen receptors. Both the DNA-binding domain and the N-terminal domain of TOX are required for this in vivo activity. Although TOX is not a T cell–specific protein, mice that lack the gene for TOX are grossly normal but have a specific block in an early stage of thymic positive selection. We are using these tools to delineate the mechanism of action of this critical regulator of the fate of T cells in the thymus. N E G AT I V E R E G U L AT I O N O F T - C E L L R E S P O N S E S The functional outcome of engagement of the T-cell antigen receptor is modulated by secondary signals, which can have costimulatory or coinhibitory functions. We isolated a gene that encodes a cell-surface protein of the immunoglobulin superfamily, now designated BTLA (B- and T-lymphocyte attenuator), that is upregulated during positive selection and that is expressed by mature lymphocytes and antigen-presenting cells. Evidence indicates that this protein can act as a negative regulator of lymphocyte activation. We produced mice that lack the gene for BTLA and panels of monoclonal antibodies specific for BTLA to analyze the in vivo function of this protein. 120 IMMUNOLOGY 2006 One of our monoclonal antibodies acts as an agonist for this inhibitory molecule, thereby inhibiting facets of T-cell activation. In vivo studies indicated that BTLA is a negative regulator of homeostatic expansion of T cells and production of CD8+ memory T cells. For vaccines against intracellular infections and tumors, development of methods to regulate CD8+ T-cell responses and memory formation is paramount, and thus BTLA may be a useful therapeutic target in this regard. Preliminary data also suggest that one of our monoclonal antibodies to BTLA prolongs allograft survival in mice, and another antibody may ameliorate disease in a murine model of inflammatory bowel disease. PUBLICATIONS Aliahmad, P., Kaye, J. Commitment issues: linking positive selection signals and lineage diversification in the thymus. Immunol. Rev. 209:253, 2006. Krieg, C., Boyman, O., Fu, Y.-X., Kaye, J. T cell intrinsic regulation of homeostasis and CD8+ memory cell generation by BTLA. Nat. Immunol., in press. Krieg, C., Han, P., Stone R., Goularte, O.D., Kaye, J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J. Immunol. 175:6420, 2005. Regulation of the Innate Immune Response in Inflammation and Infection U.G. Knaus, B. Desnues, M. Lehmann, K. von Loehneysen, S. Luxen, M. Manukyan, S. Pacquelet, M. Ruse, M. Tsatmali, M. Valo, M. Ye nnate immune cells are the first line of defense in the fight against invading pathogens. We focus primarily on understanding molecular mechanisms that phagocytes and the pulmonary epithelium use to protect the host from the injury and how some responses wind up damaging the host. For example, second messengers such as reactive oxygen species (ROS) or nitric oxide that are produced during infection can have beneficial as well as detrimental effects. The overall outcome depends on precise spatial and temporal regulation of these second messengers by the affected cell populations. The intracellular signaling pathways that control these turn on–turn off mechanisms are an ideal target for intervention in disease. Almost all processes connected to pathogen uptake, pathogen elimination, and sustained inflammation are governed by small GTPases of the Ras superfamily. Our research centers on the Rho GTPases Rac, Cdc42, I THE SCRIPPS RESEARCH INSTITUTE and Rho, which are essential regulators for various leukocyte functions ranging from production of ROS to chemotaxis and phagocytosis. Generation of superoxide is accomplished by a Rac-dependent NADPH oxidase (Nox) upon stimulation with chemotactic factors or phagocytic stimuli. GTPases of the Rho family are also involved in signaling cascades, which originate from pathogen-activated Toll-like receptors. Toll-like receptors 2, 3, and 4 stimulated by microbial products derived from bacteria and viruses activate Rac1, Cdc42, and RhoA, which regulate pathways required for activation of gene transcription. We are studying different aspects of signaling by Toll-like receptors in several primary human cell types, including macrophages and neutrophils, and in genetically altered mouse models. We are also examining the impact of this signaling on the functions, such as apoptosis and upregulation of proinflammatory mediators, of innate immune cells. Another area of research is the interaction and communication between innate immune cells and the pulmonary epithelium. To this end, we established an in vitro reconstitution system for lung epithelium that we use to examine signaling mechanisms initiated by pathogens. The differentiated and fully functional lung epithelium also serves as a model for studies of lung barrier function and the influence of bacteria-derived ligands and toxins on transmigration of neutrophils. In addition, we are investigating processes that lead to uptake of pathogens or environmental particles and the impact of these pathogens or particles on airway epithelial functions. Recently, ROS-generating Nox proteins have been identified in epithelial cells, and work is in progress to study the molecular basis for ROS generation by these novel proteins. Nox proteins may serve as compartmentalized signaling modules, thereby activating or inhibiting signaling cascades via superoxide, or as an epithelial host defense mechanism via hydrogen peroxide–generating Nox/Duox isoforms. Because of their tissue-specific distribution and distinct localization patterns, Nox proteins might have highly specialized functions and undergo isoform-dependent regulation. For example, Nox4, an oxidase expressed in colon tissue and melanomas, is constitutively active in certain conditions and does not require any of the known oxidase components for superoxide generation. Elucidating physiologic stimuli and control mechanisms for these Nox proteins combined with structure-function studies will help define the biological functions of Nox in health and disease. IMMUNOLOGY 2006 PUBLICATIONS Chan, A.Y., Coniglio, S.J., Chuang, Y.Y., Michaelson, D., Knaus, U.G., Philips, M.R., Symons, M. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene 24:7821, 2005. Martyn, K.D., Frederick, L.M., von Loehneysen, K., Dinauer, M.C., Knaus, U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 18:69, 2006. Martyn, K.D., Kim, M.J., Quinn, M.T., Dinauer, M.C., Knaus, U.G. p21-Activated kinase (Pak) regulates NADPH oxidase activation in human neutrophils. Blood 106:3962, 2005. Ruse, M., Knaus, U.G. New players in TLR-mediated immunity: PI3K and small Rho GTPases. Immunol. Res. 34:33, 2006. Regulatory Mechanisms for Tumor Carcinogenesis M. Hayashi, J.-F. Lo, S.-W. Kim, J.-D. Lee T H E F O U R T H M A P K I N A S E PAT H WAY ig mitogen-activated kinase 1 (BMK1), also called extracellular signal–regulated kinase 5, a newer member of the mammalian MAP kinase family, is activated by angiogenic growth factors. Using a mouse model in which expression of the gene for BMK1 can be deleted, we showed that the BMK1 pathway is required for tumor-associated angiogenesis and consequent tumor growth through the BMK1/ribosomal S6 kinase/ribosomal protein S6 pathway. To investigate and define the function and molecular actions of the BMK1 pathway in endothelial cells and in angiogenesis, we are investigating the signaling pathways that interact with or are regulated by the BMK1 cascade in endothelial cells and are exploring the mechanism of action of the BMK1 cascade during angiogenesis. These studies should provide new information on the regulatory mechanisms of neovascularization. We hope to use the results to identify novel and important targets for a more effective and specific therapeutic intervention for human cancer by inhibition of tumor-associated angiogenesis. B THE TUMOR SUPPRESSOR/PROTEIN CHAPERONE TID1 Tid1 is the human counterpart of the Drosophila tumor suppressor Tid56. Mutations that cause loss of function of the gene for Tid56 result in tumorous imaginal discs due to continuous cell proliferation without differentiation. Using yeast 2-hybrid screening, we found that Tid1 interacts with the signaling domain of the receptor protein-tyrosine kinase ErbB2/Her2. Subsequent studies indicated that increased expression of THE SCRIPPS RESEARCH INSTITUTE 121 Tid1 in breast cancer cells overexpressing ErbB2 facilitated the ubiquitination and degradation of ErbB2 and growth inhibition of the cells. Moreover, using RNA interference to deplete the physiologic levels of Tid1 in breast cancer cells, we discovered that the metastatic potential of Tid1-depleted cells was substantially enhanced. This enhancement was due to increased production of IL-8 through upregulation of the nuclear transcription factor NF-κB in the Tid1depleted cells. Thus, because Tid1 attenuates signals generated from the ErbB2 receptor and negatively regulates the activity of NF-κB, we hypothesize that Tid1, like its Drosophila counterpart, may be an important tumor suppressor, especially in breast carcinogenesis and metastasis. To evaluate this hypothesis in an animal model, we have established a mouse model in which the gene for Tid1 can be deleted specifically in mammary epithelial cells. We are investigating and evaluating the mechanistic role of Tid1 as a tumor suppressor in the regulation of tumorigenesis and metastasis of breast cancer not only at the molecular and cellular levels but also in an organismal context. PUBLICATIONS Abbasi, S., Lee, J.-D., Su, B., Chang, X., Alcon, J.L., Yang, J., Kellems, R.E., Xia, Y. Protein kinase-mediated regulation of calcineurin through the phosphorylation of modulatory calcineurin-interacting protein 1. J. Biol. Chem. 281:7717, 2006. Hayashi, M., Fearns, C., Eliceiri, B., Yang, Y., Lee, J.-D. Big mitogen-activated protein kinase 1/extracellular signal-regulated kinase 5 signaling pathway is essential for tumor-associated angiogenesis. Cancer Res. 65:7699, 2005. Hayashi, M., Imanaka-Yoshida, K., Yoshida, T., Wood, M., Fearns, C., Tatake, R.J., Lee, J.-D. A critical role of mitochondrial Hsp40 in preventing dilated cardiomyopathy. Nat. Med. 12:128, 2006. Kim, S.W., Hayashi, M.l., Lo, J.F., Fearns, C., Xiang, R., Lazennec, G., Yang, Y., Lee, J.-D. Tid1 negatively regulates the migratory potential of cancer cells by inhibiting the production of interleukin-8. Cancer Res. 65:8784, 2005. Zhou, H., Luo, Y., Lo, J.-F., Kaplan, C.D., Mizutani, M., Mizutani, N., Lee, J.-D., Primus, F.J., Becker, J.C., Xiang, R., Reisfeld, R.A. DNA-based vaccines activate innate and adaptive antitumor immunity by engaging the NKG2D receptor. Proc. Natl. Acad. Sci. U. S. A. 102:10846, 2005. Host-Pathogen Interactions: Mechanisms and Applications E. Li, S.P. Lad, J. Li icrobial pathogens can be classified into 2 broad categories: those that infect the host accidentally and those that do so for growth. The outcome of an infection by “accidental” pathogens is commonly associated with severe host inflammatory M 122 IMMUNOLOGY 2006 responses and is often lethal. In contrast, obligate intracellular pathogens such as chlamydiae and rickettsiae have developed efficient yet poorly defined mechanisms to evade host immune surveillance and secure a favorable habitat. We focus on host responses to viral and bacterial infections. We use infections with adenovirus and the obligate intracellular bacterial pathogen Chlamydia trachomatis as models systems. Chlamydia trachomatis infection affects 140 million persons worldwide. It is also the most notifiable disease in the United States; it leads to 50,000–100,000 new cases of pelvic inflammatory diseases and infertility each year. Although an inflammatory cellular response and chronic inflammation are the underlying mechanisms of chlamydial diseases, a hallmark of a chlamydial infection is its asymptomatic nature. We found that Chlamydia can downregulate host inflammatory responses by converting a regulatory molecule of the inflammation pathway to a negative inhibitor of the same pathway. The host responds to viral and bacterial infections by using the nuclear transcription factor NF-κB to modulate genes involved in inflammation and innate immunity. NF-κB consists of a heterodimeric complex composed of 2 subunits, commonly p50/NF-κB1 and p65/RelA, which are sequestered in the cytoplasm and are made inactive through their association with inhibitory molecules, including IκBα. Bacterial and viral infections, proinflammatory cytokines, and stimulation with lipopolysaccharide all can induce rapid degradation of IκBα, resulting in the release and nuclear translocation of the NF-κB complex for gene regulation. In addition to protecting IκBα from degradation induced by TNF-α, chlamydial infections promote p65/RelA cleavage. The N-terminal cleavage product functions as a dominant negative inhibitor of the NF-κB pathway and hence can block NF-κB–modulated gene expression associated with inflammatory responses. We are identifying and characterizing the bacterial protease that causes the cleavage. We are also designing and modifying adenovirus for targeted gene delivery. Although widely used for gene therapy studies, adenovirus-based vectors cannot target specific tissues. We generated modified adenoviruses that are equipped with a tumor-targeting antibody, enabling the selective delivery of therapeutic genes to tumor cells by antibody-guided “missiles.” PUBLICATIONS Li, J., Lad, S., Yang, G., Lou, Y., Iacobelli-Martinez, M., Primus, F.J., Reisfeld, R.A., Li, E. The adenovirus fiber shaft contains a trimerization element supporting peptide fusion for targeted gene delivery. J. Virol., in press. THE SCRIPPS RESEARCH INSTITUTE The Cysteine Protease Network in Tumor Progression and Therapy C. Liu, W. Wu, Y. Liu, F. Guo, K. Shumilak, Q. Fang eoplasms in humans arise via a multistage process that involves the functions of a protease network. We first reported that legumain, a lysosomal asparaginyl endopeptidase with a caspaselike catalytic site, is highly expressed in a majority of rodent and human solid tumors. Compared with adjacent normal tissues, both tumor cells and tumor stromal cells must survive under substantially lower oxygen tension within the tumor microenvironment. We found that legumain expression is induced by hypoxia and occurs early during tumor development. We showed that legumain enhances tumor cell metastasis and invasion and protects cells from apoptosis through a complex and precise regulation of a cathepsin and caspase network (Fig. 1). N F i g . 1 . Legumain promotes tumor cell invasion and metastasis by binding to cell-surface integrins and activates both matrix metalloproteinase 2 (MMP2) and cathepsin L. It also protects cells from programmed cell death by catalytically inactivating caspase 9. It prevents Bid activation by cathepsin B by binding to and modulating the activity of the cathepsin. IMMUNOLOGY 2006 S U P P R E S S I O N O F T U M O R I N VA S I O N , M E TA S TA S I S , AND ANGIOGENESIS BY INHIBITION OF LEGUMAIN We showed that legumain is present in the tumor microenvironment, where it binds to cell-surface integrins such as αvβ3 and α5β1. Complexes consisting of legumain and αvβ3 are predominantly at the front of migrating cells. Binding of legumain to αvβ3 significantly enhances the activity of legumain toward physiologic substrates such as pro–matrix metalloproteinase 2 and procathepsin L. Therefore, integrins are both receptors and cofactors of legumain. Inhibition of legumain activity by a high-affinity asparaginyl endopeptidase inhibitor suppressed angiogenesis and tumor cell invasion in vitro and in vivo. Systemic administration of the inhibitor in mice reduced tumor growth, vascular density, and tumor invasiveness in breast cancer models. Importantly, this treatment inhibited both spontaneous and experimental lung metastasis. These data indicate that legumain is a critical modulator of pericellular proteolytic cascades and an effective target for cancer therapy. L E G U M A I N P R O T E C T I O N A G A I N S T M U LT I P L E P R O G R A M M E D C E L L D E AT H PAT H WAY S Caspases do not occur in plants, and legumain is the effector protease for plant cell apoptosis. However, we found that in mammals legumain evolved to have an antiapoptotic activity. Overexpression of legumain protects cells from multiple programmed cell death pathways. We showed that during TNF-induced cell death, lysosomal proteases diffuse into cytoplasm. Legumain exerts its antiapoptosis activity by depleting procaspase 9 and inactivating caspase 9 in apoptosomes. Prolegumain can be activated by caspase 3. Therefore, the presence of legumain serves as a brake for the caspase cascade. In addition, legumain binds directly to cathepsin B and suppresses autoactivation of the cathepsin and cathepsin B–mediated activation of the proapoptotic protein Bid. These findings indicate that legumain plays a critical role in mitochondria and in apoptosis mediated by death receptors. Inhibition of legumain activity and expression sensitizes tumor cells to natural death cues and chemotherapeutic agents, providing a novel technique for cancer intervention. PUBLICATIONS Bhattacharjee, G., Ahamed, J., Pedersen, B., El-Sheikh, A., Mackman, N., Ruf, W., Liu, C., Edgington, T.S. Regulation of tissue factor-mediated initiation of the coagulation cascade by cell surface Grp78. Arterioscler. Thromb. Vasc. Biol. 25:1737, 2005. THE SCRIPPS RESEARCH INSTITUTE 123 Wu, W., Luo, Y., Sun, C., Liu, Y., Kuo, P., Varga, J., Xiang, R., Reisfeld, R., Janda, K.D., Edgington, T.S., Liu, C. Targeting cell-impermeable prodrug activation to tumor microenvironment eradicates multiple drug-resistant neoplasms. Cancer Res. 66:97, 2006. Role of the Tissue Factor–Thrombin Pathway in Thrombosis, Inflammation, and Cardiac Ischemia-Reperfusion Injury R. Pawlinski, R.E. Tilley, J.P. Luyendyk, L. Kidd, E. Romeo, N. Mackman e are interested in the role of tissue factor (TF) in hemostasis, thrombosis, inflammation, myocardial infarction, and cardiac remodeling. TF is the primary initiator of blood coagulation and plays an essential role in hemostasis by activating blood coagulation after vessel injury. However, aberrant TF expression within the vasculature induces thrombosis in a variety of diseases, including sepsis (Fig. 1). In W F i g . 1 . Role of the tissue factor–thrombin pathway in thrombo- sis and cardiac remodeling. Abbreviations: LPS, lipopolysaccharide; I/R, ischemia-reperfusion. addition, we have shown that TF-dependent generation of thrombin contributes to the size of infarcts and cardiac remodeling after cardiac ischemia-reperfusion injury. THROMBOSIS In sepsis, bacterial lipopolysaccharide released by gram-negative bacteria induces TF expression by intravascular cells. This expression leads to disseminated intravascular coagulation, tissue ischemia, and inflammation. Notably, inhibition of TF reduces both coagulation and inflammation in animal models of sepsis. We are interested in the relative contribution of TF expression by monocytes, endothelial cells, and platelets to 124 IMMUNOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE lipopolysaccharide-induced coagulation. In collaboration with A. Weyrich, University of Utah, Salt Lake City, we recently found that lipopolysaccharide-stimulated human platelets express TF. To delete the gene for TF in the different cell types, we generated floxed TF mice. These mice have the TF gene flanked by specific sequences (loxP sites) that can be used for tissue-specific expression of TF via the Cre recombinase system. In the first experiments, floxed TF mice were crossed with mice that express the Cre recombinase in myeloid cells. These mice had reduced levels of TF expression in lipopolysaccharide-stimulated macrophages in a murine endotoxemia model, indicating that monocytes are a major source of intravascular TF in endotoxemia. Future studies will determine how deleting the TF gene in either endothelial cells or platelets affects lipopolysaccharide-induced coagulation. We plan to design novel strategies targeting intravascular TF that will reduce lipopolysaccharide-induced coagulation without increasing the risk of bleeding. remodeling and improved left ventricular function 2 weeks after ischemia-reperfusion injury. These results suggest that PAR1 contributes to cardiac remodeling after ischemia-reperfusion injury. In parallel studies, we determined the effect of overexpressing PAR1 in cardiomyocytes on heart morphology and function. Cardiac hypertrophy developed in a transgenic mouse model, and heart function was impaired. These data indicate that PAR1 signaling in cardiomyocytes induces hypertrophy and cardiac remodeling. Future studies will determine the role of TF-dependent generation of thrombin in the activation of PAR1 in the heart. I N F L A M M AT I O N Kamimura, M., Viedt, C., Dalpke, A., Rosenfeld, M.E., Mackman, N., Cohen, D.M., Blessing, E., Preusch, M., Weber, C.M., Kreuzer, J., Katus, H.A., Bea, F. Interleukin-10 suppresses tissue factor expression in lipopolysaccharide-stimulated macrophages via inhibition of Egr-1 and a serum response element/MEK-ERK1/2 pathway. Circ. Res. 97:305, 2005. We are interested in the role of the phosphatidylinositol-3′-kinase (PI3K)–Akt intracellular signaling pathway in lipopolysaccharide-induced inflammation and coagulation. We have shown that activation of the PI3K-Akt pathway inhibits lipopolysaccharide induction of inflammatory mediators and TF in human monocytic and endothelial cells and in a murine endotoxemia model. Interestingly, several compounds that improved survival in sepsis also activated the PI3K-Akt pathway. For example, we found that a low dose of insulin, which did not affect blood glucose levels, reduced inflammation and improved survival in a murine endotoxemia model in a PI3K-dependent manner. More recently, we found that simvastatin, a widely used cholesterol-lowering drug, inhibits lipopolysaccharide induction of inflammation and coagulation in mice. Importantly, the protective effect of simvastatin was abolished by inhibition of PI3K. Future studies will determine how insulin and simvastatin reduce lipopolysaccharide-induced TF and cytokine expression in vitro and in vivo. MYOCARDIAL INFARCTION AND CARDIAC REMODELING We have shown that the TF-thrombin pathway contributes to infarction after cardiac ischemia-reperfusion injury. More recently, we found that a deficiency of protease-activated receptor 1 (PAR1), a receptor for thrombin, did not affect the size of infarcts. However, PAR1 deficiency was associated with reduced cardiac PUBLICATIONS Bhattacharjee, G., Ahamed, J., Pedersen, B., El-Sheikh, A., Mackman, N., Ruf, W., Liu, C., Edgington, T.S. Regulation of tissue factor-mediated initiation of the coagulation cascade by cell surface Grp78. Arterioscler. Thromb. Vasc. Biol. 25:1737, 2005. Hayashi, M., Matsushita, T., Mackman, N., Ito, M., Adachi, T., Katsumi, A., Yamamoto, K., Takeshita, K., Kojima, T., Saito, H., Murohara, T., Naoe, T. Fatal thrombosis of antithrombin-deficient mice is rescued differently in the heart and liver by intercrossing with low tissue factor mice. J. Thromb. Haemost. 4:177, 2006. Luyendyk, J.P., Tilley, R.E., Mackman, N. Genetic susceptibility to thrombosis. Curr. Atheroscler. Rep. 8:193, 2006. Mackman, N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol. Dis. 36:104, 2006. Mackman, N. Tissue-specific hemostasis in mice. Arterioscler. Thromb. Vasc. Biol. 25:2273, 2005. Motton, D.D., Mackman, N., Tilley, R.E., Rutledge, J.C. Postprandial elevation of tissue factor antigen in the blood of healthy adults. Thromb. Haemost. 94:504, 2005. Rushworth, S.A., Chen, X.-L., Mackman, N., Ogborne, R.M., O’Connell, M.A. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. 175:4408, 2005. Schabbauer, G., Mackman, N. Tissue factor expression by the endothelium. In: The Endothelium: A Comprehensive Reference. Aird, W. (Ed.). Cambridge University Press, New York, in press. Tilley, R.E., Mackman, N. Tissue factor in hemostasis and thrombosis. Semin. Thromb. Haemost. 32:5, 2006. Tilley, R.E., Pedersen, B., Pawlinski, R., Sato, Y., Erlich, J.H., Shen, Y., Day, S., Huang, Y., Eitzman, D.T., Boisvert, W.A., Curtiss, L.K., Fay, W.P., Mackman, N. Atherosclerosis in mice is not affected by a reduction in tissue factor expression. Arterioscler. Thromb. Vasc. Biol. 26:555, 2006. IMMUNOLOGY 2006 Regulating Adaptive Immunity M.G. McHeyzer-Williams, L.J. McHeyzer-Williams, L.P. Malherbe, N.R. Fazilleau, N. Pelletier R E G U L AT I O N O F B - C E L L I M M U N I T Y B Y H E L P E R T CELLS elper T cells are the master regulators of adaptive immunity that control the development of antigen-specific B-cell immunity. We seek to define the cellular and molecular details of the major developmental checkpoints that regulate the fate of these helper T cells and B cells in vivo. We recently extended our studies to the earliest innate immune events that initiate and shape this adaptive immune response. If we can understand the rules that control adaptive immunity, we can design safe and effective protein subunit vaccines. H SELECTION OF ANTIGEN-SPECIFIC HELPER T CELLS We recently described an affinity threshold model for selecting antigen-specific helper T cells in the response to protein antigens. The unique characteristic of this model is how clonal diversity is achieved and maintained through the progressive loss of lower affinity cells rather than through the preferential expansion of the high-affinity clonotypes. Our evidences suggests that selection is not due to interclonal competition but is a more intrinsic threshold with a “set point” for selection. Hence, in order to manipulate antigen-specific clonal diversity, this affinity threshold must be altered. We are examining multiple variables of vaccination to define these rules, with emphasis on the impact of immune adjuvant on the priming of adaptive immunity. EVOLUTION OF MEMORY B CELLS The rapid evolution of memory B cells occurs at the cellular level and is controlled by multiple cognate checkpoints during the development of adaptive immunity. Although clonal selection is the fundamental process that underpins adaptive immunity, surprisingly little is understood about the mechanism of its action across multiple junctures of development of antigen-specific memory B cells in vivo. Our recent studies focus on the consolidation of B-cell memory upon antigen recall in the boost phase of vaccination with protein antigens. Cellular and molecular analysis of the memory response reveals the developmental staging of memory B cells that express different antibody subtypes. These studies highlight the importance of antibody isotype as a fundamental cellular division within antigen-specific Bcell memory. THE SCRIPPS RESEARCH INSTITUTE 125 ANTIGEN-EXPERIENCED DENDRITIC CELLS Dendritic cells are the most efficient innate initiators of adaptive immunity. Immune adjuvants substantially affect the developmental response of subsets of dendritic cells that in turn regulate separable functions in the helper T cell and B-cell compartments in vivo. Using antibodies that reveal specific peptide–MHC II complexes on dendritic cells, we can directly isolate antigen-experienced dendritic cells to evaluate the effect of immune adjuvants on priming of the cells in vivo. PUBLICATIONS Alfonso, C., McHeyzer-Williams, M.G., Rosen, H. CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors. Eur J. Immunol. 36:149, 2006. McHeyzer-Williams, L.J., Malherbe, L.P., McHeyzer-Williams, M.G. Checkpoints in memory B-cell evolution. Immunol. Rev. 211:255, 2006. McHeyzer-Williams, L.J., Malherbe, L.P., McHeyzer-Williams, M.G. Helper T cell regulated B cell immunity. Curr. Trends Microbiol. Immunol. 311:59, 2005. McHeyzer-Williams, L.J., McHeyzer-Williams, M.G. Memory B cell development. In: The Autoimmune Diseases, 4th ed. Rose, N.R., Mackay, I.R. (Eds.). Elsevier, St. Louis, 2006, p. 157. McHeyzer-Williams, M.G., McHeyzer-Williams, L.J., Malherbe, L.P. B cells discriminate the rules of engagement. Immunity 24:125, 2006. Adaptive and Innate Responses to Alloantigens D.B. McKay, A. Shigeoka urgical and medical advances have provided an opportunity for life to patients with end-stage organ disease. One of the most remarkable advances has been the replacement of organs through transplantation. Despite remarkable technological advances, the host immune system must be suppressed to prevent rejection of the allogeneic transplanted organ. The suppression of host immunity requires the life-long use of toxic nonspecific immunosuppressive medications. One experimental method has allowed survival of transplanted organs without the use of immunosuppressive medications: intravenous exposure of the organ recipient to donor antigens before transplantation. In several animal models and human clinical trials, exposure to donor antigens before transplantation downregulated the T-cell responses of recipients to donor antigens. One focus of our research is the intracellular signaling events that lead to the induction of peripheral T-cell tolerance by exposure to donor antigens. We found that intravenous infusion of semiallogeneic donor cells into S 126 IMMUNOLOGY 2006 recipient mice leads to a series of events that culminate in acquired unresponsiveness to donor antigens and tolerance to allografts. We discovered that several proximal T-cell receptor–coupled signaling molecules are altered in peripheral T cells of the recipient mice. We are investigating how these proximal molecules may be regulated in T cells from recipients that do not reject their transplanted organs. In addition, we are interested in the initial events that regulate activation of recipient T cells, namely, the events that regulate activation of the cells that present donor antigens. Several recent studies in animals have suggested that the family of evolutionarily conserved cell-surface Toll-like receptors might respond to ligands released by apoptotic and necrotic tissue and that such ligands might provide an important molecular trigger for adaptive responses to ischemic injury. More recently, we have been investigating how binding of Toll-like receptors leads to activation of responses to donor antigens and how to target these mechanisms to prevent allograft recognition and rejection. PUBLICATIONS Josephson, M.A., McKay, D.B. Management of pregnancy in the transplant recipient. Adv. Chronic Kidney Dis., in press. McKay, D.B., Adams, P., Bumgardner, G.L., Davis, C.L., Fine, R.N., Krams, S.M., Martinez, O.M., Murphy, B., Pavlakis, M., Tolkoff-Rubin, N., Sherman, M.S., Josephson, M. Reproduction and pregnancy in transplant recipients: current practices. Prog. Transplant. 16:127, 2006. McKay, D.B., Josephson, M. Pregnancy in transplant recipients of solid organs: effects on mother and child. N. Engl. J. Med. 354:1281, 2006. McKay, D., Shigeoka, A., Rubinstein, M., Surh, C., Sprent, J. Simultaneous deletion of MyD88 and Trif delays major histocompatibility and minor antigen mismatch allograft rejection. Eur. J. Immunol. 36:1994, 2006. Zambricki, E., Zal, T., Yachi, P., Shigeoka, A., Sprent, J., Gascoigne, N., McKay, D. In vivo anergized T cells form altered immunological synapses in vitro. Am. J. Transplant. 6:2572, 2006. Blocking CCR5 to Inhibit HIV Type 1 Infection D.E. Mosier, R. Nedellec, C. Pastore, A. Ramos, J. Salkowitz-Bokal, S. Pontow,* L. Ratner,* O. Hartley,** R. Offord,** J. Overbaugh,*** M. Lederman**** * Washington University School of Medicine, St. Louis, Missouri ** Centre Médical Universitaire, Geneva, Switzerland *** University of Washington, Seattle, Washington **** Case Western Reserve University, Cleveland, Ohio H IV type 1 (HIV-1), the cause of the AIDS pandemic, infects human cells by sequential binding of the viral envelope protein to the cell-surface THE SCRIPPS RESEARCH INSTITUTE receptors CD4 and CCR5. Because CCR5 binding occurs after CD4 binding, CCR5 is defined as a coreceptor. The importance of CCR5 in HIV-1 infection was first appreciated because some persons have a natural mutation that prevents expression of CCR5. These persons are resistant to HIV-1 infection, and they have few clinical consequences of lacking CCR5. These observations led to the development of CCR5-blocking agents to prevent HIV-1 infection. However, HIV-1 can undergo mutations that allow a second chemokine receptor, CXCR4, to replace the binding function of CCR5. We have studied the costs to viral fitness of those mutations and their importance in resistance to CCR5 inhibitors. A N T I V I R A L C O M P O U N D S T H AT TA R G E T C C R 5 The normal function of CCR5 is to bind chemokines and signal cell migration. RANTES (CCL5) is the CCR5binding chemokine with the most potent activity against HIV-1, but its activity is limited. We prepared synthetic modifications of the N-terminal domain of RANTES. We found that the most potent of these compounds, PSC-RANTES, is 1000 times more effective than native RANTES at inhibiting HIV-1 infection. A single injection of PSC-RANTES before inoculation of virus prevented HIV-1 infection in 100% of mice with severe combined immunodeficiency repopulated with human peripheral blood leukocytes. Brief exposure of human cells to PSC-RANTES leads to prolonged internalization of CCR5. These properties led to the formulation of PSCRANTES as a topical microbicide to prevent sexual transmission of HIV-1. Treatment with PSC-RANTES can prevent vaginal transmission of a chimeric virus consisting of simian immunodeficiency virus and HIV in the rhesus macaque model. To ensure that PSCRANTES will be effective against HIV-1 variants present in Africa, we examined the sensitivity of a number of recently transmitted isolates from Kenya. Fortunately, all of the isolates were more sensitive to PSC-RANTES inhibition than were laboratory HIV-1 isolates from the United States. M U TAT I O N A L C O S T S O F C O R E C E P T O R S W I T C H I N G One concern about CCR5-blocking agents such as PSC-RANTES is that they might select for resistant viruses that can infect via other chemokine receptors, such as CXCR4. Although previously we showed that such “coreceptor switch” mutants can arise during treatment, a detailed analysis revealed that most mutants have a loss of fitness during coreceptor switching that IMMUNOLOGY 2006 coincides with a period when neither CCR5 nor CXCR4 supports efficient virus infection. The mutations in the HIV-1 envelope that drive coreceptor switching occur mainly in the exposed variable loops (V1/V2 and V3), and different HIV-1 isolates require as few as 1 mutation or as many as 7 mutations to switch from use of CCR5 to use of CXCR4. Poor replication on both CCR5- and CXCR4-expressing target cells and increased sensitivity to both CCR5 and CXCR4 inhibitors were common features of viruses that were switching coreceptors. To more fully understand the cost of each mutation associated with changing coreceptor binding from CCR5 to CXCR4, we reconstructed all possible mutational pathways between 4 parental CCR5-using viruses and their CXCR4-using descendants separated by 3–7 mutations. We used site-directed mutagenesis to introduce all possible combinations of single and multiple mutations in the HIV-1 envelope gene. These mutated envelopes were combined with an envelope-deficient reporter virus to make HIV-1 particles capable of only a single cycle of infection. Mutations in variable loops 1 and 2 of the envelope improved the use of CCR5 but did not permit infection via CXCR4. Mutations in variable loop 3 led to use of CXCR4 for viral entry, but only poorly. Combinations of mutations in all 3 variable loops improved the ability of the virus to use CXCR4. The sequence in which mutations were introduced was critical. The probability of coreceptor switching is thus constrained by having to make the right mutation at the right place at the right time. Using chimeric coreceptors with the 4 extracellular domains derived from either CCR5 or CXCR4, we also mapped the domains of CCR5 or CXCR4 required for infection by each of the mutated envelopes. The initial stage of coreceptor switching favored use of the second extracellular domain of CXCR4, and 2 mutants required only this domain for infection. This result allows the mapping of mutations near the tip of variable region 3 of the envelope that must confer binding to extracellular loop 2 of CXCR4. The results from these mapping studies of envelope interactions with coreceptors will enable us to predict the likelihood of resistance-conferring mutations in patients during clinical trials of CCR5 inhibitors. PUBLICATIONS Pastore, C., Nedellec, R., Ramos, A., Pontow, S., Ratner, L., Mosier, D.E. Human immunodeficiency virus type 1 coreceptor switching: V1/V2 gain-of-fitness mutations compensate for V3 loss-of-fitness mutations. J. Virol. 80:750, 2006. THE SCRIPPS RESEARCH INSTITUTE 127 Control of Cytokine Expression by Arginine Methylation K.A. Mowen, D.M. Hill, S. Hemmers, K. Bonham elper T cells can be divided into 2 distinct populations on the basis of their immune specificity and cytokine profiles. Type 1 helper T cells produce IFN-γ and are responsible for cell-mediated immunity; type 2 helper T cells secrete IL-4 and are associated with the humoral immune response. These 2 types of cells have been associated with susceptibility to malignant, infectious, allergic, and autoimmune diseases. The improper development of type 2 helper T cells can lead to allergy and asthma, and an overactive response by type 1 helper T cells can lead to autoimmune diseases such as type 1 diabetes. Because of the opposing roles of the 2 types in immune function, the development and migration of helper T cells must be tightly regulated. Indeed, the discrete subsets, type 1 and type 2, reciprocally antagonize the maturation and behavior of each other in the immune response, resulting in a population of helper T cells that is primarily type 1 or type 2. Thus, manipulating the ratio of type 1 to type 2 T helper cells provides an intriguing avenue of therapy, and understanding the molecular events that control lineage-specific cytokine expression may provide useful tools for modulating the helper T cell response. Although several lineage-specific and nonspecific transcription factors are required for the development and function of type 1 and type 2 helper T cells, less is known about the events that occur after the reactivation of type 1 and type 2 effector populations and result in the disparate cytokine profiles of the 2 types of helper T cells. Signal transduction pathways use posttranslational modifications to translate changes in the extracellular milieu into environment-sensitive gene expression in a timely and efficient fashion. Phosphorylation of serine, threonine, and tyrosine residues and protein ubiquitination has been widely studied. Although methylation of arginine residues was discovered more than 30 years ago, it has only recently aroused renewed interest. Arginine methylation of proteins by members of the protein arginine methyltransferase (PRMT) family regulates the subcellular localization of the methylated proteins and modulates protein-protein interactions. H 128 IMMUNOLOGY 2006 We discovered a unique contribution of arginine methylation to cytokine gene expression downstream of signaling by T-cell receptors. Our goal is to investigate more broadly the role for arginine methylation in immune function, including further study of helper T cells and other immune cell types. We also plan to examine the upstream regulation of PRMT expression and activity and to characterize the effects of ablation or suppression of PRMT expression. Understanding the role of posttranslational modifications, such as arginine methylation, of proteins that are key in regulating cytokine production will give us novel targets in diseases induced or exacerbated by the cytokine environment, such as inflammatory arthritis. Analysis of Immune Learning in B Lymphocytes D. Nemazee, A. Gavin, D. Aït-Azzouzene, C. Huber, T. Ota, J. Vela, B. Duong, P. Skog, M. Lim he main goal of our research is to understand how lymphocytes distinguish between self and nonself antigens. Because antigen receptors on lymphocytes are assembled from component parts through an essentially random mechanism, many lymphocytes have self-reactive receptors. Regulation of such autoreactive specificities may be important to prevent autoimmune disease and to ensure efficient response to microbes. The development of B lymphocytes is a multistep process punctuated by the somatic generation of antibody heavy and light chain genes through DNA recombination, which is catalyzed by the products of recombinase activator gene 1 (RAG-1) and RAG-2. Because V(D)J recombination is imperfect and error prone, pre-B and B cells are endowed with sensing mechanisms to detect protein expression of heavy chains and assembled heavy and light chains (i.e., intact surface IgM). A major function of the expression of immunoglobulin in immature B cells is signaling to downregulate recombinase activity and to stimulate developmental progression. Newly formed B-cell receptors are also screened for autoreactivity. These quality control mechanisms rely on signaling by antigen receptors. Previously, we showed that B cells with autoreactive receptors do not downregulate recombination because of excessive signaling through the antigen receptor, T THE SCRIPPS RESEARCH INSTITUTE resulting in “receptor editing,” a process in which previously expressed genes for antibody light chains are inactivated and replaced by secondary DNA recombination. More recent data indicated that editing can also play an important role in inactivating and replacing receptor genes that are underexpressed at the protein level. In this situation, subnormal expression of unligated surface immunoglobulin does not provide a needed signal. These recent results suggest that quality control of newly formed B lymphocytes is surprisingly stringent and that through recombinase regulation B cells are often able to “repair” unacceptable light-chain genes by replacing the unacceptable genes with new genes. Because of the apparent efficiency of the editing process, we suspect that we have uncovered a major cellular “proofreading” pathway. A key question of current interest is how signaling through the antigen receptor regulates editing. A major nuclear end point is the regulation of RAG transcription. We are assessing the biochemical signaling pathways by which the signal from antigen receptors regulate RAG transcription. Recent studies suggested that NF-κB and rel transcription factors may be involved in both positive and negative regulation of the RAG genes. We have also made progress in our understanding of the triggering involved in B-cell positive selection, in which innocuous B-cell receptors, via tonic signaling, activate a signaling cascade that involves the activity of phosphatidylinositol3′-kinase and recruited effectors, including phospholipase C γ2 and Akt. Tonic signaling refers to the weak signal generated by the unoccupied B-cell receptor for antigen. This pathway appears to be inactivated in autoreactive immature B cells, a finding that probably explains why the time frame of editing is limited. To assess the role of receptor editing in preventing unwanted autoreactivity, we generated mice that have a defect in receptor editing. These mutant mice lack a functional recombining sequence/κ light chain–deleting element, which is involved in destructive editing of loci for κ light chains in cells that go on to rearrange either a second allele for κ light chains or genes for λ light chains. These mice are being assessed for their ability to produce autoantibodies and to accelerate autoimmune disease when crossbred with mice that are prone to autoimmunity. In other studies, we focused on the cues that mature B cells use to distinguish self from nonself. Fully mature recirculating B cells can be rapidly inactivated and IMMUNOLOGY 2006 induced to apoptosis when confronted with tissue antigen, whereas the same cells are able to respond to antigens expressed by microbes. We are investigating both the death pathway involved in self-tolerance and the nature of the signals that prevent this pathway in responses to nonself antigens. Recently, we found that the ability of B cells to distinguish self from nonself in this setting is independent of T lymphocytes and instead most likely involves a novel pathway of self-recognition. We are testing the hypothesis that signaling by Toll-like receptors is not required for this discrimination. We are also exploring the idea that immune tolerance in mature B cells depends on specific costimulation by self-tissue, a mode of signaling akin to missing selfrecognition by natural killer cells. PUBLICATIONS Aït-Azzouzene, D., Gavin, A.L., Skog, P., Duong, B., Nemazee, D. Effect of cell:cell competition and BAFF expression on peripheral B cell tolerance and B-1 cell survival in transgenic mice expressing a low level of Igκ-reactive macroself antigen. Eur. J. Immunol. 36:985, 2006. Aït-Azzouzene, D., Verkoczy, L., Duong, B., Skog, P., Gavin, A.L., Nemazee, D. Split tolerance in peripheral B cell subsets in mice expressing a low level of Igκreactive ligand. J. Immunol. 176:939, 2006. Collins, C.E., Gavin, A.L., Migone, T.S., Hilbert, D.M., Nemazee, D., Stohl, W. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res. Ther. 8:R6, 2005. Gavin, A.L., Hoebe, K., Duong, B., Ota, T., Martin, C., Beutler, B., Nemazee, D. Minor role for toll-like receptor signaling in adjuvant-enhanced antibody responses. Science, in press. Nemazee, D. Receptor editing in lymphocyte development and central tolerance. Nat. Rev. Immunol. 6:728, 2006. Nemazee, D., Gavin, A., Hoebe, K., Beutler, B. Immunology: Toll-like receptors and antibody responses. Nature 441:E4, 2006. Watson, L.C., Moffatt-Blue, C.S., McDonald, R.Z., Kompfner, E., Aït-Azzouzene, D., Nemazee, D., Theofilopoulos, A.N., Kono, D.H., Feeney, A.J. Paucity of V-DD-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT-/- and Tdt+/+ mice. J. Immunol. 177:1120, 2006. Toll-like Receptor 9 Signaling by Adenoviruses That Use CD46 Receptors M. Martinez-Iacobelli, G.R. Nemerow uman adenoviruses are potent activators of the innate immune response, a feature that limits their use for in vivo gene transfer. However, the precise mechanisms by which different adenoviruses trigger innate immunity have not been fully elucidated. H THE SCRIPPS RESEARCH INSTITUTE 129 In recent studies, we analyzed different types of adenoviruses that use coxsackievirus-adenovirus receptors or CD46 as their primary receptors for the ability of the viruses to trigger innate immune responses as measured by production of type I interferon in human peripheral blood mononuclear cells. We found that adenoviruses that use CD46 preferentially induced production of IFN-α and that this finding most likely was due to activation of Toll-like receptor 9, because an oligonucleotide antagonist of the receptor inhibited cytokine expression. Moreover, empty/immature adenovirus particles that lacked double-stranded DNA did not induce interferon production even though they were fully capable of binding to and entering host cells. In further studies, we found that epithelial cells that supported equivalent levels of infection by adenoviruses that used coxsackievirus-adenovirus receptors and by adenoviruses that used CD46 produced significantly higher amounts of interferon upon infection by the viruses that used CD46. These findings indicate that distinct receptor-mediated entry pathways may play a pivotal role in activation of Toll-like receptor 9 by adenovirus. The findings also have implications for the development of safer adenovirus vectors for clinical applications. Improving Adenovirus Transduction of Human Myeloid Cells R. Nepomuceno, L. Pache, G.R. Nemerow endritic cells are ideal targets for immunomodulatory regimens to treat genetic or acquired diseases. However the lack of coxsackievirusadenovirus receptors on dendritic cells has stymied transfer of genes to these cell types via type 5 adenoviruses. In recent studies, we found that the fiber knob derived from adenovirus type 37 (37FK) could significantly enhance adenovirus-mediated gene transfer to primary human monocytes and to dendritic cells but not to T and B lymphocytes. Fiber knobs derived from other adenovirus strains, including type 5 and type 16, did not have this ability. Enhancement of gene transfer by 37FK depended on sialic acid, because removal of sialic acid residues by treatment with neuraminidase abrogated gene transfer. Moreover, lectins with speci- D 130 IMMUNOLOGY 2006 ficity for α2,6-linked sialic acid residues, but not lectins with specificity for α2,3-linked sialic acid residues, could inhibit gene transfer by 37FK. In further investigations, we found that 37FK bound directly to adenovirus particles and thereby increased virus binding to monocytic cells. We concluded that an electrostatic interaction between the positively charged 37FK and the negatively charged virus capsid and cell-surface sialic acid residues results in the formation of a ternary complex that potentiates adenovirus infection and gene transfer. These findings may point the way for improving gene delivery to dendritic cells. Adenovirus-Mediated Disruption of Endosomes and Development of Nanoparticle Delivery Methods J.G. Smith, C. Wiethoff,* S. Police,** C.Y. Lai, V. Kickhoefer,*** L. Rome,*** G.R. Nemerow * Loyola University Medical Center, Maywood, Illinois ** Cell Genesys, Inc., San Francisco, California *** University of California, Los Angeles, California he mechanisms by which nonenveloped viruses, including adenovirus, penetrate the barrier of the host cell endosomal membrane are not well understood. In previous studies, we identified an internal capsid protein, designated protein VI, that may mediate the disruption of the early endosome upon partial disassembly of the virion at low pH. To test this hypothesis, we recently examined the infectivity of a panel of mutant adenoviral particles that contain single amino acid substitutions in the putative membrane-reactive domain of protein VI. Using a quantitative fluorescence imaging device, we found that several of the particles with mutant protein VI molecules had mildly reduced infectivity compared with that of wild-type virions. Further biochemical assays revealed that the reduced infectivity was not due to defects in virion assembly or disassembly. Currently, we are evaluating the membrane lytic and endosomedisrupting properties of the mutant adenoviruses; we expect that some of the mutants will have defects in these activities. In keeping with this expectation, we found that mutations in protein VI that affect virus infection also reduce binding of protein VI to liposomes. Finally, efforts are under way to determine if wild-type T THE SCRIPPS RESEARCH INSTITUTE protein VI molecules can be incorporated into naturally occurring nanoparticles in an attempt to improve gene delivery to host cells. Structure Analyses of Adenovirus via Electron Cryomicroscopy and X-ray Diffraction S. Saban,* P. Stewart,* V. Reddy, G.R. Nemerow * Vanderbilt University School of Medicine, Nashville, Tennessee denovirus is one of the largest macromolecular complexes whose structure has been analyzed by using electron cryomicroscopy or x-ray diffraction. In ongoing studies, we are using electron cryomicroscopy and images of approximately 2000 particles to determine the adenovirus structure to 6–7 Å. At this level of resolution, we can clearly resolve multiple α-helices present in the penton base, protein VI, hexon, and protein IIIa. These studies have also revealed the location and potential associations of the adenovirus proteins located on the inner capsid surface that help stabilize the virus before its disassembly in the early endosome. The pseudoatomic model generated from the electron cryomicroscopy data was used to facilitate structural analyses of the virus by x-ray diffraction. We found that large single crystals of adenovirus diffracted to about 5-Å resolution at different synchrotron beam lines. Recent studies indicated that the adenovirus crystals can be frozen, thereby allowing the collection of nearly complete electron cryomicroscopy data sets from single crystals. Generation of electron density maps from x-ray diffraction data revealed distinct features of the inner core of the virus. This new information may provide further insights into the assembly and disassembly of adenovirus as well as the mode of viral DNA uncoating and recognition by Toll-like receptor 9. A PUBLICATIONS Maginnis, M.S., Forrest, J.C., Kopecky-Bromberg, S.A., Dickeson, S.K., Santoro, S.A., Zutter, M.M., Nemerow, G.R., Bergelson, J.M., Dermody, T.S. β1 Integrin mediates internalization of mammalian reovirus. J. Virol. 80:2760, 2006. Nepomucena, R., Pache, I., Nemerow, G.R. Enhancement of gene transfer to human myeloid cells by adenovirus-fiber complexes. Mol. Ther., in press. Nicklin, S.A., Wu, E., Nemerow, G.R., Baker, A.H. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 12:384, 2005. Saban, S.D., Silvestry, M., Nemerow, G.R., Stewart, P.L. Visualization of α-helices in a 6 Å resolution cryoEM structure of adenovirus allows refinement of capsid protein assignments. J. Virol., in press. IMMUNOLOGY 2006 Wodrich, H., Cassany, A., D‚Angelo, M.A., Guan, T., Nemerow, G., Gerace, L. Adenovirus core protein pVII is translocated into the nucleus by multiple import receptor pathways. J. Virol. 80:9608, 2006. Enhancement of Lymphocyte Cross Talk by Engagement of the NKG2D Receptor H. Zhou, Y. Luo, C.D. Kaplan, J.A. Krueger, S.H. Lee, R. Xiang, R.A. Reisfeld cells targeting tumor-associated antigens are readily detectable in cancer patients who have received cancer vaccines, but often the cells do not eradicate tumors. Thus, established tumors can apparently induce immune tolerance through as yet poorly defined mechanisms. We hypothesized that immunization strategies that target different areas of the immune system could overcome this immune tolerance. We used interactions between the NKG2D receptor and its ligand to test this hypothesis, because the receptor occurs at the crossroad between innate and adaptive immunity. NKG2D, a stimulatory lectinlike receptor, is expressed on natural killer cells, activated CD8+ T cells, γζ T cells, and activated macrophages and mediates costimulatory signals for CD8+ T cells and stimulatory signals for natural killer cells and macrophages. In addition, NKG2D ligands are related to MHC class I molecules, which in mice include products of the H60 ligand. Importantly, in syngeneic mice, ectopic expression of NKG2D ligands causes natural killer cell–mediated rejection of transfected tumor cells and primes cytotoxic T lymphocytes, which are responsible for rejecting subsequent challenges by tumor cells that do not express the NKG2D ligand. In previous studies, we showed that engagement of the NKG2D receptor markedly improved the antitumor efficacy of a DNA vaccine encoding both the NKG2D ligand H60 and the inhibitor of apoptosis protein survivin. This combination vaccine activated both innate and adaptive antitumor immunity and resulted in improved protection against tumors of different origins and with different levels of expression of NKG2D. More recently, we found that this combination vaccine induces intense cross talk between dendritic cells, natural killer cells, and T cells. Depletion of natural killer or CD8 + T cells led to a decrease in activation T THE SCRIPPS RESEARCH INSTITUTE 131 of dendritic cells, indicating the positive helper function of these cells in the activation of dendritic cells in vivo. We also found that depletion of CD8+ T cells or natural killer cells during the priming, but not the effector phase, led to reduced activity of natural killer or cytotoxic T cells, respectively. Thus, indirect cross talk exists between these cells and is mediated through dendritic cells or by direct interactions between natural killer cells and CD8+ T cells. In contrast to natural killer and CD8+ T cells, CD4+ T cells appear to negatively regulate these effector cells. Depletion of CD4+ cells during the priming phase led to the activation of dendritic cells, natural killer cells, and CD8 + T cells and to enhanced activity of natural killer cells, suggesting the existence of CD4+CD25+ T regulatory cells. Because CD4+CD25+ cells lack expression of the NKG2D receptor, even after activation, this finding could explain the lack of enhancement of activation and activity of T regulatory cells despite the profound activation of dendritic cells, natural killer cells, and CD8+ T cells. Thus, by engaging the NKG2D receptor, our vaccine preferentially activated natural killer and CD8+ T cells, a situation that might have tilted the balance toward immune surveillance and breakage of peripheral tolerance to tumor antigens mediated by T regulatory cells. Our finding that depletion of CD4+ T cells during the effector phase also reduced the activity of cytotoxic T cells suggests that CD4+ T-cell help is required to maintain antigen-specific CD8+ T cells in vivo. In our experimental model in which attenuated Salmonella typhimurium specifically delivers the DNA encoding the pH60/survivin vaccine to Peyer’s patches, these secondary lymphoid tissues most likely are the location for T-cell priming. The ability of the vaccine to increase homing of dendritic cells and natural killer cells to Peyer’s patches but decrease the homing of CD4 + T cells could presumably be due to changes in the homing receptor profile of the cells. The upregulation of the chemokine receptor CCR7 we observed especially on CD8+ T cells could occur if a greater percentage of naive CD8+ T cells home to Peyer’s patches, because CCR7 is highly expressed on naive T cells and mediates their homing to these secondary lymphoid tissues. In this situation, these findings may indicate that the pH60/survivin vaccine also induced changes in stromal cells in Peyer’s patches, especially because such cells are the source of ligands of CCR7, such as chemokines CCL19 and CCL21. Because lymphocytes activated in Peyer’s patches express homing receptors, 132 IMMUNOLOGY 2006 the cells most likely home to the periphery, where they can combat tumor cells or become quiescent in the absence of antigen. Currently, determining their exact fate is difficult because such lymphocytes lack specific and stable markers. Taken together, we showed that by preferentially activating and attracting positive regulators and reducing negative regulators in Peyer’s patches, our pH60/survivin DNA vaccine induced increased lymphocyte cross talk and thereby established a microenvironment more suitable for activation of natural killer cells and T-cell priming. The success of this vaccine in combating tumors of different origins and with different levels of NKG2D expression in prophylactic and therapeutic models and in inducing long-lived immune memory indicates that activation of the innate and adaptive arms of the immune system is an attractive strategy to overcome tumorinduced peripheral tolerance. PUBLICATIONS Abdollahi, A., Griggs, D.W., Zieher, H., Roth, A., Lipson, K.E., Saffrich, R., Grone, H.J., Hallahan, D.E., Reisfeld, R.A., Debus, J., Niethammer, A.G., Huber, P.E. Inhibition of αvβ3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin. Cancer Res. 11:6270, 2005. THE SCRIPPS RESEARCH INSTITUTE protective effects of APC is incompletely understood. Protein C is activated to APC by the key procoagulant enzyme thrombin bound to thrombomodulin on the surface of endothelial cells. APC in turn downregulates generation of thrombin in a negative feedback loop. Previously, we showed that APC signaling in endothelial cells requires binding to endothelial protein C receptor and activation of protease-activated receptor 1 (PAR1), the thrombin receptor. Thrombin-PAR1 signaling has well-established proinflammatory effects, including disruption of endothelial barrier function, raising the question of how the same receptor can also mediate protective effects of APC. Large-scale gene expression profiling indicated that APC-PAR1 downregulated transcript levels of proapoptotic proteins and that some of these transcripts were upregulated by thrombin-PAR1. Furthermore, APC-PAR1 had powerful endothelial barrier protective effects through cross-activation of the sphingosine 1-phosphate (S1P) signaling pathway (Fig. 1). Incubation of an endothelial monolayer with Allen, B.J., Raja, C., Rizvi, S., Li, Y., Tsui, W., Graham, P., Thompson, J.F., Reisfeld, R.A., Kearsley, J. Intralesional targeted alpha therapy for metastatic melanoma. Cancer Biol. Ther. 4:1318, 2005. Mahanivong, C., Krueger, J.A., Bian, D., Reisfeld, R.A., Huang, S. A simplified closing strategy for the generation of an endothelial cell selective recombinant adenovirus vector. J. Virol Methods 135:127, 2006. Osenga, K.L., Hank, J.A., Albertini, M.R., Gan, J., Sternberg, A.G., Eickhoff, J., Seeger, R.C., Matthay, K.K., Reynolds, C.P., Twist, C., Krailo, M., Adamson, P.C., Reisfeld, R.A., Gillies, S.D., Sondel, P.M. A phase 1 clinical trial of the hu14.18IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: a study of the Children’s Oncology Group. Clin. Cancer Res. 12:1750, 2006. Schrama, D., Reisfeld, R.A., Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 5:147, 2006. Schrama, D., Voigt, H., Eggert, A.O., Xiang, R., Reisfeld, R.A., Becker, J.C. Therapeutic efficacy of tumor-targeted IL2 in Ltα–/– mice depends on conditioned T cells. Cancer Immunol. Immunother. 55:861, 2006. Zhou, H., Luo, Y., Kaplan, C.D., Krueger, J.A., Lee, S.H., Xiang, R., Reisfeld, R.A. A DNA-based cancer vaccine enhances lymphocyte cross talk by engaging the NKG2D receptor. Blood 107:3251, 2006. Protective Protease-Activated Receptor 1 Signaling by the Protein C Pathway M. Riewald, C. Feistritzer, R.A. Schuepbach, R. Lenta A ctivated protein C (APC), an anticoagulant serine protease, has been approved for treatment of severe sepsis, but the molecular basis for the F i g . 1 . Inflammatory disorders such as sepsis are associated with increased permeability of the endothelial cell monolayer at the blood-tissue interface. APC-mediated enhancement of endothelial barrier integrity depends on binding of APC to endothelial protein C receptor and activation of PAR1, cellular sphingosine kinase-1 (SK1), and S1P receptor-1 (S1P1). Thrombin can affect barrier integrity in at least 3 ways: proinflammatory signaling by higher concentrations can disrupt endothelial barrier integrity, incubation with low concentrations has a barrier-enhancing effect, and activation of protein C by thrombin on the endothelial cell surface is linked to powerful autocrine protective signaling by the generated APC. low concentrations of thrombin had a similar barrier protective effect. Taken together, these results establish that PAR1 can mediate opposite effects on gene expression and barrier integrity, and they reveal an unexpected role for cross-communication between the prototypical barrierprotective S1P and barrier-disruptive PAR1 pathway. IMMUNOLOGY 2006 Using the anticoagulant double mutant thrombin W215A/E217A, we recently showed that activation of protein C by thrombin on the endothelial cell surface is mechanistically linked to highly efficient PAR1-dependent autocrine protective signaling by the generated APC. These results suggest that W215A/E217A may have powerful protective effects in systemic inflammation through signaling by the generated APC. To dissect how signaling by the same receptor leads to different biological outcomes, we are using a panel of monoclonal antibodies to analyze how specific populations of PAR1 are affected by APC and thrombin on the endothelial surface. Genetically modified mouse strains that express in endothelial cells PAR1 variants that are efficiently activated by APC but not by thrombin are currently used in models of systemic inflammation to dissect the in vivo role of PAR1 signaling by exogenous and endogenously generated APC. PUBLICATIONS Feistritzer, C., Lenta, R., Riewald, M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J. Thromb. Haemost. 3:2798, 2005. Feistritzer, C., Mosheimer, B.A., Sturn, D.H., Riewald, M., Patsch, J.R., Wiedermann, C.J. Endothelial protein C receptor-dependent inhibition of migration of human lymphocytes by protein C involves epidermal growth factor receptor. J. Immunol. 176:1019, 2006. Chemical and Genetic Approaches to Disease H. Rosen, G. Sanna, E. Jo, P. Gonzalez-Cabrera, A. Don, S. Cahalan, D. Marsolais, S. Brown, M.-T. Schaeffer, J. Chapman ymphocytes develop in the thymus (T cells) and bone marrow (B cells) and upon maturation egress from their sites of development to enter the bloodstream. Because the numbers of lymphocytes with specific receptors for antigen are limited, the probability of random productive collision of specific lymphocyte, antigen, and antigen-presenting cell in a permissive environment for an efficient immune response is low. In the immune system, this probability is enhanced by rapid recirculation of lymphocytes through secondary lymphoid organs, so that each lymphocyte has many opportunities to respond to its specific antigen. A sufficient number of blood lymphocytes are therefore essential for the development of efficient immune responses and are maintained by the recirculation of lymphocytes through the secondary lymphoid organs. L THE SCRIPPS RESEARCH INSTITUTE 133 Using small synthetic druglike organic molecules, we elucidated specific molecular gatekeepers that control the numbers of recirculating lymphocytes. These compounds alter lymphocyte trafficking and induce clinically useful immunosuppression by activating a single sphingosine 1-phosphate (S1P) receptor subtype, S1P1. Using 2-photon fluorescence and selective agonists and antagonists of this receptor, we directly imaged the control of lymphocyte egress from lymph nodes in living systems. M O L E C U L A R C O N T R O L O F LY M P H O C Y T E M I G R AT I O N Molecular control of the migration of lymphocyte subsets within the recirculation pathway is a fundamental issue of therapeutic importance. Although transplantation involves the sensitization of an immunologically naive host, treatment of most autoimmune diseases requires intervention in a sensitized host that already has autoreactive effector T cells in the periphery. We approached this problem by examining the role of the S1P system in the control of lymphocyte egress from lymph nodes and thymus, and using chemical approaches, we revealed differences between intrinsic lymphocyte and barrier mechanisms that alter lymphocyte migration. The rapid reversibility of agonist-mediated lymphocyte arrest coupled with its competitive reversal by molar excess of antagonist strongly support an endothelial barrier mechanism. ROLE OF SIGNALING LIPIDS IN THE CONTROL OF LUNG INTEGRITY Pulmonary abnormalities, including acute respiratory distress syndrome, are characterized by disruption of pulmonary integrity and edema that compromise respiratory function. S1P is a lipid mediator synthesized and/or stored in mast cells, platelets, and epithelial cells, and its production is upregulated by the proinflammatory cytokines IL-1 and TNF. We used agonists and antagonists of receptors to examine this system. S1P1, found on lung capillaries, tightens capillary junctions and protects from leakage. Antagonists of S1P1 therefore promote lung leakage from the vascular side. We found that changes in signaling-lipid regulation of lung barrier function from either vascular or endothelial interfaces induce acute pulmonary edema. The S1P-receptor axis may therefore be an important independent variable in the control of lung barrier function, and its activation and modulation in serious human diseases such as acute respiratory distress syndrome are under study. S T R AT E G I C O U T L O O K The S1P system thus regulates adaptive immunity in at least 3 discrete ways: egress of naive cells from 134 IMMUNOLOGY 2006 lymph nodes, sequestration of effector T cells in lymph nodes, and egress of mature medullary T cells from the thymus. The system can therefore alter both the peripheral diversity of lymphocyte responses and the efficiency of T-cell activation by misdirecting T cells to the wrong lymph nodes and by inhibiting the egress of antigenspecific effector T cells from lymph nodes after antigen activation and clonal proliferation. These effects can alter adaptive immune responses and the expression of tissue damage while providing potentially significant advantages to patients by sparing innate host defenses to bacteria and pathogenic fungi. The fine molecular control of this system and its effect on immune responses as a fundamental approach to organization of the immune system and potential therapeutic agents will remain our primary focus. The recent discovery of a critical role for chemically tractable S1P receptors in the innate immune system is a new focus in molecular pathogenesis of inflammatory lung disease that is of long-term interest to us. THE SCRIPPS RESEARCH INSTITUTE PUBLICATIONS Alfonso, C., McHeyzer-Williams, M.G., Rosen, H. CD69 down-modulation and inhibition of thymic egress by short- and long-term selective chemical agonism of sphingosine 1-phosphate receptors. Eur. J. Immunol. 36:149, 2006. Chun, J., Rosen, H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr. Pharm. Des. 12:161, 2006. Gong, Q., Ou, Q., Ye, S., Lee, W.P., Cornelius, J., Diehl, L., Lin, W.Y., Hu, Z., Lu, Y., Chen, Y., Wu, Y., Meng, Y.G., Gribling, P., Lin, Z., Nguyen, K., Tran, T., Zhang, Y., Rosen, H., Martin, F., Chan, A.C. Importance of cellular microenvironment and circulatory dynamics in B cell immunotherapy. J. Immunol. 174:817, 2005. Li, Z., Chen, W., Hale, J.J., Lynch, C.L., Mills, S.G., Hajdu, R., Keohane, C.A., Rosenbach, M.J., Milligan, J.A., Shei, G.J., Chrebet, G., Parent, S.A., Bergstrom, J., Card, D., Forrest, M., Quackenbush, E.J., Wickham, L.A., Vargas, H., Evans, R.M., Rosen, H., Mandala, S. Discovery of potent 3,5-diphenyl-1,2,4-oxadiazole sphingosine-1-phosphate-1 (S1P1) receptor agonists with exceptional selectivity against S1P2 and S1P3. J. Med. Chem. 48:6169, 2005. Martinez, X., Kreuwel, H.T., Redmond, W.L., Trenney, R., Hunter, K., Rosen, H., Sarvetnick, N., Wicker, L.S., Sherman, L.A. CD8+ T cell tolerance in nonobese diabetic mice is restored by insulin-dependent diabetes resistance alleles. J. Immunol. 175:1677, 2005. Rosen, H., Goetzl, E.J. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5:560, 2005. Sanna, M.G., Wang, S.K., Gonzalez-Cabrera, P.J., Don, A., Marsolais, D., Matheu, M.P., Wei, S.H., Parker, I., Jo, E., Cheng, W.C., Cahalan, M.D., Wong, C.H., Rosen, H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat. Chem. Biol. 2:434, 2006. THE SCRIPPS RESEARCH INSTITUTE MOLECULAR SCREENING CENTER The Scripps Research Institute Molecular Screening Center is a national center for small-molecule screening and is part of the National Institutes of Health (NIH) Molecular Libraries Screening Centers Network of the NIH Roadmap. The Scripps center is distributed between the La Jolla and the Florida campuses; its component parts are assay development, chemistry, assay implementation, and pharmacokinetics. These 4 cores are unified in a single data environment by the Informatics Core. The mission of the center is to use the NIH library of more than 60,000 individual compounds to screen molecular and cell-based targets, which are accepted through an NIH-wide peer-reviewed application process, for proof-of-concept small-molecule probes. Researchers at the Scripps center have successfully identified and published proof-of-concept molecules in the center’s first year of operations. Compounds discovered by this process are public information that can be accessed by all scientists through the PUBCHEM database of the National Center for Biotechnology Information. The Scripps center joins human excellence with state-of-the-art robotics and informatics. The combination can provide new insights into the basic science of small-molecule probes of physiologic and pathologic function, move scientific fields forward, and, over time, provide new, significant insights into therapies for human diseases. Wei, S.H., Rosen, H., Matheu, M.P., Sanna, M.G., Wang, S.K., Jo, E., Wong, C.-H., Parker, I., Cahalan, M.D. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat. Immunol. 6:1228, 2005. Protease Pathways in Inflammation, Angiogenesis, and Cancer J. Ahamed, M. Kerver, T. Kurokawa, Y. Kurokawa, F. Niessen, H. Petersen, H. Versteeg, P.J. Hogg,* M. Friedlander, B.M. Mueller,** W. Ruf * University of New South Wales, Sydney, Australia ** La Jolla Institute for Molecular Medicine, San Diego, California D I S U L F I D E / T H I O L E X C H A N G E A S A R E G U L AT O R Y SWITCH FOR RECEPTOR FUNCTION ctivation of coagulation by the cell-surface receptor tissue factor (TF) is induced by binding of its ligand, the serine protease factor VIIa. In addition, the TF-VIIa complex triggers cell signaling by cleaving and activating the G protein–coupled protease-activated receptor 2 (PAR2), a highly relevant promigratory receptor in tumor biology. However, the physiologic significance of direct TF-VIIa signaling remained unclear, because activation of coagulation generates a number of proteases that may override direct TF signaling pathways. We identified a surprisingly simple mechanism A IMMUNOLOGY 2006 by which TF-dependent coagulation is disabled while preserving TF-VIIa signaling. An extracellular disulfide (Cys186-Cys209) in TF fits the criteria for an “allosteric” disulfide; these disulfides are typically labile because of their bond geometry. Activation of coagulation requires this disulfide, but mutational breaking of the disulfide did not impair TF-VIIa signaling. Protein disulfide isomerase (PDI) targets this disulfide to disable coagulation. PDI is an abundant intracellular chaperone and thiol/disulfide exchange catalyst required for protein folding, but TF-VIIa is regulated by extracellular PDI. PDI suppresses TF coagulant activity by nitric oxide–dependent redox pathways and protein S nitrosylation, indicating an unexpected link between oxidative cardiovascular stress and thrombosis. These data also exemplify a new role for PDI as an extracellular switch for the functional specificity of a receptor. THE SCRIPPS RESEARCH INSTITUTE Koizume, S., Jin, M.S., Miyagi, E., Hirahara, F., Nakamura, Y., Piao, J.H., Asai, A., Yoshida, A., Tsuchiya, E., Ruf, W., Miyagi, Y. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 66:9453, 2006. Ruf, W. Flow perturbation is linked to endothelial PAR signaling. Arterioscler. Thromb. Vasc. Biol. 26:962, 2006. Ruf, W. Is APC activation of endothelial cell PAR1 important in severe sepsis? Yes. J. Thromb. Haemost. 3:1912, 2005. Ruf, W., Mueller, B.M. Thrombin generation and the pathogenesis of cancer. Semin. Thromb. Hemost. 32(Suppl. 1):61, 2006. Versteeg, H.H., Ruf, W. Emerging insights in tissue factor-dependent signaling events. Semin. Thromb. Hemost. 32:24, 2006. Structural Analysis of the Host-Pathogen Interface E. Ollmann Saphire, D.M. Abelson, M.L. Fusco, P R O T E A S E S I G N A L I N G PAT H WAY S I N A N G I O G E N E - C.R. Kimberlin, J.E. Lee, D.R. Burton, M.K. Hart* SIS AND CANCER * U.S. Army Medical Research Institute for Infectious Diseases, Frederick, We are interested in protease pathways in cancer and angiogenesis, with a particular focus on signaling by PARs. Previously, in ex vivo models, we showed that mice lacking the cytoplasmic domain of TF have enhanced angiogenesis. Recently, we analyzed the role of PARs in angiogenesis in vivo and found that PAR2, but not the thrombin receptor PAR1, plays a central role in angiogenesis. We further found that blockade of TF-VIIa suppresses angiogenesis in vivo and thus established that TF-VIIa signaling through PAR2 is the major pathway in angiogenesis. We plan to further analyze the respective roles of PARs in tumor progression and angiogenesis. We have also characterized species-specific antibodies that block TF-VIIa signaling through PAR2 on tumor cells. We found that these antibodies suppress tumor growth in xenograft models, providing evidence that PARs not only regulate angiogenesis in host cells but are an important determinant in tumor progression. We will evaluate the role of PARs in transgenic mouse models to provide additional genetic evidence for the specific role of protease pathways in tumor progression. PUBLICATIONS Ahamed, J., Versteeg, H.H., Kerver, M., Chen, V.M., Mueller, B.M., Hogg, P.J., Ruf, W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc. Natl. Acad. Sci. U. S. A. 103:13932, 2006. Bhattacharjee, G., Ahamed, J., Pedersen, B., El-Sheikh, A., Mackman, N., Ruf, W., Liu, C., Edgington, T.S. Regulation of tissue factor-mediated initiation of the coagulation cascade by cell surface Grp78. Arterioscler. Thromb. Vasc. Biol. 25:1737, 2005. Chen, V.M., Ahamed, J., Versteeg, H.H., Berndt, M.C., Ruf, W., Hogg, P.J. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry 45:12020, 2006. 135 Maryland e are crystallizing proteins that play key roles in the pathogenesis and lethality of viruses that cause hemorrhagic fever. The resulting crystal structures will provide (1) information for the design of vaccines and inhibitors against the viruses as the microbes exist naturally and (2) structural templates that will enable us to anticipate and rapidly respond to newly emerging and synthetic versions of the viruses and viral proteins. W EBOLA VIRUS At least 10 recognized outbreaks of Ebola virus in humans have occurred; in each outbreak, 50%–90% of those infected died. Survival depends on the ability of the host to mount early and strong immune responses. However, filoviruses have evolved mechanisms by which the host immune system is suppressed. For example, the viral nucleocapsid proteins VP35 and VP24 block interferon-mediated activation of immunomodulatory genes. Structural analysis of these proteins, alone and in complex with the human proteins that they bind, will provide insights into viral replication and immunosuppression and will provide the structural basis for the design of antiviral compounds and attenuated viral strains. An additional, unusual feature of the Ebola viral genome is its ability to encode 2 different glycoproteins, sGP and GP, from the same gene. These 2 glycoproteins share 295 amino acids of N-terminal sequence, but a transcriptional editing event causes them to have 136 IMMUNOLOGY 2006 different C-terminal sequences that result in unique patterns of disulfide bonding, structures, and roles in pathogenesis. Comparative structural analysis of sGP and GP should explain how 2 structures arise from the same sequence, provide templates for the design of vaccines that elicit antibodies that target the virus rather than the secreted proteins, and illustrate structural mechanisms by which the virus escapes immune surveillance. Additional crystal structures of these proteins in complex with rare human antibodies derived from survivors of Ebola virus infection or with immunotherapeutic agents under development by the U.S. Army will assist in vaccine design. We recently determined the crystal structure at 2.0-Å resolution of a potential immunotherapeutic agent, 13F6-1-2, in complex with its GP epitope (Fig. 1). 13F6-1-2 contains a rare Vλx THE SCRIPPS RESEARCH INSTITUTE determine structural features of epitopes associated with neutralization and enhancement (Fig. 2). F i g . 2 . Crystals of envelope protein E of dengue virus serotype 1. F i g . 1 . Crystal structure of Fab 13F6-1-2 in complex with its Ebola virus glycoprotein epitope. The antibody light chain is black, the heavy chain is gray, and the GP peptide is illustrated in balland-stick form. light chain and has several unusual structural features in its combining site. DENGUE VIRUS Dengue virus is a mosquito-borne flavivirus that causes up to 100 million infections each year. Infection with dengue virus results in either dengue fever or the much more severe disease dengue hemorrhagic fever. Dengue hemorrhagic fever usually occurs upon secondary infection with a different viral subtype or in infants born to dengue virus–immune mothers. This potential antibody-mediated enhancement of infection is a major concern in the testing and use of vaccines against dengue virus because antibodies elicited by the vaccines could trigger severe disease. To aid in vaccine design, we are determining crystal structures of envelope proteins of contemporary field isolates of dengue virus, alone and in complex with antibodies, to PUBLICATIONS Cárdenas, W.B., Loo, Y.-M., Gale, M., Jr., Hartman, A.L., Kimberlin, C.R., Martínez-Sobrido, L., Saphire, E.O., Nichol, S.N., Basler, C.F. Ebola virus VP35 protein binds double-stranded RNA and inhibits α/β interferon production induced by RIG-I signaling. J. Virol. 80:5168, 2006. Autoimmune Mechanisms and Compensatory Responses N. Sarvetnick, M. Cleary, S. Dabernat, S. Datta, D. Dietz, C. Fine, N. Hill, H. Hua, M. Kritzik, A. Marleau, P. Secrest, A. Stotland, D. Yadav, Y.Q. Zhang ype 1 diabetes occurs when self-reactive T cells destroy the insulin-producing beta cells in the islets in the pancreas. The assumption has been that the fault lies exclusively in the immune system, but increasingly findings suggest that the targets of autoimmunity, the islets, may also be defective. Genetic linkage analysis of nonobese diabetic mice has led to the identification of critical intervals that confer susceptibility to diabetes. One of these regions, Idd9, is associated with strong protection from disease when it T IMMUNOLOGY 2006 is replaced with the B10 allele. Interestingly, we found that genes at the Idd9 locus associated with susceptibility to diabetes control islet resilience to CD8+ T cell–mediated autoimmunity. Susceptible islets are hyperresponsive to the cytokines TNF and IFN-γ, resulting in increased expression of the death receptor Fas. Fas upregulation in beta cells is mediated by TNF receptor 2 (TNFR2), and in nonobese diabetic mice, colocalization of the receptor with the adaptor TNF receptor–associated factor 2 in beta cells is altered. The gene for TNFR2 lies within the candidate Idd9 interval, and the diabetes-associated variant contains a mutation adjacent to the binding site for TNF receptor– associated factor 2. A component of diabetes susceptibility is therefore determined by the target of the autoimmune response, and protective TNFR2 signaling in islets may inhibit early cytokine-induced damage required for the development of destructive autoimmunity. Because insulin-dependent diabetes mellitus is due to selective destruction of insulin-producing cells, strategies that promote growth of beta cells provide a means to prevent or reverse this type of diabetes. One approach is to replace insulin-producing cells by using genetic engineering or by guiding stem cells (pancreas progenitors) to differentiate into beta cells. The progression of pancreatic progenitor cells to beta cells is governed by basic helix-loop-helix transcription factors, which are regulated by inhibitor of differentiation proteins that bind to and inhibit the function of the factors. Transcription of inhibitor of differentiation proteins is induced by bone morphogenetic proteins (BMPs). We showed that BMP signaling is necessary and sufficient for proliferation of pancreatic progenitor cells and that this signaling is correlated with an increase in the expression of inhibitor of differentiation proteins. Using a mouse model of regenerating pancreas, we found that injection of an antibody that inhibits BMP4 significantly reduced cell proliferation and caused an increase in NeuroD, a basic helix-loop-helix factor required for the differentiation of pancreatic islet cells. Therefore, our results indicate that stimulation by BMP4 blocks the differentiation of endocrine progenitor cells and instead promotes their expansion, thereby revealing a novel model of signaling that explains the balance between expansion and differentiation of pancreatic duct epithelial progenitors. PUBLICATIONS Flodstrom-Tullberg, M., Hultcrantz, M., Stotland, A., Maday, A., Tsai, D., Fine, C., Williams, B., Silverman, R., Sarvetnick, N. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J. Immunol. 174:1171, 2005. THE SCRIPPS RESEARCH INSTITUTE 137 Horwitz, M.S., Ilic, A., Fine, C., Sarvetnick, N. Induction of antigen specific peripheral humoral tolerance to cardiac myosin does not prevent CB3-mediated autoimmune myocarditis. J. Autoimmun. 25:102, 2005. Hua, H., Zhang, Y.Q., Dabernat, S., Kritzik, M.N., Dietz, D., Sterling, L., Sarvetnick, N. BMP4 regulates pancreatic progenitor cell expansion through Id2. J. Biol. Chem. 281:13574, 2006. Kayali, A.G., Stotland, A., Gunst, K.V., Kritzik, M., Liu, G., Dabernat, S., Zhang, Y.Q., Wu, W., Sarvetnick, N. Growth factor-induced signaling of the pancreatic epithelium. J. Endocrinol. 185:45, 2005. Kim, S.H., Gunst, K.V., Sarvetnick, N. STAT4/6-dependent differential regulation of chemokine receptors. Clin. Immunol. 118:250, 2006. Marleau, A.M., Sarvetnick, N. T cell homeostasis in tolerance and immunity. J. Leukoc. Biol. 78:575, 2005. Martinez, X., Kreuwel, H.T., Redmond, W.L., Trenney, R., Hunter, K., Rosen, H., Sarvetnick, N., Wicker, L.S., Sherman, L.A. CD8+ T cell tolerance in nonobese diabetic mice is restored by insulin-dependent diabetes resistance alleles. J. Immunol. 175:1677, 2005. Solomon, M., Flodstrom-Tullberg, M., Sarvetnick, N. Differences in suppressor of cytokine signaling-1 (SOCS-1) expressing islet allograft destruction in normal BALB/c and spontaneously-diabetic NOD recipient mice. Transplantation 15:1104, 2005. Zhang, Y.Q., Kritzik, M., Sarvetnick, N. Identification and expansion of pancreatic stem/progenitor cells. J. Cell. Mol. Med. 9:331, 2005. Promotion of Cell Migration and Invasion by Tyrosine Kinase Signaling D.D. Schlaepfer, J.A. Bernard-Trifilo, X.L. Chen, A. Chi, D.A. Hanson, S. Hou, S.T. Lim, Y.M. Lim, S.K. Mitra, J.E. Molina, S. Uryu, A. Wang e wish to understand how intracellular signaling networks promote complex biological processes such as cell motility, cell invasion, and tumor metastasis. We hypothesize that critical intracellular signaling proteins exist within cells that act as signal “integrators” to process environmental stimuli that control cell movement. These proteins should be activated by various extracellular inputs and act to regulate multiple downstream signaling pathways. One such integrator is focal adhesion kinase (FAK), an intracellular protein-tyrosine kinase that is associated with both transmembrane integrin and growth factor receptors. W CONNECTIONS TO GROWTH FACTOR RECEPTORS The ErbB family of protein-tyrosine kinase receptors includes epidermal growth factor receptor (ErbB-1), ErbB-2, ErB-3, and ErB-4. Overexpression of ErbB-2 is associated with poor prognosis and invasiveness in cancer in humans. An important early event implicated in controlling cell migration induced by growth factors 138 IMMUNOLOGY 2006 is FAK activation. Using cells that lacked the gene for FAK, FAK-reconstituted fibroblasts, and human breast carcinoma cells in which FAK expression was inhibited by short inhibitory RNA, we dissected the function of FAK in ErbB-2/ErbB-3 oncogenic transformation and cell invasion. We found that these processes depend on FAK. In many cells, FAK activation promotes binding of the protein-tyrosine kinase c-Src to FAK, thereby generating a dual FAK-Src signaling complex. In these studies, ErbB-2/ErbB-3–induced oncogenic transformation depended on a FAK-Src and MAP kinase activation, whereas ErbB-2/ErbB-3–induced cell invasion was FAK-Src dependent and independent of MAP kinase. CONNECTIONS TO INTEGRINS Overexpression of FRNK, the FAK C-terminal domain, can inhibit FAK activity, in part by disrupting FAK association with integrins. Analyses of breast tumor samples revealed that elevated FAK expression occurs with the development of benign ductal hyperplasia into invasive carcinomas. We inhibited FAK activity or FAK expression in murine 4T1 breast carcinoma cells via transient expression of FRNK or stable expression of anti-FAK short hairpin RNA, respectively. Expression of anti-FAK short hairpin RNA resulted in the inhibition of 4T1 cell invasion in vitro and spontaneous 4T1 metastasis after implantation of the cells in Balb/c mice.* Transient reexpression of wild-type but not kinase-inactive FAK in 4T1 cells with the anti-FAK short hairpin RNA promoted in vivo lung metastasis, and this finding was associated with increased expression of urokinase plasminogen activator. Because the inhibition of FAK within 4T1 cells was also associated with increased host survival after tumor cell implantation, our results support the pharmacologic targeting of FAK activity as a means to inhibit tumor spread. TA R G E T S O F FA K A C T I V I T Y P R O M O T I N G T U M O R PROGRESSION Using stable FRNK overexpression to inhibit FAK in 4T1 breast carcinoma cells, we found that FRNK overexpression was not associated with alterations in cell proliferation or anchorage-independent cell survival in vitro. Instead, FRNK-expressing 4T1 cells secreted less vascular endothelial cell growth factor (VEGF) and formed small avascular tumors, findings associated with the inhibition of a signaling linkage involving FAK, phosphorylation of FAK at tyrosine 925, and MAP kinase that regulates expression of VEGF. The biological importance of this FAK signaling pathway was confirmed through reconstitution experiments with Src transformation of THE SCRIPPS RESEARCH INSTITUTE fibroblasts lacking the gene for FAK in which point mutations affecting FAK catalytic activity or phosphorylation disrupted the ability of FAK at tyrosine 925 to promote tumor growth–associated MAP kinase and VEGF expression. Inhibition of FAK in breast, prostate, and neuroblastoma cells also resulted in reduced VEGF expression. These studies provide the first biological support for Y925 FAK phosphorylation and define a novel role for FAK activity in promoting a MAP kinase–associated angiogenic switch during tumor progression. INTEGRIN SIGNALING INDEPENDENT OF FAK In cell culture, fibroblasts that lack the gene for FAK have defects in motility but not in proliferation. The fibronectin-binding integrins α5β1 and α4β1 generate signals pivotal for cell migration through distinct yet undefined mechanisms. For α5β1, β1-mediated activation of FAK promotes c-Src recruitment to FAK and the formation of a FAK-Src signaling complex that promotes motility. Interestingly, expression of human α4 integrin in fibroblasts that lack the gene for FAK forms a functional α4β1 receptor that promotes motility of the cells, equal to that of wild-type FAK-containing fibroblasts stimulated with α5β1. This α4β1-stimulated signaling connection was initiated by the cytoplasmic domain of α4 integrin and involved the activation of Src-family protein-tyrosine kinases in the absence of FAK. Currently, we are elucidating the molecular linkage of α4 integrin to Src and how this signaling pathway promotes neuroblastoma motility, invasion, and tumor progression. PUBLICATIONS Benlimame, N., He, Q., Jie, S., Xiao, D., Xu, Y.J., Loignon, M., Schlaepfer, D.D., Alaoui-Jamali, M.A. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J. Cell Biol. 171:505, 2005. Bernard-Trifilo, J.A., Lim, S.T., Hou, S., Schlaepfer, D.D., Ilic, D. Analyzing FAK and Pyk2 in early integrin signaling events. In: Current Protocols in Cell Biology. Bonifacino, J.S., et al. (Eds.). Wiley, New York, 2006, Chap. 14.7.1. Hsia, D.A., Lim, S.T., Bernard-Trifilo, J.A., Mitra, S.K., Tanaka, S., den Hertog, J., Streblow, D.N., Ilic, D., Ginsberg, M.H., Schlaepfer, D.D. Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domainspecific activation of c-Src. Mol. Cell. Biol. 25:9700, 2005. Hu, B., Jarzynka, M.J., Guo, P., Imanishi, Y., Schlaepfer, D.D., Cheng, S.Y. Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the αvβ1 integrin and focal adhesion kinase signaling pathway. Cancer Res. 66:775, 2006. Mitra, S.K., Lim, S.T., Chi, A., Schlaepfer, D.D. Intrinsic focal adhesion kinase activity controls orthotopic breast carcinoma metastasis via the regulation of urokinase expression in a syngeneic tumor model system. Oncogene 25:4429, 2006. Mitra, S.K., Mikolon, D., Molina, J., Hsia, D.A., Hanson, D.A., Chi, A., Lim, S.T., Bernard-Trifilo, J.T., Ilic, D., Stupack, D.G., Cheresh, D.A., Schlaepfer, D.D. Intrinsic FAK activity and Y925 phosphorylation facilitate an angiogenic switch in tumors. Oncogene 25:5969, 2006. Urbinati, C., Bugatti, A., Giacca, M., Schlaepfer, D., Presta, M., Rusnati, M. αvβ3 integrin-dependent activation of focal adhesion kinase mediates NF-κB activation and motogenic activity by HIV-1 Tat in endothelial cells. J. Cell Sci. 118(Pt. 17):3949, 2005. IMMUNOLOGY 2006 The Consequences of T-Cell Recognition of Self-Antigens and Tumor Antigens L.A. Sherman, X. Martinez, C.-H. Wei, J. Wong, E. Hamilton-Williams, J.A. Biggs, K.L. Marquardt, R.L. Trenney he consequence of antigen recognition by naive CD8+ T cells can be either tolerance or immunity, depending on the activation status of the antigenpresenting dendritic cells. If a CD8+ T cell recognizes antigen on a quiescent dendritic cell that has relatively low levels of expression of costimulatory molecules, then activation of the T cell results in deletion and tolerance. Inflammatory signals, such as those due to the presence of foreign pathogens and activated lymphocytes, activate dendritic cells to express cell-surface costimulatory molecules and cytokines. If CD8 + T cells recognize antigen on activated dendritic cells, the costimulatory molecules and cytokines prevent deletion and promote the clonal expansion of the T cells and the development of effector functions. Understanding the signals that result in either T-cell deletion or immunity is of importance in preventing autoimmunity, which represents a failure to control selfdestructive T lymphocytes. This understanding is also important in promoting tumor immunity, in which the goal is to promote the autoimmune destruction of tumor cells. We are comparing the consequence of the interaction of naive CD8+ T lymphocytes with a transgenic self-antigen (the influenza virus hemagglutinin) expressed by the insulin-producing beta cells in the pancreatic islets in 3 different types of mice: normal mice, diabetesprone nonobese diabetic (NOD) mice, and mice in which the beta cells express an oncogene that promotes spontaneous transformation and production of tumors. In all 3 types of mice, the interaction between antigen and naive CD8+ T lymphocytes specific for hemagglutinin first occurs in the pancreatic lymph nodes. There antigen is recognized on dendritic cells that obtain it in from beta cells in the islets and cross-present it to naive T cells in the lymph nodes. In normal mice, this interaction results in an abortive activation of the T cells and subsequent deletion of the potentially autoreactive T cells specific for hemagglutinin. T TOLERANCE OF TISSUE-RESIDENT MEMORY CD8+ T CELLS Memory CD8 + T cells are compartmentalized on the basis of their ability to circulate through either THE SCRIPPS RESEARCH INSTITUTE 139 lymphoid tissue (central memory cells) or blood and parenchymal tissue (tissue-resident memory cells). Compartmentalization is based on cell-surface expression of CD62L, an adhesion molecule required for migration of T cells across high endothelial venules into secondary lymphoid tissue. Tissue-resident memory cells express low levels of CD62L and are therefore excluded from secondary lymphoid tissue. Previously, we showed that central memory CD8 + T cells are efficiently tolerized by either soluble peptide or tissue-derived antigen that is cross-presented in draining lymph nodes. More recently, we determined whether tissue-resident memory cells are also tolerized by these 2 forms of antigen. Soluble peptide was highly efficient at tolerizing tissue-resident memory cells in all tissues tested except the brain. This finding may be due to the inability of soluble peptide to cross the blood-brain barrier. Crosspresented antigen was able to tolerize central memory cells but not tissue-resident memory cells. This difference in tolerance may occur because the cells are excluded from entry into secondary lymphoid tissue, the site at which dendritic cells cross-present antigen derived from tissue. Our results are also consistent with the possibility that some tissue-resident memory cells may not circulate out of tissue. MECHANISMS OF PROTECTION FROM TYPE 1 D I A B E T E S B Y G E N E T I C P O LY M O R P H I S M S The spontaneous diabetes that develops in NOD mice is similar to type 1 diabetes in humans. The disease process involves destruction of the insulin-producing beta cells in the pancreas by CD8+ T lymphocytes. In humans and mice, genetic regions have been identified in which allelic polymorphism predisposes individuals to type 1 diabetes. We are studying the effects of such allelic polymorphism, designated insulin-dependent diabetes (Idd) loci, on the establishment of CD8 + T-cell tolerance. Congenic mice that express protective alleles at Idd3/5 have normal abortive activation of islet antigenspecific CD8+ T cells in the pancreas, suggesting that tolerance is restored at the earliest time when naive CD8 + T cells first encounter antigen. In contrast, in NOD mice, such CD8+ T cells accumulate in the pancreatic lymph nodes and then enter the islets. This difference in the accumulation of CD8+ T cells in the pancreatic lymph nodes occurs in the absence of all CD4 + T cells. We are testing the hypothesis that this difference may be intrinsic to the interaction between 140 IMMUNOLOGY 2006 T cells and cross-presenting dendritic cells in NOD and Idd3/5 mice. ROLE OF CD4+ HELPER T CELLS IN PROMOTING TUMOR CELL DESTRUCTION BY CD8+ T CELLS CD4 + helper T cells can enhance the performance of CD8 + T cells in different ways, including enhanced clonal expansion during activation of CD8 + T cells, enhanced tissue infiltration by the activated effector CD8 + T cells, and enhanced survival of the effector CD8 + T cells. We are assessing the effects of CD4 + helper T cells at various times after activation of CD8+ T cells to evaluate the ability of the helper cells to promote destruction of tumor cells by CD8+ T cells. PUBLICATIONS Cohen, C.J., Zheng, Z., Bray, R., Zhao, Y., Sherman, L.A., Rosenberg, S.A., Morgan, R.A. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR [published correction appears in J. Immunol. 177:5746, 2006]. J. Immunol. 175:5799, 2005. Martinez, X., Kreuwel, H.T.C., Redmond, W.L., Trenney, R., Hunter, K., Rosen, H., Sarvetnick, N., Wicker, L.S., Sherman, L.A. CD8+ T cell tolerance in nonobese diabetic mice is restored by insulin-dependent diabetes resistence alleles. J. Immunol. 175:1677, 2005. Wei, C.-H., Trenney, R., Sanchez-Alavez, M., Marquardt, K., Woodland, D.L., Henriksen, S.J., Sherman, L.A. Tissue resident memory CD8+ T cells can be deleted by soluble, but not cross-presented antigen. J. Immunol. 175:6615, 2005. Regulation of Homeostasis of Mature T Cells C.D. Surh, C. Ramsey, J. Purton, E.M.M. van Leeuwen, J.Y. Lee, D. Kim, O. Boyman,* C. Ahn,** J. Sprent*** * Universitaire Vaudois, Lausanne, Switzerland ** Seoul National University Hospital, Seoul, Korea *** Garvan Institute of Medical Research, Darlinghurst, Australia he homeostasis of mature T cells is largely governed by 2 related cytokines, IL-7 and IL-15, which bind to the receptors belonging to the common γ-chain (CD132) receptor family. Other members of the CD132 family include receptors for IL-2, IL-4, IL-9, and IL-21. In conjunction with signals from contact with self-peptide–MHC ligands, IL-7 controls survival of naive T cells. Memory T cells, which are at a higher state of activation than naive T cells, depend on both IL-7 and IL-15 for survival and for intermittent cell division. Memory CD4+ T cells are generally more dependent on IL-7 than on IL-15 for their homeostasis, whereas memory CD8 + T cells rely primarily on IL-7 for survival and on IL-15 for periodic cell division. Indica- T THE SCRIPPS RESEARCH INSTITUTE tive of the cells’ cytokine requirements, the dimeric receptor for IL-7 (CD127-CD132) is expressed at high levels on naive and memory T cells, whereas the dimeric receptor for IL-15 (CD122-CD132) is expressed at negligible levels on naive T cells, low levels on memory CD4+ T cells, and high levels on memory CD8+ T cells. Neither IL-7 nor IL-15 is produced by T cells; both are produced by epithelial, stromal, and antigen-presenting cells. IL-2, which is produced by T cells for autocrine purposes, is closely related to IL-15. Recognition of IL-2 by T cells is mediated by the high-affinity trimeric receptor CD25-CD122-CD132; the dimeric form of the recepter (CD122-CD132) recognizes IL-15. Indeed the IL-15 receptor can also recognize IL-2 at a lower affinity. Interestingly, injecting a monoclonal antibody to IL-2, a technique that is thought to deplete the cytokine, increases the background turnover rate of memory CD8 + T cells. This finding was interpreted to indicate that IL-2, unlike IL-15, dampens the homeostasis of memory CD8+ T cells. Recent work, however, indicates that this interpretation is untrue. Instead of depleting IL-2, the monoclonal antibody actually boosts the biological activity of the interleukin. Hence, the ability of the monoclonal antibody to IL-2 to elevate turnover of memory CD8 + T cells does not occur in the absence of IL-2, and, more important, injecting the monoclonal antibody complexed with IL-2 dramatically induces rapid proliferation of memory CD8+ T cells. Indeed, administration of the antibody–IL-2 complex induced 100- to 200-fold greater expansion of memory CD8+ T cells than did administration of IL-2 alone. The complex stimulated CD8+ T cells through the dimeric IL-15 receptor rather than through the trimeric IL-2 receptor, because stimulatory activity of the complex was also evident on CD25−CD8 + T cells. Exactly how the bound monoclonal antibody enhances the activity of IL-2 is unknown. Nonetheless, it is clear that monoclonal antibodies augment the cytokine activity in vivo but not under in vitro conditions, and that the Fc part of the antibody is required for its intensifying role. These findings suggest that the monoclonal antibody boosts the cytokine activity by concentrating the cytokine onto the cell surface of antigen-presenting cells and/or by prolonging the half-life of the cytokine. The specificity of the monoclonal antibody also determines its ability to enhance the activity of IL-2 to IL-2 receptors. Thus, one particular monoclonal antibody to IL-2 could stimulate T cells expressing the IMMUNOLOGY 2006 trimeric IL-2 receptor, such as regulatory T cells, but not memory CD8 + T cells that express the dimeric IL-15/IL-2 receptor. The ability of the monoclonal antibody to boost the activity of the bound cytokine appears to be generally applicable because the biological activity of other cytokines, such as IL-4 and IL-7, can also be enhanced by binding to specific monoclonal antibodies. The ability to induce expansion and activation of specific subsets of T cells by administering complexes composed of cytokine plus monoclonal antibody may offer a new way to modulate the immune response for therapeutic purposes. The immune response can be boosted against tumors and infectious agents by activating naive and memory T cells; alternatively, allergens and tissue grafts can be suppressed by inducing expansion of regulatory T cells and the immune responses against self. PUBLICATIONS Boyman, O., Kovar, M., Rubinstein, M.P., Surh, C.D., Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311:1924, 2006. Davey, G.M., Starr, R., Cornish, A.L., Burghardt, T., Alexander, W.S., Carbone, F.R., Surh, C.D., Heath, W.R. SOCS-1 regulates IL-15-driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J. Exp. Med. 202:1099, 2005. Gattinoni, L., Finkelstein, S.E., Klebanoff, C.A, Antony, P.A., Palmer, D.C., Spiess, P.J., Hwang, L.N., Yu, Z., Wrzesinski, C., Heimann, D.M., Surh, C.D., Rosenberg, S.A., Restifo, N.P. Removal of homeostatic cytokine links by lymhodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 202:907, 2005. Lee, S.K., Surh, C.D. Role of interleukin-7 in bone and T-cell homeostasis. Immunol. Rev. 208:169, 2005. Ramsey, C., Hässler, S., Marits, P., Kämpe, O., Surh, C.D., Peltonen, L., Winqvist, O. Increased antigen presenting cell-mediated T cell activation in mice and patients without the autoimmune regulator. Eur. J. Immunol. 36:305, 2006. Surh, C.D., Boyman, O., Purton, J.F., Sprent, J. Homeostasis of memory T cells. Immunol. Rev. 211:154, 2006. THE SCRIPPS RESEARCH INSTITUTE 141 on artificial bilayers with recombinant forms of TCRαβ, CD3δε, CD3γε, and CD8αβ is in progress. We use a combination of single-molecule, multicolor imaging by total internal reflection fluorescence microscopy, in collaboration with K. Fish, University of Pittsburgh, Pittsburgh, Pennsylvania, and electron microscopy to examine the dynamics and membrane relationships of each subunit within the complex. Similar observations are carried out in the presence of MHC ligands displayed in solution or at the surface of polystyrene beads and liposomes. Interactions of MHC and TCR molecules with their respective membrane could provide simple switches essential to T-cell activation. This hypothesis is supported by our determination, in collaboration with A.K. Mitra, University of Auckland, Auckland, New Zealand, of the structure of an MHC molecule attached to a phospholipid bilayer that shows parallel orientation of the long axis of the molecule with the lipid leaflet. In collaboration with I.A. Wilson, Department of Molecular Biology, we are determining 3-dimensional structures of CD3, TCR complexes, and CD8αβ. AUTOIMMUNE DIABETES We are using MHC multimers to detect antigen-specific T-cell populations in diabetes-prone nonobese diabetic mice. Pathogenic T cells are characterized by analyzing secretion of cytokines and use of TCRs by single cells. We are also trying to treat insulin-dependent diabetes by depleting antigen-specific T cells in vivo during the preclinical phase of the disease. For this therapy, we are using MHC molecules to deliver doxorubicin liposomes to autoreactive T cells. The specificity of the intervention will limit side effects and complications of general immunosuppression. L I N K S B E T W E E N I N N AT E A N D A D A P T I V E I M M U N I T Y Surh, C.D., Sprent, J. On the TRAIL of homeostatic memory T cells [published correction appears in Nat. Immunol. 7:672, 2006]. Nat. Immunol. 7:439, 2006. Tan, J.T., Surh, C.D. T cell memory. Curr. Top. Microbiol. Immunol., in press. Structure-Function Studies of Innate and Adaptive Immunity L. Teyton, B. Atteberry, K. Bennett, C. Cantu, S.Y. Chang, L. Develioglu, S. Freigang, H. Issafras, M. Holt, E. Landais, M. Ota, N. Schrantz, J. Sim, R. Stefanko, N. Von Allmen-Zurcher, C. Wang, K. Yoshida A C T I VAT I O N O F T - C E L L R E C E P T O R S O ur goal is to understand the molecular switches that lead to activation of T cells. Assembly of functional complexes of T-cell receptors (TCRs) We are studying lipid binding to CD1 to determine the factors that govern the presentation of the lipids to T cells. A family of lipid transfer proteins known as saposins, which are involved in the catabolism of lipids, are critical for the loading of natural glycolipids onto CD1 and the selection of natural killer T cells. Other lipid transfer proteins most likely account for the loading of other endogenous and exogenous ligands. In collaboration with A. Bendelac, University of Chicago, and P.B. Savage, Brigham Young University, Provo, Utah, we are using RNA interference, genetic techniques, and recombinant biochemistry to study CD1 within the context of lipid metabolism. At a structural level, we are examining recognition of dissimilar ceramides such as α-galactosyl ceramide (Fig. 1) and isoglobotrihexosyl ceramide (β-linked) by a TCR bearing a unique 142 IMMUNOLOGY 2006 THE SCRIPPS RESEARCH INSTITUTE Genetics of Systemic Autoimmunity and T-Cell Homeostasis in Autoimmunity, Aging, and Cancer A.N. Theofilopoulos, D.H. Kono, R. Baccala, R. Gonzalez-Quintial, M.K. Haraldsson, D. Aït-Azzouzene, J. Schettini, R.M. Chintalapati, C.A. Louis-Dit-Sully e have continued our research on predisposing and effector genes in murine models of systemic lupus erythematosus (SLE), homeostatic T-cell disturbances in systemic autoimmunity and aging, and the potential use of homeostatic T-cell proliferation for inducing efficient antitumor responses. W F i g . 1 . Side view of α-galactosyl ceramide bound to murine CD1d. The galactose is accessible for TCR recognition. α-chain (Vα14 in mice, Vα24 in humans) and a limited set of Vβ partners. I N N AT E I M M U N E R E C E P T O R S Recognition of unique features of the prokaryotic world is embedded in a series of receptors of the innate immune system called pattern recognition molecules. Each of these receptors can sense the presence of a family of unique prokaryotic compounds such as glycolipids, proteoglycans, DNA, or RNA and allow activation of macrophages, dendritic cells, and neutrophils. We are collaborating with R. Ulevitch and P. Tobias, Department of Immunology, to decipher the structural basis of this mode of recognition. We expressed recombinant forms of receptor family members from Drosophila, mice, and humans to compare the biophysical and structural characteristics of the receptors and to delineate new activation pathways. PUBLICATIONS Benlagha, K., Wei, D.G., Veiga, J., Teyton, L., Bendelac, A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 202:485, 2005. Shore, D.A., Teyton, L., Dwek, R.A., Rudd, P.M., Wilson, I.A. Crystal structure of the TCR coreceptor CD8αα in complex with monoclonal antibody YTS 105.18 Fab fragment at 2.88 Å resolution. J. Mol. Biol. 358:347, 2006. Wei, D.G., Lee, H., Park, S.H., Beaudoin, L., Teyton, L., Lehuen, A., Bendelac, A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202:239, 2005. Zajonc, D.M., Cantu, C. III, Mattner, J., Zhou, D., Savage, P.B., Bendelac, A., Wilson, I.A., Teyton, L. Structure and function of a potent agonist for the semiinvariant natural killer T cell receptor. Nat. Immunol. 8:810, 2005. GENETIC BASIS OF SYSTEMIC AUTOIMMUNITY Susceptibility to SLE is inherited as a multifactorial trait, and genetic predisposition is a major if not an essential factor in the disease. We are defining the role and identity of susceptibility genes that promote SLE in both spontaneous and induced mouse models of the disease. Previously, we identified loci that predispose mice to SLE in NZB, NZW, BXSB, and MRL strains, which are SLE prone, and in C57BL/6 mice, which are not predisposed to autoimmune disease. We also identified a disease-resistance locus in DBA/2 mice. We subsequently analyzed the contribution of several of these loci to autoimmunity in interval congenic lines. The NZB x NZW F2-related Lbw2, Lbw5, and Lbw7 (chromosomes 4, 7, and 1, respectively); the MRL x B6-Fas lpr F2-related Lmb1–Lmb4 (chromosomes 4, 5, 7, and 10), and the NZB x DBA/2 and SJL x DBA/2 F 2 -related Hmr1 (chromosome 1) were among those analyzed. On the basis of these studies. we concluded that lupus-prone strains have substantial genetic heterogeneity, strains not predisposed to autoimmune disease can harbor susceptibility genes, a single locus often promotes multiple traits, and specific combinations of loci determine clinical manifestations.* Moreover, phenotypes do not always correlate with the initial mapping results, and the genetic background plays a major role in shaping locus-induced phenotypes. Further fine-mapping studies indicated that identifying the underlying genes by screening for alterations in gene expression still requires the evaluation of subcongenics. Substantial progress was also made in defining Lmb3 as a nonsense mutation of the gene that encodes IMMUNOLOGY 2006 coronin-1A, and actin-binding protein that plays a role in the formation of filaments. Surprisingly, this mutation was present in the C57BL/6-Faslpr/Scr substrain, which is not predisposed to autoimmunity, and not in the MRL strain, indicating that Lmb3 most likely is a diseasemodifying or resistance allele. Currently, we are determining the role of coronin-1 in normal immune responses and autoimmunity. We are also more precisely mapping and identifying the genes for other loci. TYPE I INTERFERONS IN SLE Type I interferons (IFN-α/β) have important effects in the innate and adaptive immune systems and may play a central role in the pathogenesis of autoimmune diseases, including SLE. We used several approaches to define the mechanisms of these interferons and to curtail their adverse effects. We previously found that deletion of the common receptor for type I interferons resulted in significant disease reduction in lupus-predisposed NZB mice. Although this finding provides strong support for the involvement of IFN-α/β in SLE, it has yet to be shown that blockade of IFN-α/β activity can inhibit active disease. We previously showed the efficacy of intramuscular injections of nonviral vectors encoding the fusion protein consisting of the IFN-γ receptor and the Fc fragment of IgG1 in ameliorating SLE in MRL-Faslpr lupus-prone mice. A similar strategy to block type I interferons is being used to treat the prototypic NZB/W strain of lupus-prone mice. Early assessments indicate that blockade of IFN-α/β can indeed cause regression of active disease. In collaborative studies with R. Schreiber and his colleagues, Washington University of Medicine, St. Louis, Missouri, we will ascertain the efficacy of a recently developed mouse monoclonal antibody to mouse IFN-α receptor 1. Overall, our previous and current studies have provided the impetus for the potential use of interferon blockers as therapies in human SLE and other autoimmune diseases. A major issue in the role of type I interferons in SLE is the stimuli that induce and sustain the production of these cytokines. Although exogenous stimuli, such as infectious agents, may induce IFN-α/β through engagement of Toll-like receptors (TLRs) and precipitate disease, the primary involvement of the endogenous stimuli remains less defined. Therefore, a major focus has been on identify the endogenous (self) stimuli acting under “sterile” conditions. Studies have shown that products of apoptotic/ necrotic cells or nucleic acids, when combined with THE SCRIPPS RESEARCH INSTITUTE 143 autoantibodies from the sera of patients with SLE, induce plasmacytoid dendritic cells to produce large quantities of IFNα/β. The requirement of forming a complex with autoantibodies is consistent with the idea that mammalian nucleic acids themselves are compartmentalized away from the endosomes where IFN-α/β–inducing TLR3, TLR7, TLR8, and TLR9 reside. Although this mechanism is important for disease perpetuation, primary inducers not predicated on preexisting autoantibodies need to be identified. A likely candidate might be apoptotic material that under certain conditions could constitute a “danger signal.” Recent studies, in collaboration with K. Hoebe and B. Beutler, Department of Immunology, indicated that early apoptotic cells can indeed act as efficient inducers of type I interferons and that the responding cells are not plasmacytoid dendritic cells, but precursors of B220 −CD8 + lymphoid-type dendritic cells. Importantly, induction was mediated by a TLR-independent pathway. On the basis of these findings, we propose that induction of IFN-α/β in SLE encompasses 2 types of apoptosis-generated stimuli that act sequentially in the disease process. The early-phase stimulus does not require autoantibodies or TLR engagement and is generated by stressed cells with propensity to apoptosis; this material is taken up by lymphoid-type dendritic cells and initiates production of IFN-α/β, leading to activation of antigen-presenting cells, priming of previously quiescent nontolerant T and B cells, and production of autoantibodies. IFN-α/β may also promote maturation and survival of T and B cells directly as well as by inducing production of B-cell trophic factors from activated dendritic cells. The late-phase stimulus consists of apoptosis/necrosis materials and associated nucleic acids complexed with autoantibodies. These complexes are directed into endosomal compartments of plasmacytoid or conventional dendritic cells and B cells, through receptors for IgG or autoreactive B-cell receptors, where they engage TLRs and amplify production of interferons and responses of autoimmune T and B cells.* We are conducting experiments to define the responsiveness of plasmacytoid and conventional dendritic cells to apoptotic materials in SLE and to assess the 2-step hypothesis in disease pathogenesis. CYCLIN KINASE INHIBITORS IN SYSTEMIC AUTOIMMUNITY We previously found that the cell-cycle inhibitor p21 is a nonredundant effector molecule for SLE in 144 IMMUNOLOGY 2006 autoimmune-prone BXSB mice; lack of p21 resulted in enhanced apoptosis of T and B lymphocytes, decreased numbers of activated/memory CD4+ T cells, and disease reduction. These findings support our earlier hypothesis that increased numbers of activated/memory CD4 + T cells in SLE could be caused by repeated stimulation by self-antigens leading to accumulation of cell cyclin kinase inhibitors, such as p21 and p27, and a replicative senescence-like state. Upon stimulation, these senescence-like T cells would be resistant to apoptosis and proliferation because of their inability to cycle but could produce autoimmunity-promoting proinflammatory factors. A more recent T-cell transfer study confirmed that lack of p21 in T cells is sufficient to reduce clinical manifestations of autoimmunity. Moreover, BXSB mice lacking the gene for p27, another cell-cycle inhibitor, also have reduced mortality compared with littermate BXSB mice that have the gene, further supporting our hypothesis. We are also examining the role of p21 in organ-specific autoimmunity, and we have generated type 1 diabetes–prone nonobese diabetic mice that lack the gene for p21. We have backcrossed (>10 generations) the p21 knockout gene onto the type 1 diabetes–prone nonobese diabetic strain of mice. Studies will continue to define in more detail the role of cyclin kinase inhibitors in autoimmunity and to determine the potential for modulation of the cell cycle in therapy for SLE. T - C E L L H O M E O S TA S I S A N D S L E Systemic autoimmunity is essentially a disease that can be defined on the basis of disturbances in homeostasis of lymphoid cells. We previously speculated that frank lymphopenia and associated excess of T-cell trophic cytokines (IL-7 and IL-15) leading to ”acute homeostatic T-cell proliferation” might be an inducing mechanism for SLE autoimmune processes. We found that acute homeostatic T-cell proliferation of adoptively transferred T cells indeed recapitulated systemic autoimmunity in lymphopenic recipient mice predisposed to SLE. Currently, we are investigating whether a similar phenomenon might be mediated by downregulation of receptors for IL-7 and/or IL-15 in accumulating chronically activated autoreactive T cells, a process that might create an excess of these cytokines and promote proliferation of newly generated autoreactive T cells. To assess this possibility, we studied MRL-Fas lpr mice in which a Fas mutation leads to massive expansion of double-negative (CD4−CD8 −) T cells. THE SCRIPPS RESEARCH INSTITUTE We found that these T cells had significantly reduced expression of CD127 (the receptor for IL-7) and CD122 (the receptor for IL-15). In accordance with our hypothesis, nonlymphopenic MRL-Fas lpr recipients supported proliferation of transferred isogenic CD8 + T cells, whereas nonlymphopenic normal hosts did not. Moreover, although single-positive CD4+ and single-positive CD8+ as well as double-negative T cells proliferated efficiently in a lymphopenic MRL-Fas lpr recipient, survival and repopulation occurred with single-positive, but not double-negative, T cells. These studies support the concept that excess of IL-7 and IL-15 created by downregulation of the corresponding receptors in expanded autoreactive T cells creates an environment that mimics the lymphopenic condition of cytokine excess, triggering proliferation of newly emerging autoreactive T cells. Verification of these results may indicate the usefulness of blocking T-cell trophic cytokines and/or the receptors of these cytokines to interfere with autoimmune responses. We have started experiments to address this possibility. T - C E L L H O M E O S TA S I S A N D A G I N G Aging has been associated with several T-cell defects, but whether these defects are intrinsic or are imposed by changes in the microenvironment is unclear. To address this issue, we adoptively transferred labeled T cells into young and old lymphopenic mice and examined degrees of “acute homeostatic expansion.” The results indicated that aging is associated with impaired homeostatic T-cell proliferation. The proliferation was not due to a primary T-cell defect but rather to changes in the microenvironment. Adoptively transferred T cells from aged donors indeed proliferated normally in lymphopenic young recipients, whereas T cells from young donors had reduced proliferation in aged lymphopenic hosts. One possibility for this aging-associated defect in the microenvironment is reduced levels of IL-7 and IL-15, which are necessary for homeostatic expansion. Therefore, we treated old, sublethally irradiated recipients with recombinant mouse IL-7. We found that the defect was largely corrected. The results suggest that T-cell trophic cytokine reconstitution may be an effective means to correct immunologic senescence. T - C E L L H O M E O S TAT I C P R O L I F E R AT I O N T O B R E A K TOLERANCE TO TUMOR ANTIGENS We previously proposed that lymphopenia-induced homeostatic proliferation mediated by excess of trophic cytokines and recognition of self-peptide–MHC may be a way to activate low-affinity T cells that recognize tumor IMMUNOLOGY 2006 antigens. Our earlier studies with a melanoma model indicated the validity of this approach when coupled with tumor cell immunization. We are now assessing the efficacy of this approach in established and metastasizing tumors, specifically in mouse models of breast and prostate carcinomas. We found that although the size of subcutaneous tumors was again reduced, metastasis was marginally affected, probably because of the time required for regeneration of lymphocytes and acquisition of a diverse repertoire. Currently, we are assessing the potential usefulness of protocols in which homeostatic proliferation as a priming event is combined with administration of complexes consisting of IL-7 and nonneutralizing antibodies to IL-7. This approach is based on the recent novel finding of our collaborators O. Boyman, C.D. Surh, and J. Sprent, Department of Immunology, that such complexes induce massive and accelerated expansion of CD8 + (and CD4+) T cells. Preliminary results indicate that this modified protocol significantly reduces both the size of the primary tumor and the degree of metastasis. We think that this and other contemplated protocols to promote priming of T cells may be promising approaches to tumor immunotherapy. PUBLICATIONS Homann, D., Dummer, W., Wolfe, T., Rodrigo, E., Theofilopoulos, A.N., Oldstone, M.B., von Herrath, M.G. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J. Virol. 80:270, 2006. Janssen, E., Tabeta, K., Barnes, M.J., Rutschmann, S., McBride, S., Bahjat, K.S., Schoenberger, S.P., Theofilopoulos, A.N., Beutler, B., Hoebe, K. Efficient T cell activation via a Toll-interleukin 1 receptor-independent pathway. Immunity 24:787, 2006. Kono, D.H., Theofilopoulos, A.N. Genetics of autoantibody production in mouse models of lupus. In: Autoantibodies and Autoimmunity: Molecular Mechanisms in Health and Disease. Pollard, K.M. (Ed.). Wiley-VCH, New York, in press. Kono, D.H., Theofilopoulos, A.N. Genetics of murine models of autoimmunity. In: Dubois’ Systemic Lupus Erythematosus, 7th ed. Wallace, D.J., Hahn, B.H. (Eds.). Williams & Wilkins, Baltimore, in press. Kono, D.H., Theofilopoulos, A.N. Genetics of SLE in mice. Springer Semin. Immunopathol. 28:83, 2006. Sfikakis, P.P., Gourgoulis, G.M., Moulopoulos, L.A., Kouvatseas, G., Theofilopoulos, A.N., Dimopoulos, M.A. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur. J. Clin. Invest. 35:380, 2005. Watson, L.C., Moffatt-Blue, C.S., McDonald, R.Z., Kompfner, E., Aït-Azzouzene, D., Nemazee, D., Theofilopoulos, A.N., Kono, D.H., Feeny A.J. Paucity of V-D-D-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT–/– and TdT+/+ mice. J. Immunol. 177:1120, 2006. THE SCRIPPS RESEARCH INSTITUTE 145 Initiation of Inflammation by the Innate Immune System P.S. Tobias, H.-K. Lee, L.K. Curtiss,* P. Dawson,** T. Kirkland,*** D. Liebler**** * Department of Immunology, Scripps Research ** Department of Cell Biology, Scripps Research *** University of California, San Diego, California **** Vanderbilt University, Nashville, Tennessee e focus on understanding the mechanisms by which cells use the innate immune system to initiate defensive inflammatory responses. First, we seek to understand the structural features of the Toll-like receptors (TLRs) and their allied proteins lipopolysaccharide-binding protein, CD14, MD-2, and CD36, which enable the receptors to bind their ligands. Second, we seek to understand the structural changes by which binding of a microbial ligand to the extracellular domain of the receptor leads to signal transduction across the cell membrane and initiation of intracellular signaling cascades. Third, we seek to understand the involvement of endogenous and exogenous inflammatory stimuli in atherosclerosis. Ten TLRs are known. For most of these, ligands derived from microorganisms are known; binding to the ligands initiates signaling, leading to expression of inflammatory mediators and other defensive responses. In addition, some TLRs that may be involved in sterile inflammatory conditions such as arthritis or atherosclerosis may have endogenous ligands. However, these ligands are not yet clearly identified. To understand the structural features of ligand-receptor binding, we use using 2 approaches. In the traditional mutation approach, amino acid residues in the proteins are mutated, and the proteins are then studied for functional changes. In the second approach, we use cross-linking agents to create covalent attachments of the ligands to the proteins. The proteins are then degraded chemically to determine the site of attachment. Currently, we are using these approaches to study binding of endotoxin, bacterial lipopeptides, and polyinosinic-polycytidylic acid to CD14. Binding of ligands to TLRs starts an intracellular signaling cascade that results in activation of a number of cellular responses. Prominent hypothesized mechanisms by which ligand binding to TLRs incurs transmembrane signaling are (1) the ligand induces dimerization of receptors and (2) binding of the ligand induces con- W 146 IMMUNOLOGY 2006 formational changes in the receptor. Our studies indicate that pairs of TLRs are associated even in the absence of ligand and that the pairs undergo a conformational change upon ligand binding. We are using a variety of approaches to understand the structural basis for associations among the TLRs and their associated intracellular signaling partners. Atherosclerosis is an inflammatory disease of the large arteries. Evidence suggests that inflammatory components derived from microbes can induce progression of atherosclerosis. However, most of the development of atherosclerotic lesions is due to endogenous inflammatory factors. Because the TLR system is so intimately involved with inflammation, we are determining whether the TLRs are involved in atherosclerosis. Our initial data clearly indicate that TLR2, whether activated by endogenous ligands or by exogenous ligands, promotes progression of atherosclerosis. Unexpectedly, we found that TLR2 expressed on non–bone marrow– derived cells detects endogenous TLR2 ligands that promote atherosclerosis, whereas bone marrow–derived cells detect exogenous TLR2 ligands that promote atherosclerosis. For these experiments, we are using mouse models of the disease and mice deficient in individual TLRs. PUBLICATIONS Lee, H.K., Dunzendorfer, S., Soldau, K., Tobias, P.S. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity 24:153, 2006. Mullick, A.E., Tobias, P.S., Curtiss, L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115:3149, 2005. Viriyakosol, S., Tobias, P.S., Kirkland, T.N. Mutational analysis of membrane and soluble forms of human MD-2. J. Biol. Chem. 281:11955, 2006. Molecular Mechanisms of Host-Pathogen Interactions R.J. Ulevitch, V.V. Kravchenko, C. Fearns, T.-H. Chuang, J.C. Mathison, Q. Pan, J. da Silva Correia, K. Iwata, K.D. Janda, G. Kaufmann, M. Meijler nfection by microbial pathogens often sets in motion chains of events that cause severe injury to the host, and nowhere is this phenomenon illustrated more dramatically than in the response by humans to infection by gram-negative bacteria. In his book Lives of a Cell, Lewis Thomas characterized the host response to the endotoxin, or lipopolysaccharide, of gram-negative bacteria as being “read by our tissues as the very worst I THE SCRIPPS RESEARCH INSTITUTE of bad news. . . . There is nothing intrinsically poisonous about endotoxin, but it must look awful, or feel awful, when sensed by cells. Cells believe that it signifies the presence of gram-negative bacteria, and they will stop at nothing to avoid this threat.” In other words, the innate immune response to infection has caused a serious disease in humans. Clearly, much human suffering could be eased if such overzealous host responses could be tempered. However, such responses, when not overzealous, are a normal part of the host’s homeostatic mechanisms, designed to respond to the threat of infection by gramnegative bacteria. Accordingly, we are attempting to (1) define the mechanisms of innate immunity and (2) learn how to control these responses without compromising host defenses against pathogens. Recently, we contributed to the understanding of innate immunity through studies of Toll-like receptors (TLRs) and of effector mechanisms that mediate host responses to infection. It is now well appreciated that the innate immune system is positioned at the intersection of multiple host pathways, including those for microbial and viral recognition, enhancement of adaptive immune responses, and, possibly, cancer immunosurveillance. Each pathway depends on ligand recognition by specific cellular receptors that are either membrane bound (plasma membrane as well as endosomal compartments) or cytosolic. The most important class of membrane-bound receptors are the TLRs. Among cytosolic receptors, an important family known as the NLR/ Nod/Caterpillar family has been identified. Within this family, 2 proteins, Nod1 and Nod2, are involved in recognition of bacterial ligands distinct from the ligands for TLRs. Activation of TLR and Nod signaling pathways leads to production of multiple cytokines with proinflammatory and anti-inflammatory activities. Such responses are central to host responses to infection. However, when a breakdown occurs in the normal regulatory mechanisms that control these pathways, disease may result. Perhaps the most well-understood link between innate immunity and human disease is in the host response to infection. When dysregulation of innate immune responses occurs, clinical syndromes such as septic shock and acute respiratory distress syndrome ensue. Dysregulation of innate immune responses may also play a role in human diseases in which chronic inflammation is responsible for disease progression, including autoimmune and autoinflammatory diseases. Genetic studies in humans have revealed strong associations IMMUNOLOGY 2006 among various members of the Nod family of proteins and human diseases. During the past year, we made considerable progress in several different areas. We have identified several unique pathways of innate immunity. These findings are briefly summarized here. C A S PA S E 1 2 A S A N E G AT I V E R E G U L AT O R O F I N N AT E I M M U N I T Y Caspases function in both apoptosis and inflammatory cytokine processing and thereby have a role in resistance to sepsis. During the past year we described a novel role for a caspase in dampening responses to bacterial infection. We showed that in mice, gene-targeted deletion of caspase-12 makes animals resistant to peritonitis and septic shock. The resulting survival advantage was conferred by the ability of the caspase12–deficient mice to clear bacterial infection more efficiently than did wild-type littermates. Caspase-12 dampened the production of the proinflammatory cytokines IL-1β, IL-18 (IFN-γ–inducing factor), and IFN-γ, but not TNF-α and IL-6, in response to various bacterial components that stimulate TLR and Nod pathways. The IFN-γ pathway was crucial in mediating survival of caspase-12–deficient mice that had sepsis; administration of neutralizing antibodies to IFN-γ receptors ablated the survival advantage that otherwise occurred in these animals. Mechanistically, caspase-12 associated with caspase-1 and inhibited its activity. Notably, the protease function of caspase-12 was not necessary for this effect, because the catalytically inactive caspase-12 mutant Cys299Ala also inhibited caspase-1 and IL-1β production to the same extent as did wild-type caspase-12. In this regard, caspase-12 seems to be the counterpart of cFLIP, an antiapoptotic protein, for regulating the inflammatory branch of the caspase cascade. In mice, caspase-12 deficiency confers resistance to sepsis, and its presence exerts a dominant-negative suppressive effect on caspase-1, resulting in enhanced vulnerability to bacterial infection and septic mortality. These findings have broad implications for new therapies for sepsis and related problems. HOMOSERINE LACTONES AND HOST IMMUNITY Receptors in the innate immune system function as sensors of infection and trigger the immune responses through ligand-specific signaling pathways. The ligands are pathogen-associated products, such as components of bacterial walls and viral nuclear acids. A common response to such ligands is the activation of mitogen- THE SCRIPPS RESEARCH INSTITUTE 147 activated protein kinase p38; double-stranded viral RNA additionally induces the phosphorylation of eukaryotic translation initiation factor 2α. Now we have shown that p38 and phosphorylation of eukaryotic translation initiation factor 2α are 2 biochemical markers of the effects induced by N-(3-oxo-acyl)homoserine lactones, the secreted products of a number of gram-negative bacteria, including Pseudomonas aeruginosa, an opportunistic pathogen in humans. Furthermore, N-(3-oxo-dodecanoyl)homoserine lactone induces distension of mitochondria and the endoplasmic reticulum and transcription of the gene for c-jun. These effects occur in a wide variety of cell types, including alveolar macrophages and bronchial epithelial cells, and require the structural integrity of the lactone ring motif and its natural stereochemistry. These findings suggest that N-(3-oxo-acyl)homoserine lactones might be recognized by receptors of the innate immune system. However, we found that signaling mediated by N-(3-oxo-dodecanoyl)homoserine lactone does not require the presence of the canonical innate immune system receptors, TLRs or Nod1 and Nod2. These data offer a new understanding of the effects of N-(3-oxo-dodecanoyl)homoserine lactone on host cells and its role in persistent airway infections caused by P aeruginosa. Our results should be useful in the development of new therapeutic interventions for devastating diseases such as cystic fibrosis in which P aeruginosa or other homoserine lactone–producing organisms are important in disease progression. ROLE OF NOD1 IN THE GROWTH OF ESTROGENSENSITIVE BREAST TUMOR CELL LINES Nod1, a cytosolic protein that senses ligands containing meso-diaminopimelic acid derived from peptidoglycan, plays a role in host responses to invasive bacteria. We have identified a novel function for Nod1: control of tumor formation. We used cell lines derived from the human breast cancer epithelial cell line MCF-7 in a xenograft model of mice with severe combined immunodeficiency to characterize a pathway linking Nod1 to the growth of estrogen-sensitive tumors. In MCF-7 cells, the absence of Nod1 correlates with tumor growth, an increased sensitivity to estrogen-induced cell proliferation, and a failure to undergo Nod1-dependent apoptosis. Conversely, overexpression of Nod1 in MCF-7 cells results in inhibition of estrogen-dependent tumor growth and reduction of estrogen-induced proliferative responses in vitro. We are using a combination of genetics and protein pathway 148 IMMUNOLOGY 2006 mapping to investigate the molecular details of this Nod1 pathway. PUBLICATIONS da Silva Correia, J., Miranda, Y., Austin-Brown, N., Hsu, J., Mathison, J., Xiang, R., Zhou, H., Li, Q., Han, J., Ulevitch, R.J. Nod1-dependent control of tumor growth. Proc. Natl. Acad. Sci. U. S. A. 103:1840, 2006. Kravchenko, V.V., Kaufmann, G.F., Mathison, J.C., Scott, D.A., Katz, A.Z., Wood, M.R., Brogan, A.P., Lehmann, M., Mee, J.M., Iwata, K., Pan, Q., Fearns, C., Knaus, U.G., Meijler, M.M., Janda, K.D., Ulevitch, R.J. N-(3-Oxy-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J. Biol. Chem. 281:28822, 2006. Pan, Q., Kravchenko, V., Katz, A., Huang, S., Ii, M., Mathison, J.C., Kobayashi, K., Flavell, R.A., Schreiber, R.D., Goeddel, D., Ulevitch, R.J. NF-κB-inducing kinase regulates selected gene expression in the Nod2 signaling pathway. Infect. Immun. 74:2121, 2006. Raetz, C.R., Garrett, T.A., Reynolds, C.M., Shaw, W.A., Moore, J.D., Smith, D.C., Jr., Ribeiro, A.A., Murphy, R.C., Ulevitch, R.J., Fearns, C., Reichart, D., Glass, C.K., Benner, C., Subramaniam, S., Harkewicz, R., Bowers-Gentry, R.C., Buczynski, M.W., Cooper, J.A., Deems, R.A., Dennis, E.A. Kdo2-lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J. Lipid Res. 47:1097, 2006. Saleh, M., Mathison, J.C., Wolinski, M.K., Bensinger, S.J., Fitzgerald, P., Droin, N., Ulevitch, R.J., Green, D.R., Nicholson, D.W. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature 440:1064, 2006. Wirz, S.A., Tobias, P.S., Ulevitch, R.J., Aribibe, L., Bartfai, T. TLR2 is required for the altered transcription of p75NGF receptors in gram positive infection. Neurochem. Res. 31:297, 2006. Prion Diseases: Insights Into the Biology of an Infectious Protein L. Solforosi, A. Bellon, Z. Cheng, P. Sidiropoulos, G. Abalos, J. Cruite, R.A. Williamson he prion diseases, or transmissible spongiform encephalopathies, are diseases of protein conformation that cause profound neurodegeneration and death. They include bovine spongiform encephalopathy, also known as mad cow disease; scrapie in sheep; and chronic wasting disease, which is spreading rapidly in deer and elk within the United States. The epidemic of bovine spongiform encephalopathy in the United Kingdom predated the emergence of a variant form of Creutzfeldt-Jacob disease (vCJD) in humans, the incidence of which can be most readily explained by the consumption of foods contaminated with the prion that causes bovine spongiform encephalopathy. The transmission of vCJD prions via blood products obtained from apparently healthy donors in whom vCJD later developed has reignited concern about the widespread dissemination of prions in humans. Alarmingly, retrospective immunocytochemical detection of prion disease T THE SCRIPPS RESEARCH INSTITUTE in archived tissue samples suggests that at least 3000 persons in the United Kingdom are currently incubating vCJD asymptomatically. Uniquely, the infectious agent in transmissible spongiform encephalopathies, the prion, is though to be composed largely of PrP Sc, an abnormally shaped version of the cellular prion protein PrPC, a molecule of unknown function found in all healthy individuals. Once established within an infected host, prions replicate by converting the normal PrPC form of the protein into additional molecules of the disease-associated form. Over time, PrPSc accumulates in the CNS, and its appearance is closely associated with profound neuropathologic changes. In the favored model of prion propagation, PrPSc acts as a template, sequestering endogenous PrPC and triggering its conformational rearrangement into nascent PrPSc and prion infectivity. Although almost none of the molecular details of this pivotal process are understood, the persistence of individual prion strains (each of which is associated with a distinct disease phenotype) suggests that assembly of the prion replicative complex is a tightly choreographed process. Implicit in this view of prion propagation is a direct and specific binding interaction between PrP C and PrP Sc . To systematically map defined regions of PrP sequence that bind tightly to PrP Sc , we generated a large and comprehensive panel of motif-grafted recombinant antibodies containing successive and overlapping polypeptide grafts that collectively span PrP residues 19–231. In PrPSc-binding studies conducted under stringent conditions with these hybrid-antibody reagents, we identified 3 distinct and independent high-affinity PrPSc recognition motifs. The first of these binding motifs lies at the N-terminal region of the mature PrP molecule, within residues PrP 23–33; the second motif, within PrP residues 98–110; and the third, within PrP residues 136–158. Mutational analyses of these PrP Sc -binding regions revealed that reactivity of the 23–33 and 98–110 PrP peptide segments depends largely on the presence of multiple positively charged amino acid residues. Intriguingly, in an acidic environment akin to that found intracellularly within the endocytic pathway in which PrPSc is found, PrP grafts corresponding to the N-terminal region of PrP, between residues 29 and 100, also acquire the ability to strongly recognize misfolded PrP conformers. These studies yield new insight into critical peptidic components composing one face of the IMMUNOLOGY 2006 prion replicative interface and suggest the potential for a 2-phase PrPC-PrPSc binding interaction that is pH dependent. Importantly, elucidating how these different PrP conformers interact now enhances the prospect of efficiently inhibiting their association and thereby halting prion replication and disease. Endoglin (CD105) as a Target for a DNA Vaccine Against Breast Cancer S.H. Lee, N. Mizutani, M. Mizutani, Y. Luo, H. Zhou, C.D. Kaplan, R. Xiang ntiangiogenic therapy has become an attractive concept for tumor therapy because the growth of new capillary blood vessels from preexisting vasculature is an essential feature of tumor growth and metastasis. The goal of this approach has been to deliver antiangiogenic agents to appropriate targets in the tumor vasculature to eliminate or suppress the blood supply to tumors, ablating or suppressing growth of the tumors without seriously disturbing blood flow to normal tissues. Endoglin (CD105) is a suitable target for such an antiangiogenic strategy because it is a coreceptor in the transforming growth factor β (TGF-β) receptor complex that is overexpressed on proliferating endothelial cells in the neovasculature of breast tumors. CD105 and its ligand TGF-β are important modulators of angiogenesis, and expression of endoglin on proliferating endothelial cells is upregulated by TGF-β and hypoxic conditions. In solid tumors, such as breast cancer, endoglin is almost exclusively expressed on endothelial cells of both peritumoral and intratumoral blood vessels and on tumor stromal components. We tested the hypothesis that antiangiogenic or antitumor effects can be achieved in a prophylactic setting by using an oral DNA vaccine encoding murine endoglin carried by double-attenuated Salmonella typhimurium to a secondary lymphoid organ, that is, Peyer’s patches in the small intestine. We found that this DNA-based vaccine elicited the activation of antigen-presenting dendritic cells and induced immune responses mediated by CD8 + T cells against endoglinpositive murine endothelial cells. For these studies, we used a syngeneic model of D2F2 murine breast carcinoma cells, which do not express endoglin. A THE SCRIPPS RESEARCH INSTITUTE 149 Expression of endoglin after oral vaccination was verified in Peyer’s patches by using confocal microscopy, indicating that CD11c+ dendritic cells express endoglin intracellularly. Moreover, the endoglin vaccine markedly suppressed D2F2 breast tumor metastases to the lung, resulting in a 60% prolongation in life span. In vivo, suppression of pulmonary metastases was abrogated by depletion of CD8+ T cells but not by depletion of CD4+ T cells. Antitumor activity of the vaccine correlated with T-cell activation as indicated by upregulation of CD28 and with activation of dendritic cells as indicated by increased expression of CD80 and CD86 on CD11c + dendritic cells. Immunization with the DNA vaccine evoked the generation of endoglin-specific cytotoxic T lymphocytes that lysed endoglin-positive murine endothelial cells. Importantly, tumor angiogenesis was markedly suppressed in Matrigel assays, indicating a significant decrease in neovascularization only in mice immunized with the endoglin vaccine. Taken together, our data suggest that a CD8 + T cell–mediated immune response effectively suppressed dissemination of pulmonary metastases of D2F2 breast carcinoma cells by eliminating proliferating endothelial cells, causing suppression of angiogenesis in the tumor vasculature. We anticipate that vaccine strategies such as this one will contribute to future therapies for breast cancer. PUBLICATIONS Lee, S.H., Mizutani, N., Mizutani, M., Luo, Y., Zhou, H., Kaplan, C.D., Kim. S.W., Xiang, R., Reisfeld, R.A. Endoglin (CD105) is a target for an oral DNA vaccine against breast cancer. Cancer Immunol. Immunother. 55:1565, 2006. Zhou, H., Luo, Y., Mizutani, M., Mizutani, N., Reisfeld, R.A., Xiang R. T cellmediated suppression of angiogenesis results in tumor protective immunity. Blood 106:2026, 2005. 150 IMMUNOLOGY 2006 The Membrane-Proximal External Region of gp41 and HIV Type 1 Vaccine Design J.D. Nelson, R. Jensen, I.A. Wilson, P.E. Dawson, D.R. Burton, M.B. Zwick IV type 1 (HIV-1) is a major world health problem for which the preferred solution is an effective vaccine. However, typical candidates for HIV-1 vaccines have not come close to eliciting the concentration of neutralizing antibodies associated with protection. HIV-1 neutralizing antibodies target the envelope glycoproteins gp120 and gp41, which assemble as a trimer, (gp120-gp41)3, on the surface of the virion. We have shown that of the roughly 6 reported broadly neutralizing monoclonal antibodies against HIV-1, 3 of them, 2F5, 4E10, and Z13, bind to the membrane-proximal external region (MPER) of gp41. These antibodies, particularly 4E10, can neutralize primary isolates of HIV-1 from around the globe with remarkable breadth and potency. Clearly, it would be desirable to exploit the MPER, and gp41 in general, for vaccine development, but eliciting neutralizing antibodies against gp41 has not been straightforward. One problem is that the structure of gp41 in the native trimer is unknown. Another difficulty is that the envelope trimer is labile and produces nonfunctional forms of gp41, including gp120-gp41 monomers and “stumps” of gp41 from which gp120 has been shed. Thus, the poor concentrations of neutralizing antibodies to gp41 elicited by vaccination and during natural infection apparently are due at least in part to a poor presentation to the immune system of native gp41 relative to nonfunctional forms of gp41. In collaboration with I.A. Wilson, Department of Molecular Biology, and P.E. Dawson, Department of Cell Biology, we identified a minimal peptide epitope that binds with high affinity to 4E10 and adopts a largely helical conformation in the 4E10-bound complex. To our knowledge, no structure-function analysis has been done on an antibody whose natural epitope is closer to the membrane than that of 4E10. Thus, we have an excellent opportunity not only to better understand the MPER of gp41 and its potential in HIV-1 vaccine design but also to gain insight into the role of lipid in antibody recognition near a membrane. H THE SCRIPPS RESEARCH INSTITUTE We are particularly interested now in performing a similarly detailed structure-function analysis of the monoclonal antibody Z13, because its epitope on the MPER overlaps those of 2F5 and 4E10 and therefore is important for understanding all 3 sites on gp41. By clarifying the effects that the membrane and constraints imposed by the envelope trimer have on the MPER, our findings should enable the design of HIV-1 vaccine candidates with stable, homogeneous, and nativelike presentations of the MPER of gp41. PUBLICATIONS Brunel, F.M., Zwick, M.B., Cardoso, R.M.F., Nelson, J.D., Wilson, I.A., Burton, D.R., Dawson, P.E. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J. Virol. 80:1680, 2006. Moore, P.L., Crooks, E.T., Porter, L., Zhu, P., Cayanan, C.S., Grise, H., Corcoran, P., Zwick, M.B., Franti, M., Morris, L., Roux, K.H., Burton, D.R., Binley, J.M. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80:2515, 2006. van Houten, N.E., Zwick, M.B., Menendez, A., Scott, J.K. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine 24:4188, 2006. Yuste, E., Sanford, H.B., Carmody, J., Bixby, J., Little, S., Zwick, M.B., Greenough, T., Burton, D.R., Richman, D.D., Desrosiers, R.C., Johnson, W.E. Simian immunodeficiency virus engrafted with human immunodeficiency virus type 1 (HIV1)-specific epitopes: replication, neutralization, and survey of HIV-1-positive plasma. J. Virol. 80:3030, 2006. Zwick, M.B. The membrane-proximal external region of HIV-1 gp41: a vaccine target worth exploring. AIDS 19:1725, 2005.