© Effects of length of seed chilling period and sowing date
advertisement

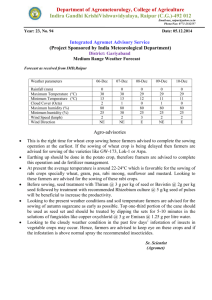
New Forests 12: 187-202, 1996. © 1996 Kluwer Academic Publishers. Printed in the Netherlands. Effects of length of seed chilling period and sowing date on family performance and genetic variances of Douglas-fir seedlings in the nursery FRANK C. SORENSEN1 1 Forestry Sciences Laboratory, Pacific Northwest Research Station, 3200 SW Jefferson Way, Corvallis, OR 97331, U.S.A. Received 16 January 1996; accepted in revised form 9 April 1996 Key words: time of emergence, date of bud set, date of bud burst, growing season length, seedling height, nursery testing, genetic testing, Pseudotsuga menziesii Application. Date of sowing and length of chilling period had a predictable effect on date of emergence, seedling phenology, and seedling size; interacted with families; and had an unpredictable effect on family and within-plot components of variance. To produce vigorous seedlings early sowing and long seed chilling are recommended. Because families interacted with treatments, it is important for genetic evaluations in the nursery that sowing date and chilling period be as consistent as possible from year to year. Abstract. Seeds of four full-sibling Douglas-fir families (F) were moist chilled (C) for 14, 33, and 77 days and sown (S) March 29, April 26, and May 24 at two densities (D = 111 and 200 seeds/m2), grown for 2 years in nursery beds and phenology and size traits recorded. The study was analyzed in two parts: part I evaluated seed treatment effects and their interactions with families; and part II investigated the effect of treatments on genetic variances, particularly among-family (u�) and within-plot (u�) components and the intraclass correlation for families C and S and F for all traits. Early S combined with long C resulted in early emergence and gave large seedlings with little loss and damage. Many interactions between C and F, and S and F, were significant. Interactions involved rank changes for size but not for phenology traits, and were larger for C x F than for S x F. Seedling density affected seedling size but not (t1). In part I there were large and highly significant differences associated with among phenology, did not interact with seed treatments, and interacted significantly but weakly with families. In part II, C and S, but not D, had significant effects on u�, u�, and t1, but not in a predictable manner. Because of significant interactions, it is recommended that standardized seed treatments be used in family nursery tests. This should aid in keeping the results from these tests as repeatable as possible. Long chilling and sowing as early as practicable are recommended to minimize disease losses and winter damages and to provide good nursery stock. Introduction Several studies have indicated, both in agricultural crops (Black and Wilkinson 1963; Alessi and Power 1975; Gray 1976; Cook 1980) and trees (Skeates 1986; Jenkinson and McCain 1993), including Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) (Sorensen 1978), that date of seedling emergence influences seedling size, as well as phenology (Dormling 1973; 188 Sorensen 1978) and survival or seedling yield (Cook 1980; Miller 1987; Jenkinson and McCain 1993). Two factors, length of growing season and synchrony between the developmental cycle of the plant and the annual climatic cycle, may be important. In addition to direct effects on first-year growth, there may be carry over effects into the subsequent year or years (Heide 1977; Sorensen 1978; Schmidt-Vogt 1995). In Douglas-fir (Sorensen 1978) and Norway spruce (Picea abies (L.) Karst.) (Dormling 1973), prove­ nances responded differently to date of sowing, and in Norway spruce to year of sowing (Schmidt-Vogt 1995). In annual crops, highly significant variety x year and variety x year x location interactions have been observed (Robin­ son and Moll 1959). These and other observations point out the intimate connection between the climatic and photoperiodic regime and the phasic developmental cycle of the plant (Rasumov 1930; Watson and Baptiste 1938; Hammerton 1975; Lawn 1979). If families react differently to these factors, it could influence genetic and environmental variances and genetic rankings in nursery evaluations (Johnson and Frey 1967). Time of emergence can be affected by date of sowing and by length of time that seeds are moist chilled or stratified prior to sowing. Chilling increases both rate and uniformity of germination, and the effect is inversely related to the incubation temperature; i.e., the lower the substrate temperature within the germinable range, the more chilling increases the speed and uniformity of germination (Weber and Sorensen 1990; Sorensen 1991). Therefore, depend­ ing on substrate temperature, genetic variances for time of emergence and for nursery growth and phenology could be affected by sowing date and chilling pretreatment. The objective of the present test was to investigate the effect of length of chilling period and date of sowing on genetic evaluation (early testing) in nurserybed trials (e.g., Robinson and van Buijtenen 1979, Bastien and Roman-Amat 1990). Particular emphasis was placed on family x sowing date and family x chilling period interactions. The test was designed so that the effect on among- and within-family variances could be analyzed. Two sowing densities were used to determine if competition influenced the magnitude of the effects, particularly effects carrying into the second year (Fowler 1984; Magnussen 1989; Mazer and Schick 1991; Miller et al. 1994). Materials and methods F amities (F) Four full-sib and unrelated families were randomly chosen from about 30 control-pollinated crosses made in a natural second-growth stand in the 189 foothills of the central Oregon Cascade Range (44°35 ' N, 122°42' W, 300 m). Number of entries was restricted to four, because of the many treat­ ments applied. Families had not been tested previously for any germination characteristics. Nursery location was 44°341 N, 123°271 W, 75 m (Corvallis, Oregon, U. S.A.). All seeds were x-rayed, and only filled, apparently healthy seeds were used in the tests. Chilling periods (C), dates of sowing (S) and densities (D) Chilling periods (14, 33, and 77 days) were spaced to be linear on the loga­ rithmic scale (Sorensen 1980, 1983). These periods span the range normally used for Douglas-fir seeds. Fourteen days is marginally adequate for germi­ nation at 25 °C, but longer periods are necessary for complete germination at lower temperatures (Allen 1960; Sorensen 1991). Seeds were soaked at room temperature (ca. 22 °C) in aerated water for 24 hours, then the water drained, and seeds placed in a cooler at 2-3 °C for the designated periods. Seeds were not treated for mold during long stratification, but none formed on them. Dates of sowing were March 29, April 26, and May 24 (28-day intervals). March 29, the first S, is a little later than the mean emergence date of autumn­ sown seeds in our nursery, which was March 11 and March 22 in two previous tests (Sorensen 1990; Sorensen and Campbell 1993), but the dates span the normal times of outdoor sowing for the area. On designated sowing days, seeds were surface dried, sown in small depressions in raised nursery beds, and covered with a thin layer (ca. 0.5 cm) of granite grit. Our usual cultural practices were followed: watering and weeding was done by hand as needed with last watering and fertilization in mid-August; balanced soluble fertilizer (20-20-20) was applied every 4 weeks at rate of 28 kg/h from time of seedcoat shed (year 1) or budfiush (year 2) to mid-August. Sowing densities were 111 (10 cm x 9 cm) and 200 (7 cm x 6.5 cm) seeds/m2 • Based on visual impression, these D would not differentially affect plant growth through shading until after bud flush in the second year. Design and analyses Experimental design included two blocks within each of two nursery beds. As described below, beds and blocks were treated as four replications in one analysis (see "Part I" below), whereas the beds were treated as the only two replications in the second analyses (Part II). Part! Experimental design was split-split-split-plot. Density treatments, D1 and D2 , were assigned to whole plots randomized within blocks. Sowing dates, 190 Si. S2 , and S3, were assigned to subplots randomized within whole plots. Chilling times, C1 , C2 , and C3, were assigned to sub-subplots randomized within subplots. Finally, families, F1 , F2 , F3, and F4, were assigned to sub-sub­ subplots randomized within sub-subplots. Each F was represented initially by an 18-seed noncontiguous plot. The test was conducted in raised nursery beds. There were two border rows between the test seedlings and the edges of the beds and eight border rows on each end of the test. Sub-subplots (different C sown on the same date) were separated by one buffer row; subplots (different S) were separated by two buffer rows, one row sown at each date; and D were separated by six buffer rows, three at each density. Split-plot design was used primarily to reduce the amount of between-plot buffering that would have been necessary without the splits. For the presentation of the results of this analysis, variability was appor­ tioned based on the percentage of total sums of squares (%SS) explained by the different sources of variation (Hicks 1982; Hiihn et al. 1987). When compared with equivalent variance components, residual %SS is usually underestimated so that the proportion of variation explained by other terms should be consid­ ered maximal (Hicks 1982). Comparison based on %SS seemed suitable for this material, because the traits represented different stages of development of the same seedlings and all analyses used the same format. Part Ila The second part of the analysis had the purpose of investigating the effect of treatments, particularly the C and S treatments, on genetic variances. Each of the 18 sub-subplots (all combinations of 2 D, 3 C, and 3 S) was analyzed as a "minitest" of the 4 F, each F represented initially by an 18-tree plot, and replicated twice within a nursery bed. The format of the analysis of variance of a single minitest is shown in Table 1. Part /lb In each of the 18 minitests, components of variance were determined for family, within-plot, block, and experimental error (block x family), and these components entered into a second analysis of variance. The goal of the analysis primarily was to determine if C and S were systematically or predictably influencing genetic variances (a} and a� in Table 1) and the intraclass correlation [tf, or a}!(aF +a�)], and if D altered the influence of C and S. Seed treatment effects on the variance components for block and exper­ imental error were small and are not presented. The analysis of variance format, which is split-split-plot, is given in Table 2. Plot coefficients of vari­ ation and skewnesses also were analyzed according to the format of Table 2. 191 Format of analyses of variance of Part Ila "minitests" - components of variance estimated for blocks (u1), families (u}), experimental error (u1p) and within plot (u�) among 4 families within each of the 18 combinations of sowing density, date of sowing, and chilling period. Variance components from the minitests are then analyzed according to the format in Table 2. Table I. are Sources of variation Degrees of freedom2 Expected mean squares 1 Total Blocks Families BxF (experimental error) Within plot error bfn-1 (b-1) (f-1) (b-l)(f-1) bf(n-1) u�+n u1F +nf u1 u�+n u1F +nb u} u�+n u1F 0"2 w 1 u2 are variance components for blocks (B), families (F) and interaction. 2 b = 2; f =4; n =seedlings per plot= 18 initially, later harmonic mean of survivors. Table 2. Part Ilb format for analyses of variance components derived from the minitests (e.g., analysis of u} 's and other components of variance obtained from each of the tests analyzed according to the format of Table l). Sources of variation Degrees of freedom2 Expected mean squares 1 Total Blocks (B) Densities (D) Error (a) beds-I (b-1) (d-1) (b-1) (d-1) u� +eds u1 u� +bes Ob 0"2 Sowing date (S) SxD Error (b) (s-1) (s-1) (d-1) d(b-1) (s-1) ut+bed 01 ut+be Oh ut Chilling period (C) CxD CxS CxDxS Error (c) (c-1) (c-1) (d-1) (c-1) (s-1) (c-1) (d-1) (s-1) ds(b-1) (c-1) u�+bds Ob u�+bs Obv u�+bd Obs u�+bObvs 0"2 c a 1 u2 is the variance component for blocks (B); 02 are fixed effects for density (D), date of sowing (S), and chilling period (C). 2 b = 2; d = 2; s = 3; c = 3. Presumably because of effective transformations (see below), neither added information to that provided by analyzing variance components alone, and they are not included in the results. Traits The following seed and seedling traits were recorded: 192 EMERG - days from sowing to emergence (seed coat or hypocotyl visible above ground surface). Observations made every second day from day of sowing. BS1 and BS2 - dates of bud set, first and second year. Observations made biweekly first year, weekly second year. Buds recorded as set when bud scales first visible between the needles of the terminal shoot. BB2-date of bud burst. Observations made every second day. Buds recorded as flushed when green needles first visible between the scales of the terminal bud. EMERG, BS1 and 2, and BB2 recorded in Julian calendar dates. GSLl and GSL2 - first- and second-year growing season lengths for the terminal shoot in days (BSl minus EMERG in year 1, and BS2 minus BB2 in year 2). H1 and H2 - heights in centimeters from ground level to base of terminal bud at end of first and second year. HI2 - height increment in centimeters during year 2 (H2 minus Hl). D2 - diameter in millimeters below cotyledons at the end of the second growing season. All measurements were tested before analysis for the need for trans­ formation, so that variance would be independent of the mean. Where transformation was necessary, log transformation was satisfactory. Results Establishment Of the 5 184 seed spots sown, 5 036 (97.1 %) emerged. Of these 53 were obvious mutants and deleted from further observation, leaving 4 984 (96.1 %) emerged usuable seedlings. At the end of the first year, 4 866 (93.9%) were alive and healthy and 4 788 (92.4%) were present at the end of year 2. Percentage of emergence by sowing date (S l is earliest) was 98.7 (S l ), 96.0 (S2), and 96.7% (S3) and by chilling period (Cl is shortest) 93.2 (Cl ), 98.7 (C2), and 99.5% (C3). Family emergence ranged from 96.0 to 98.1%. Mutant seedlings were predominantly curly needle phenotype (35 of 53), which we typically find at low frequencies in Douglas-fir beds, particularly if the seeds have been stored for several years as these had. It occurred in 193 all families, but frequencies differed among them (x2 = 10.99; d.f. 2; p 0.012). Other mutants were fused cotyledons (8 seedlings, 7 in one family) and unclassified (10 mutants). First-year mortality was 2.3% and most of it (1.9%) was due to late-season damping off. S1 had less mortality than S2 and S3 (0.5% vs 2.6 and 2.6%; x2 27.48; d.f. 2; p < 0.001). Longer C also had less mortality (1.3 and 1.4%) than Cl (3.0%) (x2 16.23; d.f. = 2; p < 0.001). The differences were small but they were consistent with other observations that early emergence decreases incidence of damage from soil-borne pathogens (Bloomberg 1973; Jenkinson and McCain 1993). = = = = = Seedling phenology and growth S and C strongly and significantly influenced seedling phenology and size. The effects on several traits and a comparison with family differences are shown in Figure 1. Early S and long C affected all traits in the same direction, except for EMERG (early S increased, long C decreased time to emerge) and H2/D2 (early S increased the size of the ratio, C had no effect). Relative importance of main effects and interactions, given as a percentage of total sums of squares, is shown in Table 3. The results were consistent with expectations and in general agreement with other reports where comparable traits have been measured. One exception is the ratio, H2/D2. Early S, compared with late S, increased the ratio in this study and decreased it in a previous one (Sorensen 1978). Some consequences and unexpected aspects of the results are pointed out below. Seeds sown later did not emerge as rapidly as expected based on temper­ ature. After long chilling (C3), the averages were 22, 18, and 18 days for S1, S2, and S3 seeds to emerge, respectively. A recording 8-day mechanical thermometer was in the nursery area. Air temperatures were summed in degree-hours (0C) above 4.4 °C from S to mean date of emergence for the C3 treatment only. Approximate degree-hours from S to EMERG were 3 500, 4 100 and 4 800 for S1, S2, and S3, respectively. The reason for the increase in degree-hours to emergence with advance of the season was not clear. Because increase of autumn frost resistance is associated with bud development (Campbell and Sorensen 1973), delayed BSl can influence susceptibility to early frosts. A frost of -5 °C on October 29 damaged terminal needles on the main shoot of 2.05% of the seedlings. The frequency of damage was not large enough to test with analysis of variance, but treat­ ment main effects (D, S, C, and F) were tested with chi-square analyses. All main effects, except D, were highly significant, with the percentage of frost-damaged seedlings increasing with later sowing and decreasing with long chilling. Family differences depended on S. With early sowing (S1), Table 3. Sums of squares and statistical significances for sources of variation in Part I analyses in seedling size and phenology traits (Table I) given in percentages of total sums of squares. Sources of variation EMERG 1 BSI Sowing date (S) Chilling period (P) 11.3***2 40.5*** 7.7*** BS2 _3 BB2 GSLI GSL2 HI H2 HI2 D2 H2/D2 30.4*** 3.5*** 68.3*** 6.1 *** 10.2*** 55.3*** 7.8*** 26.7*** 5.4*** 5.2*** 5.3*** 31.6*** 30.4*** 30.9*** Density (D) - Family (F) 4.9*** 38.4*** 67.6*** 27.3*** 5.3* 13.l*** 4.2*** 9.2* 14.8*** 65.0*** 12.8*** 34.9*** 44.4*** SxF CxF 0.8*** 2.2*** 0.3*** 0.4*** 2.3*** l.7*** 0.9** l.7*** 0.8*** 0.6** 0.4*** 0.7*** 0.9*** 1.0*** 0.5*** 1.0*** 0.3* l.7*** - 0.4 * - l.6** 0.9*** - 0.6** 0.4*** DxF SxC SxD 37.0*** - - - - - 1.3*** - - 1.0*** 0.8*** - - - 2.0*** - - - 0.9** 0.3** 0.8 ** l.9*** 0.3* 0.7** 4.6*** 0.4 * 0.7 *** 0.9** l.7 *** 29.0** 4.9*** 0.7** 2.0*** 0.8*** 1.2** CxD SxCxF - - - SxDxF 0.8** CxDxF SxCxD SxCxDxF Percent SS explained4 Residual 58.5 88.1 72.5 65.9 91.3 79.5 1.7 2.7 10.7 8.4 2.1 6.2 78.4 2.3 76.1 3.3 74.7 4.3 81.8 68.6 7.3 4.9 1 Traits are, EMERG, days from sowing to seedling emergence; BS, date of bud set; BB, date of bud burst; GSL, growing season length of terminal shoot in days; H, total height; HI, height increment; D, diameter below cotyledons; HID, height diameter ratio. Numbers I and 2 after the trait abbreviations refer to first and second year in the nursery bed. 2 Significance, *,p < 0.05; **,p < 0.01; ***,p < 0.001. 3 Value not given if mean square is not significant. 4 Percentage of total sum of squares explained by significant terms. ....... \0 ..;... 195 Cl c "i � 40 E 30 g Figure 1. Comparative effects of sowing date (S1 March 29, S2 April 26, S3 May 24) and seed chilling period (C l , C2, and C3 14, 33, and 77 days, respectively) and family (F l through F4 four biparental crosses) on several seedling phenological and size traits: EMERG, days from sowing to seedling emergence; BS and BB, date of bud set and bud burst; GSL, growing season length for terminal shoot extension; H and D, height and diameter; and FROST, percentage of seedlings frost damaged in first autumn. A number 1 or 2 after trait symbols refers to 1 or 2 years after sowing. If the effect was not significant, the overall mean value was given to all levels of the treatment (e.g., trait BS 2). = = = = = percentage of frost-damaged seedlings was 0.1 % and there was no difference among F (x2 = 3.00; d.f. = 3; p = 0.392); after S3, the range among F was zero to 10. 2% damaged seedlings, and family variance was highly significant <x2 60.48; p < 0.001). In addition to the direct effect of date of emergence on BS1 timing, there was a carryover influence on date of BB2. Seed treatments that gave seedlings = 196 with early BS1 gave seedlings with late BB2. The regression relation was close (BB2 132.7-0.149 BS l ; t 13.65; d.f. 7; p < 0.001); i.e., a 10-day advance in BSl 1.5-day delay in BB2. Early emergence and accompanying longer GSLl seemed to have a disproportionately large affect on H l . For example, mean GSLl for S3 seedlings was 122.1 days; GSLl for S l seedlings was 153.3 days or 25.6% longer. For S3 seedlings, Hl was 8.8 cm; H l for S l seedlings was 16.6 cm or 88.6% greater. Two factors could have contributed to the disproportionality. First, part of GSLl was used in seedling establishment (radicle elongation, cotyledon elongation and seed shed, and early needle elongation) before any epicotyl extension begins. The S3-Sl difference in time allotted to epicotyl extension probably was proportionately greater than was the difference in GSL as I recorded it. Also, elongation tends to be exponential, with the result that loss of GSL at the end of the extension period had a larger-than-equivalent effect on H1. Second, relative to S1 seedlings, growing season of S3 seedlings continued late into the autumn when conditions for shoot extension were less favorable. Seedling form was analyzed as the second-year height/diameter ratio. Density and sowing date had strong effects (Table 3). On average, stockier seedlings were in less dense plots and in plots whose seeds had emerged later. In an earlier study (Sorensen 1978), early emerging, early budsetting plants were stockier than late emerging plants. Because diameter increment continues after bud set, the discrepancy presumably was due to differences in dates of bud set and in conditions for growth after bud set between the two studies. In any case, seedling quality and health were not positively related to stockiness in this study, because the stockiest plants were the small seedlings from late sowing. Seedlings grown at wide spacing were significantly taller and had signifi­ cantly greater second-year height increment than seedlings grown at narrow spacing. Since GSL2 did not differ with density, the greater increment at wide spacing must have been due to greater rate of elongation. = = = = Interactions between seed treatments and families Many interactions between F and C and F and S were highly significant (Table 3). Of the interactions, C x F tended to be larger than S x F interactions, particularly for size traits, even where S main effects were larger than C main effects. Interactions for size traits, compared with phenology traits, tended more often to involve rank changes or to have more potential for rank changes. S x C x F interactions were highly significant for size traits, but insignificant for GSLl and GSL2 (Table 3). As observed in connection with BS1 and frost damage, however, even small interactions involving phenological traits can be 197 rather important if a damaging event is timed right. Density, or competition, did not affect interactions between C and F, and S and F (Table 3, 3- and 4-way interactions involving D were not significant). Family and within-family variances Part II of the study investigated the effect of seed treatments on variance components, specifically among-family (a°}) and within-plot (o-�) and on the intraclass correlation (t1) for families. F-values from the analyses of the components (see Table 2 for format) are presented in Table 4 for several traits frequently measured in nursery genetic evaluations. The F-values show that C and S do indeed affect genetic variances, but the significance of both main effects and interactions differs greatly from trait to trait. With regard to changes in variances, no pattern emerges to indicate that certain seed treat­ ments should be used for family nursery tests. The effect of seed treatments on components of variance and intraclass correlations for H2 are shown in Figure 2, which illustrates the lack of pattern. Discussion Both early S and long C promoted early emergence. In addition, long C gave more rapid, complete, and uniform emergence. With earlier emergence, first-year seedlings had a longer extension season, became taller, set buds earlier, had less loss from nursery diseases, and escaped damage from a late-October frost. In the second year, seedlings that had emerged early had a shorter extension season, but greater overall shoot extension and larger diameters. Clearly, largest and healthiest seedlings were associated with early emergence, as also has been reported for survival (Miller 1987) and seedling vigor in other species (references given in "Introduction"). The inverse relation in the second year between amount of extension and extension period is probably due to early emerging first-year seedlings setting buds early and developing more preformed growth (Tranquillini et al. 1980). At the same time, the longer year-2 extension period of seedlings that emerged late the first year suggested a compensation mechanism that dampens early environmentally caused size differences. This is apparently analogous to the seed-weight environmental effect on Douglas-fir seedling size, which also was observed to decrease over time (Sorensen and Campbell 1993). Long C is particularly important if the seeds are sown early when the nursery soil is cold (McLemore 1969; Tanaka et al. 1986; Sorensen 1990; Weber and Sorensen 1990; Barnett 1993). Compared to short C, long C 198 Table 4. Listing of F-values and their significances for sources of variation (date of sowing, chilling period, and sowing density) that affected among-family and within-plot components of variance and the intraclass correlation coefficient for families in several seedling size and phenology traits. A significant F-value means that the indicated source of variation influenced the among-family or within-plot components of variance or influenced the intraclass correlation coefficient. Sources of variation BS11 BB2 BS2 HI H2 D2 A. Among-family component of variance (a}) Date of sowing (S) 3.15 9.90** 5.71*2 0.54 7.96** 2.89 7.09** 2.30 0.73 4.45* 2.59 2.74 2.15 2.40 1.25 2.99* 2.35 1.10 0.29 3.67* 0.68 S x C 0.06 6.08** S x D 4.34* 1.79 0.85 0.13 0.25 0.04 C x D 0.30 0.14 0.26 0.18 0.81 0.98 S x C x D 1.14 0.39 0.58 0.15 0.73 0.73 Chilling period (C) Sowing density (D) B. Within-plot component of variance (a;) Date of sowing (S) 4.56* Chilling period (C) 0.45 0.70 3.86* Sowing density (D) 0.99 12.51** 3.13 0.54 7.42* S x C 1.65 0.45 0.62 16.93*** 0.88 15.28*** 10.00*** 28.25*** 0.33 3.08 3.11* 2.69 6.05** 0.55 6.68** S x D 3.76* 1.51 0.28 0.18 0.96 3.61* C x D 1.05 3.94 0.39 0.18 0.12 0.10 S x C x D 1.04 0.76 0.87 0.28 0.51 0.71 2.05 6.68** 5.57* 4.48* 3.85 5.68** C. Intraclass correlation coefficient [t1 = a}/(u} + 5.18* u;)] Chilling period (C) 6.73** 5.47* 0.04 3.02 Sowing density (D) 0.03 0.00 0.68 S x C 2.64 0.77 5.66* 4.17* 2.76 0.15 4.59** Date of sowing (S) 1 2.80 0.80 23.32*** 11.38*** S x D 1.28 0.82 1.73 0.51 0.65 1.91 C x D 2.28 0.84 1.37 3.94* 1.85 4.11* S x C x D 1.75 0.59 1.00 0.59 0.53 1.35 Traits are, BS 1 and BS2, dates of bud set, years 1 and 2; BB =date of bud burst; H1, H2 and D2, total heights or diameters, year 1 or year 2. 2 Significance of F-values, *, p < 0.05; **, p < 0.01; ***, p < 0.001. both increases rate of emergence and reduces the variation among seeds in emergence time. Long C is still beneficial but less important under conditions when the substrate is warm. Seed treatment x family interactions for height were similar to those observed for provenances (Sorensen 1978). Different genetic entries appar­ ently differ in sensitivity to date of emergence. In the current family test, the same family (Fl) showed the least difference in response to both S and C. 199 0.04 Oc1· II C2 .C3 0.03 2 A F CJ 0.02 0.01 0.06 �-------� B 0.05 0.04 2 w CJ 0.03 0.02 0.01 o.s ..---, 0.5 t, 0.4 0.3 $1 $2 S3 (ai) and within-plot (a�) components of variance and intraclass correlation coefficients [tf = ai/(ai + a�)] for families for second-year height (loge cm) after 3 dates of sowing (SI = March 29, S2 = April 26, S3 = May 24) and 3 seed chilling periods (Cl, C2, and C3 14, 33, and 77 days, respectively). Figure 2. Among-family = 200 Because of the effect of seed treatments on both rate and uniformity of emergence, it was anticipated that varying S and C might alter the relation between a} and a� in the seedling traits (Prout and Barker 1989; Mazer and Schick 1991). Although both S and C did indeed influence a� , a� , and t1 (the intraclass correlation coefficient for families), they did so mostly in an unpredictable way (e.g., Figure 2). The only apparent generality was that long C caused the effect of S on a} and a � to be small (Figure 2, A and B). Conclusions The study was designed to investigate the effect of C, S, and D on family evaluations in the nursery. Different C and S caused family rank changes for size but not for phenological traits. Interactions were larger for C x F than for S x F and smallest for D x F. C and S affected among-family and within-plot variances, but not in a predictable manner, except that long C reduced the effect of S on variances. Consistent C and S are needed for consistent genetic evaluations and long C and early S are recommended because they promoted early emergence, and gave the largest seedlings with least disease incidence and least autumn frost damage. Independent observation in Weyerhaeuser Company Douglas-fir nurseries support these recommendations (Yasuomi Tanaka, 1995, personal communication). Pacific Northwest seed plants and nurseries stratify Douglas-fir seeds up to 13 weeks. A caution with longer chilling is that seeds with low stratification requirements can germinate during chilling (Tanaka 1976) if seeds have to be held for any reason after an already long chilling. Outdoor nursery sowing times are dictated by rainfall and frost. Early emerging (late February to early March) seedlings from autumn sowing have been damaged by spring frost in our beds. Sowing probably could be advanced 2-3 weeks prior to the March 29 date used in this study, but this will vary with nursery. The C3/S1 treatment seemed optimal or nearly so for our conditions, but the results indicated that longer chilling and earlier sowing both could be beneficial provided they stayed with safe limits for the particular material and nursery (Jenkinson et al. 1993). Acknowledgments Richard S. Miles maintained the test and made many of the measurements; Nancy Mandel ran and summarized many analyses; Roger G. Petersen reviewed the statistical procedures. Yasuomi Tanaka and three anonymous referees provided helpful comments on an earlier draft. Their contributions are gratefully acknowledged. 201 References Alessi, J. and Power, J.F. 1975. Response of an early-maturing com hybrid to planting date and population in the Northern Plains. Agron. J. 67: 762-765. Allen, G.S. 1960. Factors affecting the viability and germination behavior of coniferous seed. IV. Stratification period and incubation temperature, Pseudotsuga menziesii (Mirb.) Franco. For. Chron. 36: 18-29. Barnett, J.P. 1993. Presowing treatments affect shortleaf pine seed germination and seedling development. Tree Planters' Notes 44: 58--62. Bastien, J.Ch. and Roman-Amat, B. 1990. Predicting Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco) volume at age 15 with early trails. Silvae Genet. 39: 29-35. Black, J.N. and Wilkinson, G.N. 1963. The role of time of emergence in determining the growth of individual plants in swards of subterranean clover (Trifolium subterraneum L.). Austral. J. Agric. Res. 14: 628--638. Bloomberg, W.J. 1973. Fusarium root rot of Douglas-fir seedlings. Phytopathology 63: 337341. Campbell, R.K. and Sorensen, F.C. 1973. Cold-acclimation in seedling Douglas-fir related to phenology and provenance. Ecology 54: 1148-1151. Cook, R.E. 1980. Germination and size-dependent mortality in Viola blanda. Oecologia 47: 115-117. Dormling, I. 1973. Photoperiodic control of growth and growth-cessation in Norway spruce seedlings. IUFRO Working Party 2.01.4, Symposium on Dormancy in Trees. K6rnik, Poland, Sept. 5-9, 1973. 16 pp. Fowler, N.L. 1984. The role of germination date, spatial arrangement, and neighbourhood effects in competitive interactions in Unum. J. Ecol. 72: 307-318. Gray, D. 1976. The effect of time to emergence on head weight and variation in head weight at maturity in lettuce (Lactuca sativa). Ann . Appl. Biol. 82: 569-575. Hammerton, J.L. 1975. Experiments with Cyperus rotundus L. III. Seasonal variations in growth. Weed Res. 15: 339-348. Heide, 0. M. 1977. Regulering av vekst of kvile hos ulike 0kotyper av gran. (Control of growth and dormancy in Norway spruce ecotypes.), pp. 1-10. In: Dormling, I. and Eriksson, G. (Eds) Experimental Genekologi. Department of Forest Genetics, Royal College of Forestry, Stockholm. Research Note 27. (Swedish with English summary and captions.) Hicks, C.R. 1982. Fundamental Concepts in the Design of Experiments. 3rd edition. Holt, Rinehart and Winston. New York. HUhn, M., Kleinschmit, J., Svolba, J. 1987. Some experimental results concerning age depen­ dency of different components of variance in testing Norway spruce (Picea abies [L.] Karst.) clones. Silvae Genet. 36: 68-71. Jenkinson, J.L. and McCain, A.H. 1993. Winter sewings produce 1--0 sugar pine planting stock in the Sierra Nevada. USDA Forest Serv., Pacific SW Res. Sta., Berkeley, Calif., Res. Pap. PSW-RP-219. 10 p. Jenkinson, J.L., Nelson, J.A., Huddleston, M.E. 1993. Improving planting stock quality-the Rumbold experience. USDA Forest Serv., Pacific SW Res. Sta., Berkeley, Calif., Gen. Tech. Rep. PSW-GTR-143. 219 p. Johnson, G.R. and Frey, K.J. 1967. Heritabilities of quantitative attributes of oats (Avena sp.) at varying levels of environmental stress. Crop Sci. 7: 43-46. Lawn, R.J. 1979. Agronomic studies on Vigna spp. in south-eastern Queensland. I. Phenological response of cultivars to sowing date. Austral. J. Agric. Res. 30: 855-870. Magnussen, S. 1989. Effects and adjustments of competition bias in progeny trials with single­ tree plots. For. Sci. 35: 532-547. Mazer, S.J. and Schick, C.T. 1991. Constancy of population parameters for life-history and floral traits in Raphanus sativus L. II. Effects of planting density on phenotype and heri­ tability estimates. Evolution 43: 1888-1907. 202 McLemore, B.F. 1969. Long stratification hastens germination of loblolly pine seed at low temperatures. J. For. 67: 419-420. Miller, T.E. 1987. Effects of emergence time on survival and growth in an early old-field plant community. Oecologia 72: 272-278. Miller, T.E., Winn, A.A. and Schemske, D.W. 1994. The effects of density and spatial distri­ bution on selection for emergence time in Prune/la vulgaris (Lamiaceae). Am. J. Bot. 81: 1-6. Prout, T. and Barker, J.S.F. 1989. Ecological aspects of the heritability of body size in Drosophila buzzatii. Genetics 123: 803-813. Rasumov, V.I. 1930. U ber die photoperiodische Nachwirkung in Zusammenhang mit der Wirkung verschiedener Aussaatterminen auf die Pflanzen. Planta 10: 345-377. Robinson, H.F. and Moll, R.H. 1959. Implications of environmental effects on genotypes in relation to breeding, pp. 24-31. Proc. 14th Annual Hybrid Corn Industry Research Conference. Robinson, J.F. and van Buijtenen, J.P. 1979. Correlation of seed weight and nurserybed traits with 5-, 10-, and 15-year volumes in a loblolly pine progeny test. For. Sci. 25: 591-596. Schmidt-Vogt, H. 1995. EinfluB der Umweltbedingungen auf die Jugendentwicklung von Fichten-Provenienzversuchen (Picea abies (L.) Karst.). Alig. Forst- u. Jagdztg 166: 121127. Skeates, D.A. 1986. Time of germination: a factor in rate of growth of accelerated transplants of black spruce (Picea mariana (Mill) B.S.P.). Tree Planters' Notes 37(1): 20-24. Sorensen, F.C. 1978. Date of sowing and nursery growth of provenances of Pseudotsuga menziesii given two fertilizer regimes. J. Appl. Ecol. 15: 273-279. Sorensen, F.C. 1980. Effect of date of cone collection and stratification period on germination and growth of Douglas-fir seeds and seedlings. USDA Forest Serv., Pacific NW Forest and Range Exp. Sta., Portland, Oregon. Res. Note PNW-346. 11 p. Sorensen, F.C. 1983. Relationship between logarithms of chilling period and germination or bud flush is linear for many tree species. For. Sci. 29: 237-240. Sorensen, F.C. 1990. Stratification requirements for germination of western larch (Larix occi­ dentalis Nutt.) seed. USDA Forest Serv., Pacific NW Research Sta., Portland, Oregon. Res. Note PNW-RN-493. 11 p. Sorensen, F.C. 1991. Stratification period and germination of Douglas-fir seed from Oregon seed orchards: two case studies. USDA Forest Serv., Pacific NW Research Sta., Portland, Oregon. Res. Note PNW-RN-499. 22 p. Sorensen, F.C. and Campbell, R.K. 1993. Seed weight - seedling size correlation in coastal Douglas-fir: genetic and environmental components. Can. J. For. Res. 23: 275-285. Tanaka, Y. 1976. Stratification and other pre-treatments of Douglas-fir for nursery germination, pp. 163-173.In: Hatano, K., Asakawa, S., Katsuta, M., et al. (Eds) Proceedings ofIUFRO International Symposium on Physiology of Seed Germination. Fuji, Japan. Tanaka, Y., Kleyn, N.J., Harper, L.M. 1986. Seed stratification of Engelmann spruce and lodgepole pine: the effect of stratification duration and timing of surface-drying. For. Chron. 62: 147-151. Tranquillini, W., Lechner, F., Oberarzbacher, P., Unterholzner, L. and Holzer, K. 1980. Uber das HOhenwachstum von Fichtenklonen in verschiedener Seeh1>he. Mitt. der forstl. Bundes­ Versuchsanst. Wien. 129: 7-25. Watson, D.J. and Baptiste, E.C.D. 1938. A comparative physiological study of sugarbeet and mangold with respect to growth and sugar accumulation. I. Growth analysis of the crop in the field. Ann. Bot. 2: 437-480. Weber, J.C. and Sorensen, F.C. 1990. Effects of stratification and temperature on seed germi­ nation speed and uniformity in central Oregon ponderosa pine (Pinus ponderosa Doug!. ex Laws.). USDA Forest Serv., Pacific NW Research Sta., Portland, Oregon. Res. Pap. PNW-RP-429. 13 p.