BoT. GAz. 136(3): 290-298. 1975. © 1975 by The University of Chicago. All rights reserved. PHENOLOGY OF BUD BURST IN DOU GLAS-FIR RELATED TO PROVENAN CE '
PHOTOPERIOD, CHILLI NG, AND FLUSHING TEMPERATURE
. ROBERT K, CAMPBELL AND ALBERT I. SUGANO
United States Department of Agriculture, Pacific Northwest Forest and Range Experiment Station '
Forestry Sciences Laboratory, Corvallis, Oregon 97331
ABSTRACT
Feasibility of deye '?pin& a method for predicting timing of spring vegetative bud burst in Douglas-fir
(Pseudotsuga llleiiZtestt (Mirb.) Franco) was assessed. Several provenances from western Washington and
Oregon
were examined in three growth-ch 3;mber experiments that used combinations of chilling, photo­
.
penod, and temperature as treatments durmg dormant and postdormant phases. Quantitative effects of
reatme!lts on rate of bud development vere complex· and differed among provenances, giving rise to many
mteractwns. However, responses were similar for all provenances. A conceptual model is proposed for the
action of temperature and photoperiod on timing of flushing in Douglas-fir.
Introduction
Synchronization of developmental and annual
climatic cycles is a feature of the adaptation of many
perennial plants to variable seasonal climates. It is
apparently accomplished by reaction of population
genotypes to environmental stimuli (VEGIS 1964).
The mechanism is by no means understood by
genecologists. Of particular interes t to the authors is
the process by which subpopulations of species with
large continuous distributions adjust to variable
climates associated with mountainous topography.
A method for predicting phenological timing would
be helpful. As a first s tep, we chose to study vegeta­
tive bud flushing in seedling Douglas-fir (Pseudotsuga
menziesii (Mirb.) Franco).
Our working hypothesis (H ESLOP-H ARRISON 1964,
p. 228) was that timing of bud flushing operates
through the interdependent action of winter chilling,
spring photoperiod, and temperature during flushing.
All conifer species tested have been reported to have
chilling requirements for dormancy release (CoviLLE
1920; W oMMACK 1964; NIENSTAEDT 1966, 1967;
W oRRALL and MERGEN 1967; DoRMLING, G usTAFS­
soN, and VoN W ETTSTEIN 1968; S TEINHOFF and
H oFF 1972). Long photoperiods sometimes com­
pensate for chilling requirements ( GusTAFSON 1938;
OLSON, S TEARNS, and N IENSTAEDT 1959; N IENSTAEDT
1966, 1967; WoRRALL and MERGEN 1967). It has
qeen suggested that bud flushing is completely
temperature mediated if chilling requirements have
been satisfied (W AREING 1956), but interacting
quantitative effects of photoperiod and chilling on
rate of bud flush have been clearly shown in several
experiments (e.g., J ENSEN and G ATHERUM 1965;
N IENSTAEDT 1967; WoRRALL and MERGEN 1967;
D ORMLING et al. 1968; FARMER 1968).
If environmental effects on rate of bud flushing are
interdependent as hypothesized, a first step in
elucidating adapta tional mechanisms is to discover
if provenances are genetically differentiated in their
response to combinations of treatments. Previous
290
work on bud flushing in Douglas-fir has shown
provenance-differentiation in effects of photoperiod
( IRGENS-MOLLER 1957), photoperiod and chilling
(WoMMACK 1964), and temperature during the
flushing period ( C AMPBELL 1974). Two questions
remain: (1) Are differences among provenances in
response to spring flushing temperatures modified by
pho toperiod, or by timing or duration of winter
chilling; and (2) do provenances react similarly to
fluctuating versus constant temperatures during
flushing?
This paper reports three experiments designed to
answer these questions and also to provide informa­
tion on the feasibility of developing a model for
bud-burs t timing.
Material and methods
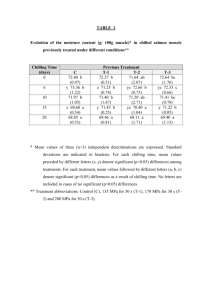
A provenance sample ( table 1) consisted of a
mixture of an equal number of wind-pollinated seed
from each of five seed trees. In early March, seeds
from samples were soaked in water for 24 h, drained,
stratified at 2 C for 14 days, and germinated on
moist filter paper in petri dishes at 30 C day/21 C
night, with 12 h per photoperiod and thermoperiod.
Germinated seeds were planted in 11.5-cm half-liter
plastic pots containing Willamette River bottom
loam mixed with 1/3 peat by volume. Early losses
were replanted to maintain six plants per pot.
Seedlings were grown in the greenhouse until late
May, then moved into a lathhouse to grow through
the summer under SO% shade.
Treatments usually started in September after all
seedlings had set buds and ended on the date of bud
flushing, determined for each seedling by observa­
tions made every 3 1/2 days (i.e., Monday A.M. and
Thursday P.M. ) . Lighting in growth chambers and
supplemental lighting in the greenhouse were from
fluorescent and incandescent lamps.
Designs used for the three experiments were all
modifications of the nested design in which subplot
treatments consisting of a factorial set were ran­
291
CAMPBELL & SUGANO-PHENOLOGY OF BUD BURST IN DOUGLAS-FIR
TABLE 1
LO CATIONS OF W EATHER BUREAU STATIONS AT WHICH PROVENANCE COLLECTIONS WERE MADE
Provenance
Latitude
(')
Longitude
(' )
Elevation
(m)
Port Townsend ................
Greenwater...................
Government Camp ............
Estacada .....................
Cloverdale....................
Cascadia ... ..... .. ..... .
Odell Lake ....................
Idleyld Park ..................
48.12
47' 13
45.30
45.27
45.22
44.02
43.58
43.37
122.75
121.63
121.75
122.32
123.90
122.48
122.05
122.97
20
527
1213
125
6
259
1460
329
. .
1
.
.
Date of last
Distance from spring frost
(days from
Pacific Ocean
December 22)8
(km)
Frost-free
season
(days)•
112
161
174
114
104
146
186
145
138
183
167
121
5
121
164
106
206
127
81
201
228
144
28
149
Average date of last spring frost of 0 C and frost-free season based on 22 yr of record,
domized within larger plot treatments. In analyses
of variance, degrees of freedom for main factors and
interactions were further separated into linear,
quadratic, etc., components by coding treatment
factors as per SNEDECOR and CoCHRAN (1967, pp.
349-359). For descriptive purposes "provenance"
was also coded (LI 1964, pp. 341-348) and used as a
dummy quantitative variable. Hypotheses· were
tested at the .OS P level with the appropriate error
term in the split-plot model (almost all significant
factors were significant at P < .01). Then a response
equation was calculated (SNEDECOR and CoCHRAN
1967, pp. 357-358) using as coefficients only those
b's for main effects and interactions that were
significant. Residuals from predicted values were
examined, and the models appeared satisfactory in
all respects.
We chose to present the graphic surfaces predicted
by equations as being preferable to regression coeffi­
cients for illustrating complex relationships. In fact,
data points deviated only slightly from predicted
points, that is, almost all variation in plot means was
explained by response surfaces-respectively, 94%,
81%, and 96% for experiments 1, 2, and 3.
Treatment results were assessed by their effect on
length of the flushing period, measured in days to
bud burst (W) from the time a plant entered the
flushing treatment. Bud burst occurred when scales
of a seedling's terminal bud first opened to expose
green needles.
Reactions of seedlings to experimental environ­
ments were induced (e.g., by chilling), as well as
immediate reactions (by flushing temperatures).
Conceptually it appeared worthwhile to treat quan­
titative results of these reactions as potential and
realized responses. Therefore, a reciprocal data
transformation, described below, was used in all
analyses. It is based on an assumption that most of
the energy needed for growth is chemical energy
released through respiration (UTAAKER 1968). Com­
monly, respiration and resulting growth rate are
directly related to temperature over a wide range of
temperatures ( KuYPER 1910). Consequently, the
period required for any growth process (e.g., flushing
of buds) can be expected to be inversely related to
temperature, and the reciprocal of this period is a
direct estimate of the process rate.
By reason of the above, the response variable in all
analyses was 1/W where W was based on plot
means. This transformation has been used by Azzi
(1956, "sprouting index"), ARNOLD (1959), and
CAMPBELL (1974). It represents the percentage daily
average rate of development (DARD) toward bud
burst. Thus, if it takes 25 days for the bud to flush,
the DARD
100/25
4% per day
4 DARD.
The higher the DARD the fewer the days to bud
burst.
Chilling requirements are met if rate of flushing is
not enhanced by additional days of chilling (WoR­
RALL and MERGEN 1967) (i.e., when DARDs
maximize). If DARDs can be related to chilling
treatments by some function, a maximum can be
obtained easily by graphical or algebraic methods.
=
=
=
Results
!.-Government Camp and Clover­
dale were chosen to represent high and low elevation
provenances, respectively (table 1). Government
Camp seedlings had completed bud set by August 10
and Cloverdale seedlings by September 15. On
September 15, all seedlings were moved to a growth
chamber with a 12-h thermoperiod of 21 C day-15 C
night and a 9-h photoperiod, where they remained
until chilling treatments began.
On October 22, plants in the first of two schedules
of chilling were moved into three chilling chambers
set with 4.4 C, 7.2 C, 10 C, and 9-h photoperiod.
The first schedule consisted of 216 pots (six plants
per pot), 108 each from Cloverdale and Government
Camp, 36 of which were placed in each chilling
chamber. Nine pots were successively removed from
each chamber after 11, 22, 33, and 44 days. The
second schedule of 216 pots went into chilling
(December 5) when the last (44-day) treatment of
schedule 1 was removed. Schedule 2 plants also came
out of chilling at intervals of 11, 22, 33, and 44 days
ExPERIMENT
292
BOTANICAL GAZETTE
or, respectively, 55, 66, 77, and 88 days after Octo­
ber 22, the start of the chilling phase for the first
schedule.
After completion of each chilling period, plants
were placed in a heated greenhouse under long days
(16 h) obtained by supplemental lighting. During
this flushing period, temperatures at plant level
ranged from 18-27 C, the average (20 C) remaining
consistent from week to week.
When data were transformed to DARDs (table 2),
analysis of variance (SE of plot means
0.17
DARD; CV
8.4%) indicated that rates of bud
flushing were highly specific to provenance and
chilling treatment. Statistically, all main factors
(chilling temperatures, chilling periods, schedules,
and provenances), many of their linear, quadratic,
and cubic components, and many interactions were
highly significant (P < .01). Regression lines cal­
culated from the resulting response equation curved
sharply upward with length of chilling. Slopes were
steeper the higher the DARD and the longer the
chilling period, giving the impression that the DARD
induced during any period might be proportional to
the DARD already attained. Therefore, logarithms
of DARDs were plotted against days of chilling.
Resulting trends were approximately linear over the
major part of the data (fig. 1). The largest deviations
occurred in 11-day chilling treatments of the Clover­
dale provenance. The "supereffectiveness" of chilling
in the first period may be related to different degrees
of "dormancy" of provenances when chilling was
1.5
[SEPTEMBER
CLOVERDALE
1.0
0.5
=
=
TABLE 2
AVERAGE DAYS TO BUD BURST AND AVERAGE DARDS (IN
PARENTHESES) AS INFLUENCED HY PROVENANCE, AND
BY TIMING (SCHEDULE), LENGTH, AND TEMPERATURE
OF CHILLING
CHILLING
PROVENANCE,
AND
(DAYS)
SCHEDULE,
PERIOD
4.4
Cloverdale:
1:
11............
22............
33............
14 ............
2:
11...........
22............
33............
44............
Government Camp:
1:
11 ............
22............
33.......
44............
2:
1\ ............
22............
33............
44............
.
TEMPERATURE
(C)
---,--- -----­
7.2
10.0
67.0 (1.55) 74.0(1.37)
69.1 (1.45) 66.1 (1.52)
63.3 (1.64) 65.4 (1.54)
45.6 (2. 22) 49.9 (2.01)
79.0 (1.27)
72.5 (1.3R)
66.0 (1.53)
57.4(1.75)
52.1 (1.95)
50.0 (2.00)
37.3 (2.68)
3 1.2 (3.21)
51.2 (1.95)
50.2 (1.99)
41.9 (2.39)
36.1 (2. 77)
60.9 (1.65)
51. 7 (1.94)
44.3 (2.26)
36.4 (2.76)
48.1 (2 .08)
43.2 (2.35)
37.7 (2.66)
32.5 (3.10)
65.7 (1.52)
58.1 ( 1.72)
46.9 (2.14)
41.6 (2.41)
67.6 (1.49)
57.7 (1.74)
so. 9 (1. 97)
44.5 (2.25)
56.2 (1.79) 62.3 (1. 61)
44.7 (2.24) 55.6 (1. 80)
36.4 (2.76) 45.6 (2.19)
26.3 (3.82) 44.6 (2.24)
62.4 (1.60)
60. 7 (1.65)
4.4.7 (2.25)
44.9 (2.23)
NoTE.-Each average is based on three replications of 18-seedling plots,
GOVERNMENT CAMP
1.0
0. 5
0
-11
22 33 44
Schedule I
11
--
-- -22 33 44
Schedule II
CHILLING PERIOD (days)
FIG. 1.-Log . DARD (% daily average rate of development
of buds) as influenced by provenance, days of chilling, chilling
temperature, and time of entry into chilling. Schedule 1
seedlings entered chilling on October 22; schedule 2 seedlings
entered chilling 44 days later, on December 5.
started. For remaining data, the logarithms of DARD
increased at approximately uniform rate per day
of chilling. Thus, in early phases of chilling the
relative rate of increase in DARD is approximately
constant for each specific combination of chilling
temperature and provenance.
Chilling effectiveness was greatest at 4.4 C com­
pared with 7.4 C and 10 C, the latter two tempera­
tures differing little in their influence on relative
increase in DARDs. Also, for Cloverdale, chilling
effectiveness per day was greater when chilling was
started later in the season. This can be seen by
carrying lines between response points for comparable
treatments in schedule 1 and schedule 2 (fig. 1) (e.g.,
from 44-day treatment response in schedule 1 to
44-day treatment response in schedule 2). Lines will
be seen to be roughly parallel and with positive slope.
For Government Camp there was no comparable,
general, promotive effect of late entry into chilling.
Cloverdale seedlings chilled at 4.4 C for 44 days
starting October 22 burst buds only after an addi­
tional 46 days in flushing conditions (January 19).
When the 44 days of chilling was started Decem­
1975]
CA:tviPBELL & SUGANQ-PHENOLOGY OF BUD BURST IN DOUGLAS-FIR
ber 6, buds burst after 31 days in flushing conditions,
or not before February 22. For Government Camp,
in identical treatments, bud-burst dates were Janu­
ary 7 and February 17.
ExPERniENT 2.-Provenances were Odell Lake
and Idleyld Park, high- and low-elevation sources
from the central Oregon Cascades Mountains (table
1). On September 9, 1970, 90 pots of each source
(18 pots in each of five replications) were moved into
a holding chamber (21 C day/15 C night, 12-h
thermoperiod, 9-h photoperiod). On October 27, the
chamber temperature was decreased to a constant
4 C.
Flushing treatments started on February 4, 11,
and 18, coincident with changes in photoperiod,
were applied in 18 combinations of six temperature
variations with three photoregimes. The plot design
included six pots (two provenances X three photo­
regimes) in each of five blocks within each growth
chamber. Data were analyzed as a split-plot experi­
ment in randomized block design with unreplicated
main-plot temperature treatments. The six growth
chambers for flushing were programmed as follows:
(a) 13 C day (12 h)-4 C night, (b) 17 C day (12 h)­
4 C night, (c) 21 C day (12 h)-4 C night, (d) 13 C
day and night, (e) 17 C day and night, (f) 21 C day
and night. Photoperiods (19,500 lx) in all six chambers were
changed on the following schedule: (a) February 4,
8.5-h day; (b) February 11, 10-h day; (c) Febru­
ary 18, 11.5-h day. The light energy increased with longer photo­
periods, as is the case with photoperiods in the field
in spring. At changes in the photoperiod schedule,
one pot per source per replication was introduced in
each of the six growth chambers. Consequently,
plants entering flushing chambers on February 4 at
8.5-h daylengths received increases in photoperiods
on February 11 and February 18. Plants entering
on February 11 started flushing treatment at 10-h
daylength, which was subsequently increased on
February 18. Since phototreatments included changes
in energy and photoperiod, they are called photo­
regimes 8.5, 10, and 11.5 by reference to the entering
photoperiod.
Bud-burst dates in chambers with constant flush­
ing temperatures were more variable than in cham­
bers with fluctuating temperatures, and homogeneity
was not achieved by transformation to DARDs
(fig. 2). Consequently, data from fluctuating- and
constant-temperature chambers were analyzed as
separate experiments of identical design. In each
analysis of variance, the quadratic terms were not
significant. Consequently, resulting response surfaces
were planes (fig. 3).
Photoregimes, as well as flushing temperatures,
significantly influenced rate of development toward
bud burst. The only exception occurred with Idleyld
Park seedlings flushed in 21 C days and 4 C nights
293
(fig. 3). Response to flushing temperature apparently
depends on the photoregime in which buds are
flushed. This interaction was consistent within each
of the provenances as shown by regression lines with
similar patterns in fluctuating and constant flushing
temperatures. In contrast, interaction patterns dif­
fered greatly between provenances (fig. 3).
A preliminary study using the conventional tem­
perature-summation method (ARNOLD 1959) for
measuring thresholds and temperature responses had
indicated that 4 C was below the threshold tempera­
ture for bud flushing in Douglas-fir. On this assump­
tion, seedlings in constant-temperature chambers,
0
80
f(/)
a:
::::>
co
Cl
::::>
co
A OBSERVED DATA
0
70
,x
0'
60_
0
0
50_
0
f(/)
40_ Cl
30_ '
'
'
'
X
'
X
'
'8
><
o
X
0
--.i- X
8
sr---- -
UJ
(.!)
.<(
a:
20
X
)(
Constant day-night temperatures o Fluctuating day-night temperatures - Average value
x
UJ
0
7
.._,....
.._,.... '-r-'
B TRANSFORMED DATA
X
6
>
(I)
"0
....... 5
)(
4
)(
)(
X
Cl
a:
<(
Cl
0
3
2
,
0
)( _,.,,.
,.
,.
lf
8
--"'if
-0
)( -
)(
--a-)(
_a...)(
8
OL-----�-----.�---.�
13C
21C
17C
DAYTIME FLUSHING TEMPERATURE
FIG. 2.-A, Observed data in constant vs. fluctuating
temperature environment. Points are replication means of
Odell Lake seedlings flushed in the 10-h photoregime at three
(13, 17, and 21 C) daytime flushing temperatures. Night
temperatures (12-h) were 4 C in the fluctuating-temperature
treatment. B, Above data transformed to DARDs. Variability
among replications within treatments is made homogeneous by
transformation but variability in constant temperatures is
average
greater than in fluctuating temperatures. Solid line
values of constant temperatures; dashed line
average
values of fluctuating temperatures.
=
=
294
BOTANICAL GAZETTE
compared with fluctuating-temperature chambers,
would be exposed to twice the degree-hours daily.
Thus, relative effectiveness at any given photo­
period-temperature combination is evaluated by the
ratio: 2(DARD in fluctuating temperature)/(DARD
in constant temperature). If degree-hours contribute
equally to promotion of bud flushing in fluctuating
and constant temperatures, the ratio should equal
unity in all combinations of photoregime and flushing
temperature.
Relative effectiveness of fluctuating temperature
was consistently > 1 as indicated by ratios calculated
from adjusted treatment means. Furthermore, the
response to fluctuating temperatures appeared to be
qualitatively different between provenances ( fig. 4).
The higher the maximum (or average) flushing
temperature and the longer the photoperiod, the
greater the relative effectiveness of fluctuating tern­
6
ODELL LAKE
5
11.5
4
3
2
Constant temperature
Fluctuating temperature
>:
(1)
"C
.......
0
0::
0
6
0
IDLEYLD PARK
11.5
10.0
8.5
5
4
3
2
O L-----�----�--
13C
17C
21C
FLUSHING TEMPERATURE
FIG. 3.-Rate of development toward bud burst(DARD) as
affected by provenance and constant and fluctuating flushing
temperatures in photoregimes of 8.5, 10.0, and 1 1.5 h.
u..
0
CJ)
CJ)
LU
z
LU
;;:::
LU
0::
:::J
!;{
0::
LU
a..
(SEPTEMBER
1.6 -
8.5
1.5-
10.0
tiLU LU
1-
1.4 _11.5
(!)
LUZ
1.3-
LU
> :::J
-�--
1.2-
u..
u..
LU -l
o:u..
.,... ......
......
......
......
......
,... """'
,. ,.
......
---ODELL LAKE
IDLEYLD PARK
13C
17C
21C
DAYTIME FLUSHING TEMPERATURE
FIG. 4.-Relative effectiveness of constant vs. variable
flushing temperatures as influenced by provenance and flushing
temperature. Effectiveness is defined as 2(DARDs in fluctuat­
ing temperature)/(DARDs in constant temperature).
peratures in increasing DARDs in the Odell Lake
population and the less the relative effectiveness in
the Idleyld Park population.
If the threshold temperature for bud flushing in
Douglas-fir is, in fact, below 4 C, the daily 12 h at
4 C would contribute degree-hours not considered in
the above model. To check, the equation for each of
the constant temperature regression lines in figure 3
was solved for the temperature at which DARD ""
0. As shown by ARNOLD (1959), this provides a best
estimate for the threshold that is conventionally
used in calculating degree-days. Thresholds were
above 4 C except for Idleyld Park seedlings flushed
in the 11.5 photoregime (table 3). Degree-days were
then obtained by summing (degrees above threshold
X days). Seedlings in different treatments required
greatly different numbers of degree-days to flush
(table 3). In all combinations, degree-days in
fluctuating temperatures were fewer than in constant
temperatures, thus agreeing with results obtained by
analysis of DARDs.
ExPERIMENT 3.-Four provenances were used:
Cascadia, Estacada, and Greenwater from the
western slopes of the Cascades Mountains, and Port
Townsend from the edge of Puget Sound (table 1).
On October 18, 1971, 60 pots (12 pots in five replica­
tions) of each provenance were moved into a holding
chamber with a 12-h thermoperiod of 21 C day-15 C
night, and a 9-h photoperiod. On December 16
temperature in. the chamber was changed to a
constant 4 C. On January 4, after 18 days of chilling,
one pot from each replication per provenance was
moved into each of four flushing chambers set at 10,
14, 18, and 22 C, with 16-h photoperiod at 18,500 lx.
Two additional lots of seedlings made up exactly
as above were moved into flushing chambers after
40 and 62 days of chilling.
Results indicate that chilling requirements, defined
as the point at which DARDs maximize, are not
uniquely characteristic of a genotype but are also
·
1975]
295
CAMPBELL & SUGANQ-PHENOLOGY OF BUD BURST IN DOUGLAS-FIR
TABLE 3
DEGREE-DAYS TO POPULATION MEAN FLUSHING DATE AS AFFECTED BY PROVENANCE, CONSTANT VERSUS FLUCTUATING TEM PERATURES, AND PHOTOREGIMES ODELL
LAKE IDLEYLD
Degree-days to mean flushing date in:
Degree-days to mean flushing date in:
DAY TEMPERATURE (C)
AND PHOTOPERIOD
21:
(h)
8.5.............
10.0 .............
11.5.............
17:
8.5.............
10.0. .. ..........
11.5.............
13:
8.5.............
10.0.............
11.5.............
PARK
Threshold
temperature
(C)
Threshold
temperature
(C)
Fluctuating
temperature
Constant
temperature
4.74
5.37
5.84
247
214
164
368
321
245
8.19
5.37
1.44
178
235
306
263 312 352 4.74
5.37
5.84
238
196
172
376
334 282
8.19
5.37
1. 44
165
222
304
274 311
372 4. 74
5.37
5.84
269
247
193
386
327 294
8.19
5.37
1.44
158
213
355
210 283
400 Fluctuating
temperature
Constant
temperature
NoTE.-In fluctuating temperatures, night temperatures were maintained at 4 C, which was below the threshold temperatures calculated for most treatments.
related to flushing temperature. In a flushing tem­
perature of 22 C, Cascadia seedlings attained maxi­
mum DARD after approximately 47 days of chilling
at 4 C (fig. 5). In an 18- C flushing temperature, 62
days were required.
At any given flushing temperature, chilling require­
ments may vary among provenances. In flushing
temperature of 22 C, maximum DARDs for Port
Townsend were reached after approximately 60 days
of chilling (fig.5) in contrast to the 47 days needed for
Cascadia. For provenances of Estacada and Green­
water, considerably more than 62 days are needed as
indicated by steepness of slopes at 62 days of chilling.
Larger chilling requirements appear to be asso­
ciated with lower flushing temperatures. The Cascadia
and Port Townsend DARDs in progressively lower
flushing temperatures tend to maximize after pro­
gressively longer periods of chilling (fig. 5). The
DARDs for lowest flushing temperatures did not
reach maximum for any provenance within the
scope of the experiment. Further experiments are
required to determine the validity of a qualitative
"chilling requirement" for high and low flushing
temperatures.
Provenances with satisfied chilling requirements
in any given flushing temperature may still differ in
time to bud burst as a result of differential growth
rates within the developing bud. In 22 C flushing
temperatures, maximum DARDs for Cascadia and
Port Townsend are approximately 5.8 and 5.2,
respectively (fig. 5), or 17 days to bud burst for
Cascadia, 19 for Port Townsend.
Within each chilling period, temperature response
curves were generally S shaped, but exact shapes
depended on provenance and length of chilling.
After 18 days of chilling, rate of development was
somewhat higher in flushing temperatures of 10 C
than in 14 C (fig. 5). In experiment 1, 10 C was
shown to be effective in increasing DARDs in
response to chilling. In experiment 3, the 10 C in
which seedlings were flushed may have acted simul­
taneously in chilling and flushing capacities.
Combined effects of chilling and flushing tempera­
tures on timing of bud flush were evaluated by adding
the days of chilling and flushing. Results tabulated
for flushing temperatures of 14 and 22 C are illustra6
5
4
3
2
ESTACADA
CASCADIA
p::
/
:
14C
14C
10C
10C
>:
"'
-c
....... Cl a:
0
6
<l:
Cl 5
GREENWATER
22C
PORT TOWNSEND
4
3
2
e::;:
0 L.___L__L____J
62
40
18 CHILLING PERIOD
10C
40
18
(days)
62
Fw. 5.-lnteracting effects on DARDs of provenance,
days of chilling at 4 C, and temperature in which buds are
flushed.
296
BOTANICAL GAZETTE
TABLE 4
TOTAL DAYS TO BUD BURST, INCLUDING CHILLING AND
FLUSHING PERIODS, AS AFFECTED BY PROVENANCE, DAYS
OF CHILLING, AND CONSTANT FLUSHING TEMPERATURES
OF 14 AND 22 C
DAYS
PROVENANCE AND
CHILLING PERIOD
(DAn
)
Cascadia:
18..........
40............. 62.............
Estacada:
18............. 40 .............
62............. Greenwater:
18.............
40.............
62.............
Port Townsend:
18.............
40.............
62.......... ..
22
TO BUD BURS'!: (AFTER DECEMBER
IN FLUSHING TEMPERATUN.E!
c
14 c
16)
Difference
(14-22 C)
---·-
46
57
80
118
90
102
72
33
22
48
62 79
129
90
96
81
28
17
57
63
80
135
99
105
78
36
25 62
62 81
129
99
103
67 37
22
tive of the potential range of flushing dates (table 4).
Chilling was started on December 16 when chilling
effectiveness per day is expected to be high because
of the lateness of season. However, even in the long
photoperiod (16 h) and highest flushing temperature
of experiment 3 (22 C), bud burst did not occur
before about February 1. With flushing temperatures
more characteristic of days during natural flushing
(10-14 C), dates of bud burst were further delayed,
ranging from the middle of March to late April,
depending on days of chilling. These dates encompass
the expected natural flushing of provenances planted
in mild areas of western Washington and Oregon.
The requirement for chilling is much more effective
in slowing bud development at low flushing tem­
peratures. This can be seen by comparing differences
in days to bud burst in 14 and 22 C flushing tem­
peratures (table 4). When additional days of chilling
no longer influence response to flushing temperature,
differences should minimize and become constant,
reflecting different growth rates at the two tempera­
tures. This point has not been reached for any
provenance (table 4).
Discussion
Effects of chilling, photoperiod, and flushing
temperature on rate of bud flushing are highly
interdependent. Effects induced by chilling depended
on timing, duration, and temperature (experiment
1). After induction by a single chilling temperature
and period, rates of bud burst were also influenced by
photoregime and temperature during flushing (ex­
periment 2). The influence of flushing temperatu
. re
[ SEPTEMBER
on the expression of induced effects of chilling
depended on duration of chilling (experiment 3).
The apparent total effect of chilling and photo­
period is one of widening the temperature response,
as hypothesized by VEGIS (1963). In coastal Douglas­
fir this is accomplished by increasing the rate of
bud burst at all flushing temperatures. The increase
associated with added chilling is greatest at the
lowest flushing temperatures (experiment 3). In
comparison to increases induced by chilling, in­
creases associated with photoperiod appear to depend
more strongly on provenance, but interaction with
flushing temperatures is still important (experiment
2). Rate of flushing may not level off and maximize
with added chilling (experiment 3). Instead, during
late stages of chilling, growth may begin at chilling
temperatures (e.g., 10 C in experiment 2) and rapid­
ity of response to forcing may increase up to the day of bud burst. Similar responses have been noted in some seed studies where, during stratification period,
seeds will germinate at progressively lower tempera­
tures until germination takes place at stratification
temperatures (S T ONE 1957; H ATA NO and As A K A WA
1964).
Interacting environmental effects were closely
fitted to polynomial response surfaces in all experi­
ments, indicating a quantitatively describable sys"
tern for controlling bud-burst.timing. Our concept of
the system is most easily explained in terms of
potential and realized DARDs as means for quan­
tifying the general types of reactions to environ­
mental stimuli called "inductive" and "immediate"
by HESLOP-HARRISON (1964).
When a bud with a given potential DARD is
exposed to sufficient heat and other requirements
are not limiting, it develops by an increment which
is dependent on its potential DARD. If the stimu­
lus is effective for the period of a day, the poten­
tial DARD becomes a realized DARD. Potential
DARDs are an induced response that depends on
duration and temperature of prechilling (Do RMLI NG
et al. 1968; SuGANO 1971) and chilling. Realized
DARDs are a function of this potential and photo­
period and temperature during the time of realiza­
tion. From our experience, the potential DARD is
specific to the environment at the time of realization;
although for any given flushing environment the
potential has been determined by temperature events
in previous months. Others (WEINBERGER 1954;
BENNETT 1960; VEGIS 1963; NIENSTAEDT 1966) have
noted dormancy reinduction in response to diurnal
temperature fluctuation during the chilling phase.
Thus, alternating periods of warm and cold during
chilling may also influence potential DARDs.
By definition, DARDs will be accumulated as they
are realized. Accumulation continues throughout the
spring season until realized DARDs sum to 100, at.
which time bud burst is achieved. We can speculate
1975]
CAMPBELL & SUGANO-PHENOLOGY OF BUD BURST IN DOUGLAS-FIR
that during autumn DARDs are accumulated
slowly, if at all, because potential DARDs are small
due to lack of chilling. During the winter, as
potential DARDs increase in response to chill­
ing, photoperiods are becoming shorter. Realized
DARDs remain small in response to short photo­
periods. Consequently, during autumn and winter,
realized DARDs accumulate slowly regardless of
flushing temperatures. As the growing season ap­
proaches, potential DARDs that are appropriate to
higher flushing temperatures may reach maximum
in response to chilling and photoperiod. However, it
appears likely that, even to the time of bud burst,
potential DARDs appropriate to lower temperatures
will be increasing in response to added chilling, and
realized DARDs will be increasing in response to
longer photoperiods.
Because winter buds are "resting," there may be
some question whether DARDs can be realized and
accumulated during warm winter days. There is
ample evidence in several species that resting vegeta­
tive buds are not inert (PERRY 1971) and may even
increase in dry weight by 100% during winter
months (YOUNG, WINNEBERGER, and BENNETT
1974). Results of experiment 2 show that develop­
ment of Douglas-fir buds is not inhibited by fluctuat­
ing daily temperatures which bridge the flushing and
chilling range but was faster than could be expected
from results in constant temperatures. This may be
explained by an increase of potential DARDs by
chilling during the night.
The regulatory basis for potential and realized
DARDs is assumed to be a changing balance of
growth inhibitors and promoters during winter rest
(LAVENDER and HERMANN 1970) and flushing
(LAVENDER et al. 1973). In our experiments, re­
sponses to environmental treatments were quantita­
tive and continuous. It is therefore unlikely that
dormancy phases in Douglas-fir are clearly separated
with unique growth-regulator patterns as proposed
in the chemical model of SMITH and KEFFORD (1964).
Our results indicate that, under natural flushing
conditions, date of bud burst is mainly a function of
spring temperature. However, timing will also be
occasionally influenced by winter chilling, especially
in years with relatively warm winters and cool
springs. In the coastal Douglas-fir region, it is not
unusual for low elevation sites to have winters with
fewer than 62 days of chilling at 4 C (WAKEFIELD
1969). Average temperatures during the flushing
period characteristically range from 7 to 10 C,
average daily maximums from 12 to 17 C, and only
about 10% of days reach maximums of 22 C. At
these flushing temperatures, potential rates of bud
development may be increased (experiment 3) by
additional days of chilling even after 62 days at 4 C.
In some years, flushing date will also be partly a
function of photoperiod. In experiment 2, after 100
297
days of chilling at 4 C (fig. 3), rates of flushing were
generally increased by longer photoperiods. In years
in which seedlings are chilled for fewer than 100
days, photoperiod effects are likely to be correspond­
ingly larger. Our experiments did not examine the
combined effects of chilling and photoperiod. How­
ever, a general type of interaction can be inferred
from other studies ( WOMMACK 1964; NIENSTAEDT
1967; WoRRALL and MERGEN 1967) which show that
after short chilling periods conifer seedlings flushed
in long days will burst buds before seedlings flushed
in short days. As chilling period is increased, differ­
ences between dates of bud burst in long versus
short days are decreased, eventually vanishing. In
other words, the promotive effect of long photo­
periods tends to zero as chilling progresses. In
experiment 2, Idleyld Park seedlings had apparently
reached the zero point in higher flushing temperatures
but not in lower (fig. 3). From the above evidence,
we can infer that the zero point at lower temperatures
would have been reached after longer chilling and,
conversely, that effects of photoperiod would have
been greater with shorter chilling.
In experiment 2, plants in photoregimes 8.5, 10,
and 11.5 received 100, 107, and 114 days of chilling,
respectively, before entering flushing treatments,
thus confounding chilling and photoregimes. From
previous information (WoMMACK 1964), chilling
requirements were supposedly satisfied by 100 days
at 4 C and additional days should not have in­
fluenced bud flushing. From experiment 3, this
assumption now appears erroneous. A few days of
additional chilling after 100 days probably did not
greatly influence rate of bud flushing, but effects
ascribed to photoregime may not be due strictly to
phototreatment.
The few provenances used to sample within-species
variation in coastal Douglas-fir were highly variable
in their response to controlling factors. Population
differences appeared in response to duration, timing
and temperature of chilling; to flushing temperature,
particularly in its interrelation with photoperiod and
length of chilling; and to fluctuating flushing tem­
perature. We used too few provenances to attempt
to correlate provenance differences with habitat
types. Other studies have shown chilling require­
ments (PERRY and WANG 1960; KRIEBEL and WANG
1962; FARMER 1968) and flushing temperature re­
sponses ( CAMPBELL 1974) to be related to latitudinal
origin of provenances and other habitat factors.
However, the strong interactions found here make it
unlikely that any single-factor analysis will provide
an understanding of synchronization as an adapta­
tional mechanism.
With further experimentation it should be possible
to develop a quantitative model for bud-burst
timing. It will be useful for investigating adapta­
tional patterns in Douglas-fir and also for gaining
BOTANICAL GAZETTE
298
insight into complex effects of temperature and
photoperiod during dormancy and dormancy release
or into the physiological basis for these effects.
Acknowledgments
Experiment 1 is a reanalysis of data taken by
A. I. SUGANO for his M.Sc. thesis at Oregon State
University. We wish to thank DENIS LAVENDER,
WILLIAM FERRELL, and HELGE lRGENs-MoLLER,
Oregon State University, for loans of growth­
chamber space and other facilities. We also gratefully
acknowledge the helpful comments of referees and
discussions with DR. lRGENs-MoLLER which led to
the design of experiment 1.
LITERATURE CITED
C. Y. 1959. The determination and significance of
the base temperature in a linear heat unit system. Proc.
Amer. Soc. Hart. Sci. 74:43Q-445.
Azzr, G. 1956. Agricultural ecology. Constable, London,
BENNETT, ]. P. 1960. Temperature and bud rest period.
Effect of temperature and exposure on the rest period of
deciduous plant leaf buds investigated. California Agr.
4(1): 11, 13, 15-16.
CAMPBELL, R. K. 1974. Use of phenology for examining
provenance transfers in reforestation of Douglas-fir. ].
Appl. Ecol. 11:1069-1080.
CoVILLE, F. V. 1920. The influence of cold in stimulating the
growth of plants. J. Agr. Res. 20(2): 151-160.
DoRMLING, I., A. GusTAFSSON,and D. VoN WETTSTEIN. 1968.
The experimental control of the life cycle in Picea abies (L)
Karst. I. Some basic experiments on the vegetative cycle.
Silvae Genet. 17:44--64.
FARMER,R. E.,]R. 1968. Sweetgum dormancy release: effects
of chilling, photoperiod, and genotype. Physiol. Plantarum
21:1241-1248.
GusTAFSON, F. G. 1938. Influence of the length of day on the
dormancy of tree seedlings. Plant Physiol. 13:655-658.
HATANO, K., and S. AsAKAWA. 1964, Physiological processes
in forest tree seeds during maturation, storage, and germina­
tion. Int. Rev. Forest Res. 1:2 79-323.
HESLOP-HARRISON, J. 1964. Forty years of genecology. Pages
159-247 i11 ]. B. CRAGG, ed. Advances in ecological re­
search. Vol. 2. Academic Press, New York.
lRGENs-MoLLER, H. 1957. Ecotypic response to temperature
and photoperiod in Douglas-fir. Forest Sci. 3:79-83.
jENSEN, K. F., and G. E. GATHERUM. 1965. Effects of tem­
perature, photoperiod, and provenance on growth and
development of Scotch pine seedlings. Forest Sci. 11(2):
189-199.
KRIEBEL, H. B., and C. W. WANG. 1962. The interaction
between provenance and degree of chilling in bud-break of
sugar maple. Silvae Genet. 11:125-130,
KuYPER,]. 1910. The influence of temperature on respiration
in higher plants. Koninkl. Ned. Akad. Wetenschap., Ser.
B., 12:219-227.
LAVENDER, D. P. , and R. K. HERMANN. 1970. Regulation of
the growth potential of Douglas-fir seedlings during dor­
mancy. New Phytol. 69:675-694.
LAVENDER, D. P., G. B. SwEET, ]. B. ZAERR, and R. K.
HERMANN. 1973. Spring shoot growth in Douglas-fir may be
initiated by gibberellins exported from the roots. Science
182:838-839.
LI, J. C. R. 1964. Statistical inference. Vol. 2. Edwards, Ann
Arbor, Mich.
NIENSTAEDT, H. 1966. Dormancy and dormancy release in
white spruce. Forest Sci. 12:374-384.
---. 1967. Chilling requirements in seven Picea species.
ARNOLD,
Silvae Genet. 16:65-68.
J, S., F. W, STEARNS, and H. NIENSTAEDT. 1959.
Eastern hemlock seeds and seedlings: Response to photo­
period and temperature. Connecticut Agr. Exp. Sta. Bull.
620.
PERRY, T. 0. 1971. Dormancy of trees in winter. Science
171:29-36.
PERRY, T. 0., and C. W. WANG. 1960. Genetic variation in the
winter chilling requirement for date of dormancy break for
Acer mbrum. Ecology 41(4): 790-794.
SMITH, H., and N. P. KEFFORD. 1964. The chemical regulation
of the dormancy phases of bud development. Amer. ].
Bot. 51(9): 1002-10 12.
SNEDECOR, G. W., and W. G. CocHRAN. 1967. Statistical
methods. Iowa State University Press, Ames.
STEINHOFF, R. ]., and R. ]. HoFF, 1972. Chilling requirements
for breaking dormancy of western white pine seedlings.
U SDA Forest Serv. Res. Note INT- 153. Intermountain
Forest and Range Experiment Station, Ogden, Utah.
STONE, E. C. 1957. Embryo dormancy of Pi11us jeffrey! Murr.
seed as affected by temperature, water uptake, stratification
and seed coat. Ecology 32:93-99.
SuGANO, A. I. 197 1. The effects of low temperatures on
dormancy release in Douglas-fir from western Oregon,
Washington and California. M. Sc. thesis, Oregon State
University, Corvallis.
UTAAKER, K. 1968. A temperature-growth index-the respira­
tion equivalent-used in climatic studies on the meso-scale
in Norway. Agr. Meteorol. 5:351-359.
VEGIS, A. 1963. Climatic control of germination, bud break,
and dormancy. Pages 265-287 ill L. T. EVANS, ed. En­
vironmental control of plant growth. Academic Press,
New York.
---. 1964. Dormancy in higher plants. Annu. Rev. Plant
Physiol. 15:185-224.
WAKEFIELD, ]. D., ed. 1969. Clim atological handbook,
Columbia Basin states temperature. Vol. 1, pt. A. Pacific
Northwest River Basins Commission, Vancouver, Wash.
WAREING, P. F. 1956. Photoperiodism in woody plants.
Annu. Rev. Plant Physiol. 7: 191-214.
WEINBERGER,]. H. 1954. Effects of high temperatures during
the breaking of the rest of Sullivan Elberta peach buds.
Proc. Amer. Soc, Hart. Sci. 63:157-162.
WoMMACK, D. E. 1964. Temperature effects on the growth of
Douglas-fir. Ph.D. thesis, Oregon State University, Corval­
lis.
WORRALL, ]., and F. MERGEN. 1967. Environmental and
genetic control of dormancy in Picea abies. Physiol. Plan­
tarum 20:733-745.
YOUNG, L. C. T., J, T. WINNEBERGER1 and J. P. BENNETT,
1974. Growth of resting buds. ]. Amer. Soc. Hart. Sci.
99:146--149.
OLSON,