steady-flow compressor. The work and heat transfer for this compression... 13-59
advertisement
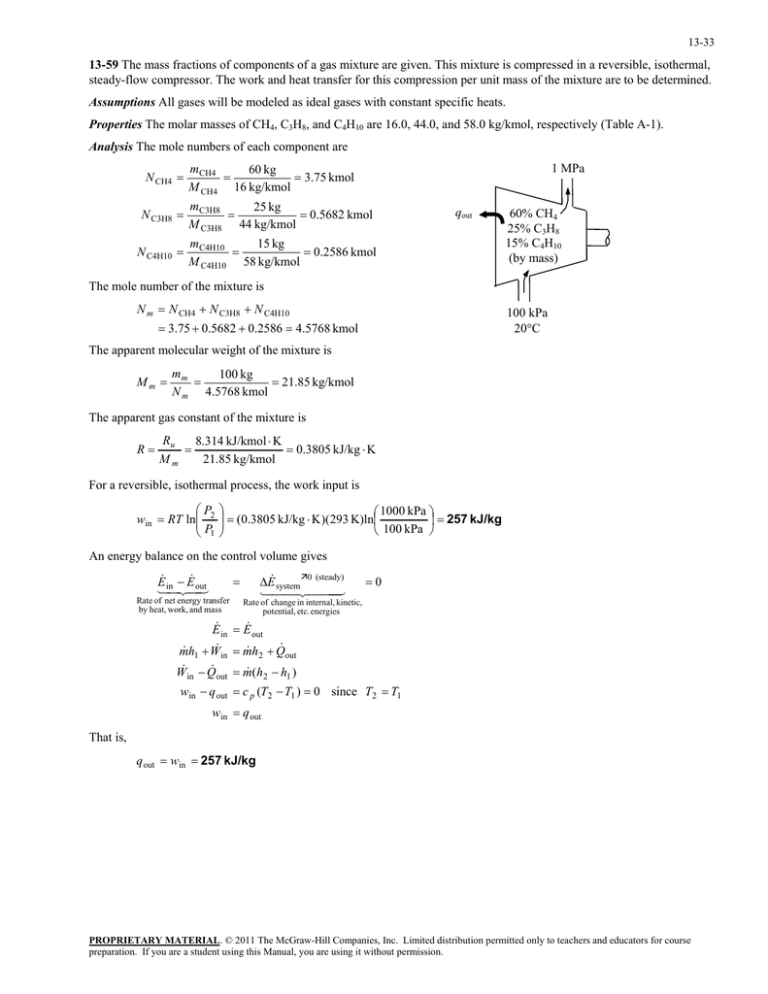
13-33 13-59 The mass fractions of components of a gas mixture are given. This mixture is compressed in a reversible, isothermal, steady-flow compressor. The work and heat transfer for this compression per unit mass of the mixture are to be determined. Assumptions All gases will be modeled as ideal gases with constant specific heats. Properties The molar masses of CH4, C3H8, and C4H10 are 16.0, 44.0, and 58.0 kg/kmol, respectively (Table A-1). Analysis The mole numbers of each component are N CH4 1 MPa mCH4 60 kg 3.75 kmol M CH4 16 kg/kmol N C3H8 mC3H8 25 kg 0.5682 kmol M C3H8 44 kg/kmol N C4H10 mC4H10 15 kg 0.2586 kmol M C4H10 58 kg/kmol qout 60% CH4 25% C3H8 15% C4H10 (by mass) The mole number of the mixture is N m N CH4 N C3H8 N C4H10 100 kPa 20C 3.75 0.5682 0.2586 4.5768 kmol The apparent molecular weight of the mixture is Mm mm 100 kg 21.85 kg/kmol N m 4.5768 kmol The apparent gas constant of the mixture is R Ru 8.314 kJ/kmol K 0.3805 kJ/kg K 21.85 kg/kmol Mm For a reversible, isothermal process, the work input is P win RT ln 2 P1 1000 kPa (0.3805 kJ/kg K )(293 K)ln 257 kJ/kg 100 kPa An energy balance on the control volume gives E E inout Rate of net energy transfer by heat, work, and mass E system 0 (steady) 0 Rate of change in internal, kinetic, potential, etc. energies E in E out m h1 W in m h2 Q out W in Q out m (h2 h1 ) win q out c p (T2 T1 ) 0 since T2 T1 win q out That is, q out win 257 kJ/kg PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.