ENERGY RELATIONS FOR THE STATES OF MATTER
advertisement
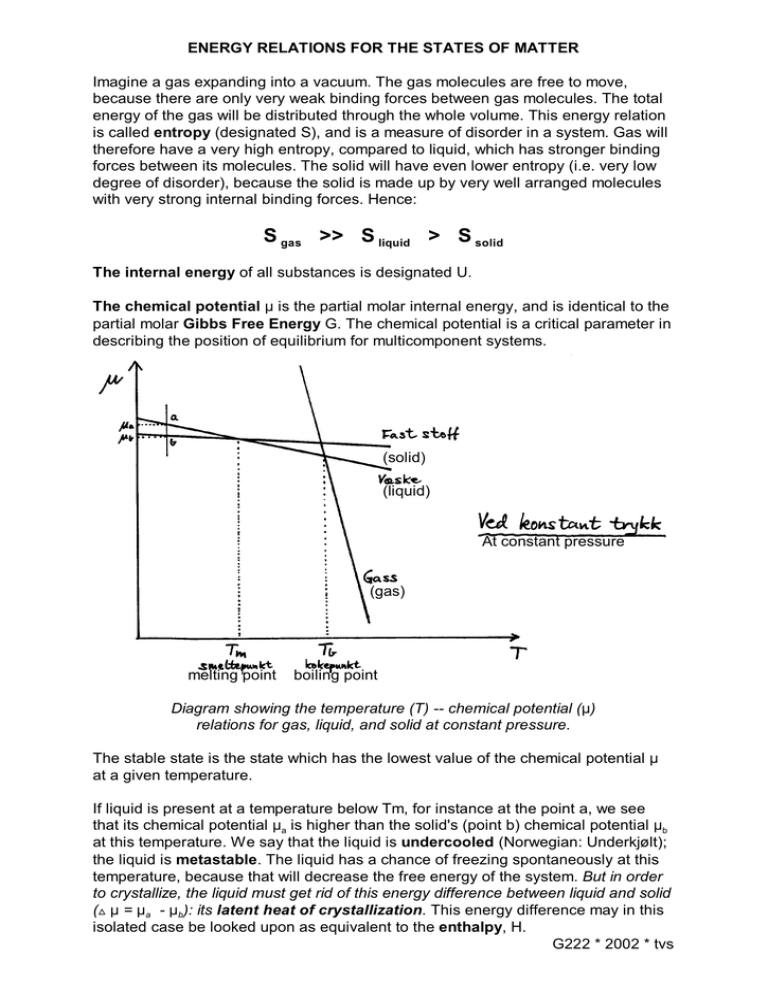
ENERGY RELATIONS FOR THE STATES OF MATTER Imagine a gas expanding into a vacuum. The gas molecules are free to move, because there are only very weak binding forces between gas molecules. The total energy of the gas will be distributed through the whole volume. This energy relation is called entropy (designated S), and is a measure of disorder in a system. Gas will therefore have a very high entropy, compared to liquid, which has stronger binding forces between its molecules. The solid will have even lower entropy (i.e. very low degree of disorder), because the solid is made up by very well arranged molecules with very strong internal binding forces. Hence: S gas >> S liquid > S solid The internal energy of all substances is designated U. The chemical potential : is the partial molar internal energy, and is identical to the partial molar Gibbs Free Energy G. The chemical potential is a critical parameter in describing the position of equilibrium for multicomponent systems. (solid) (liquid) At constant pressure (gas) melting point boiling point Diagram showing the temperature (T) -- chemical potential (:) relations for gas, liquid, and solid at constant pressure. The stable state is the state which has the lowest value of the chemical potential : at a given temperature. If liquid is present at a temperature below Tm, for instance at the point a, we see that its chemical potential :a is higher than the solid's (point b) chemical potential :b at this temperature. We say that the liquid is undercooled (Norwegian: Underkjølt); the liquid is metastable. The liquid has a chance of freezing spontaneously at this temperature, because that will decrease the free energy of the system. But in order to crystallize, the liquid must get rid of this energy difference between liquid and solid (ª : = :a - :b): its latent heat of crystallization. This energy difference may in this isolated case be looked upon as equivalent to the enthalpy, H. G222 * 2002 * tvs











