S02Final.doc
advertisement
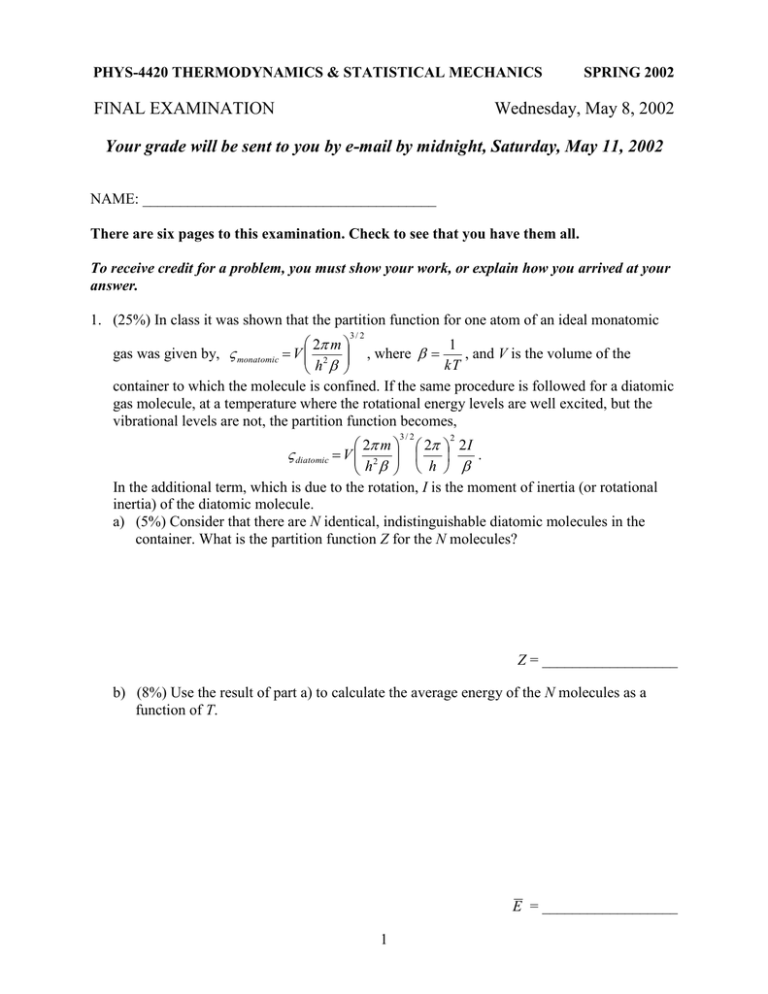
PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS FINAL EXAMINATION SPRING 2002 Wednesday, May 8, 2002 Your grade will be sent to you by e-mail by midnight, Saturday, May 11, 2002 NAME: _______________________________________ There are six pages to this examination. Check to see that you have them all. To receive credit for a problem, you must show your work, or explain how you arrived at your answer. 1. (25%) In class it was shown that the partition function for one atom of an ideal monatomic 3/ 2 2 m 1 gas was given by, monatomic V 2 , where , and V is the volume of the kT h container to which the molecule is confined. If the same procedure is followed for a diatomic gas molecule, at a temperature where the rotational energy levels are well excited, but the vibrational levels are not, the partition function becomes, 3/ 2 2 m 2 2 I diatomic V 2 . h h In the additional term, which is due to the rotation, I is the moment of inertia (or rotational inertia) of the diatomic molecule. a) (5%) Consider that there are N identical, indistinguishable diatomic molecules in the container. What is the partition function Z for the N molecules? 2 Z = __________________ b) (8%) Use the result of part a) to calculate the average energy of the N molecules as a function of T. E = __________________ 1 c) (5%) Use the result of part a) to calculate the Helmholtz function F for the N molecules. F = __________________ d) (7%) Use the Helmholtz function to calculate the pressure of the gas as a function of temperature and volume. This should give the same ideal gas formula as a monatomic gas. p = __________________ 2 2. (30%) A quantity of gas is taken reversibly around the cycle a-b-c-d-a shown on the T-S diagram shown in the figure below. a) (4%) The system goes around the cycle in the direction a-b-c-d-a. Is it operating as a heat engine or a refrigerator? (Circle the correct answer.) HEAT ENGINE REFRIGERATOR b) (12%) Calculate the heat transferred in each step of the cycle. The sign of the heat transferred in each step is important. You may leave your answers in terms of R. Qa-b = __________________ Qb-c = __________________ Qc-d = __________________ Qd-a = __________________ c) (5%) How much work is done by the system in the complete cycle? You may leave your answer in terms of R. W = __________________ 3 d) (5%) From your results in parts b) and c), determine the efficiency of the system when it operates as a heat engine. = __________________ e) (4%) The cycle shown in the figure is actually a Carnot cycle. Calculate the efficiency of the cycle with the formula for the efficiency of a Carnot engine to see if it gives the result that you obtained in part d). Carnot = __________________ 4 3. (30%) A container is divided into two equal chambers, each of the same volume V. One chamber contains N molecules of an ideal gas at temperature T, and the other chamber is completely empty, a perfect vacuum. A small hole, of area A, is punched in the wall separating the two chambers, and gas begins to leak into the empty chamber. The temperature of the gas is kept constant. a) (10%) Find an expression for the rate at which molecules leave the filled chamber at the instant the hole is punched. Express your answer in terms of A, V, N, and v , the average speed of an air molecule. (Hint: the flux of molecules moving in the + x direction can be written, f x 14 v , where is the number of molecules per volume.) dN = ________________ dt b) (10%) The number of molecules in the chamber that was initially filled will decrease until the two chambers are equally populated (each with N/2 molecules). Then, the rate at which molecules leave the chamber will be equal to the rate at which they enter it from the other side. Derive an expression for n, the number of molecules in the initially filled chamber, as a function of time after the hole is punched. Again, the result can be in terms of A, V, N, and v . c) (10%) As the gas redistributes itself between the two chambers, the entropy of the system increases. Calculate the difference between the entropy of the final state with both chambers holding N/2 molecules, and the initial state when all the molecules were in one chamber. S =__________________ 5 4. (15%) Assume that Earth and the Sun are perfect blackbodies, and that Earth’s only source of heat is the Sun. What will be the temperature of the surface of Earth when it is radiating energy at the same rate that it is absorbing it from the sun, i.e. when it reaches steady state. The following information may be useful. Temperature of the surface of the Sun: TS = 5800 K Radius of the Sun: RS = 6.96 ×108 m Radius of Earth: RE = 6.37 ×106 m Distance from Earth to the Sun: r = 1.50 ×1011 m Stephan-Boltzmann constant: = 5.67 ×10-8 W/m²·K4 Be sure to make your work clear. In case you do not complete this correctly, that will make it possible to assign partial credit. 6




