electrically at constant pressure. The minimum work by which this... 8-36
advertisement

8-21
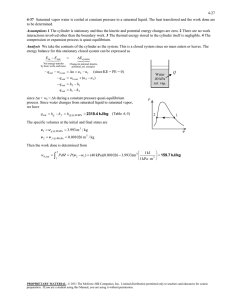
8-36 An insulated cylinder is initially filled with saturated liquid water at a specified pressure. The water is heated
electrically at constant pressure. The minimum work by which this process can be accomplished and the exergy destroyed
are to be determined.
Assumptions 1 The kinetic and potential energy changes are negligible. 2 The cylinder is well-insulated and thus heat
transfer is negligible. 3 The thermal energy stored in the cylinder itself is negligible. 4 The compression or expansion
process is quasi-equilibrium.
Analysis (a) From the steam tables (Tables A-4 through A-6),
u1 u f @120 kPa = 439.27 kJ / kg
3
P1 120 kPa v 1 v f @120 kPa = 0.001047 m /kg
sat. liquid h1 h f @120 kPa = 439.36 kJ/kg
s1 s f @120 kPa = 1.3609 kJ/kg K
Saturated
Liquid
H2O
P = 120 kPa
The mass of the steam is
We
3
0.008 m
V
m
7.639 kg
v 1 0.001047 m 3 / kg
We take the contents of the cylinder as the system. This is a closed system since no mass enters or leaves. The energy
balance for this stationary closed system can be expressed as
E E
E system
inout
Net energy transfer
by heat, work, and mass
Change in internal, kinetic,
potential, etc. energies
We,in W b,out U
We,in m(h2 h1 )
since U + Wb = H during a constant pressure quasi-equilibrium process. Solving for h2,
We,in
1400 kJ
h2 h1
439.36
622.63 kJ/kg
m
7.639 kg
Thus,
h2 h f 622.63 439.36
x2
0.08168
h fg
2243.7
P2 120 kPa
s 2 s f x 2 s fg 1.3609 0.08168 5.93687 1.8459 kJ/kg K
h2 622.63 kJ/kg
u 2 u f x 2 u fg 439.24 0.08168 2072.4 608.52 kJ/kg
v 2 v f x 2v fg 0.001047 0.08168 (1.4285 0.001047) 0.1176 m 3 /kg
The reversible work input, which represents the minimum work input Wrev,in in this case can be determined from the exergy
balance by setting the exergy destruction equal to zero,
X X out
in
Net exergy transfer
by heat, work,and mass
X destroyed0 (reversible) X system Wrev,in X 2 X1
Exergy
destruction
Change
in exergy
Substituting the closed system exergy relation, the reversible work input during this process is determined to be
Wrev,in m(u1 u 2 ) T0 ( s1 s 2 ) P0 (v 1 v 2 )
(7.639 kg){(439.27 608.52) kJ/kg (298 K)(1.3609 1.8459) kJ/kg K
+ (100 kPa)(0.001047 0.1176)m 3 / kg[1 kJ/1 kPa m 3 ]}
278 kJ
(b) The exergy destruction (or irreversibility) associated with this process can be determined from its definition Xdestroyed =
T0Sgen where the entropy generation is determined from an entropy balance on the cylinder, which is an insulated closed
system,
S in S out S gen S system
Net entropy transfer
by heat and mass
Entropy
generation
Change
in entropy
S gen S system m( s 2 s1 )
Substituting,
X destroyed T0 S gen mT0 ( s 2 s1 ) (298 K)(7.639 kg)(1.8459 1.3609)kJ/kg K = 1104 kJ
PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course
preparation. If you are a student using this Manual, you are using it without permission.