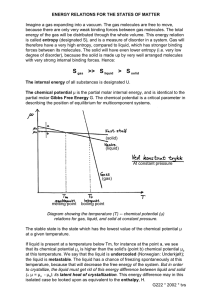
7 entopy and free energy :there are two statements of... 1. Heat alaways flows from a hotter to a...
advertisement

7 entopy and free energy :there are two statements of the second law of thermodynamics: 1. Heat alaways flows from a hotter to a colder body never in the reverse direction. 2. An isolated system always tend to take up a more disordered form . The physical example of this experience is shown by the molecules of a gas enclosed in a chamber which is connected by a tap to an evacuated chamber. If the tap is opened the gas molecules by their random motion in the space available to them , will enter the second chamber until .on the average there will be the same number of molecules in each chamber. In other words , a gas always tend too fill any chamber in which it is placed. The second condition is more probable than the first condition and is more disordered than the first condition.A spontaneous change has taken place accompanied by an increase in probability and therefore an increase in the disorder of the system. Thermodynamics is not concerned with the behavior of the individual molecules of a gas system only the statistical average behavior of the system. In addition to an energy change ,we can therefore have a change in the state of order accompanying a chemical or physical change in a system, and this change in the state of order can be expressed as a change in a property of the system the entropy S. S=kln W +So k:boltzman constant Wthe probability So the entropy at the Absolut zero temperature