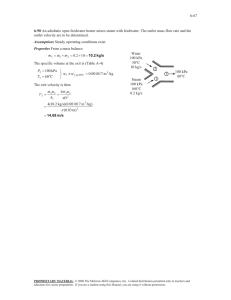
Steam Mixing Problem: Thermodynamics Solution
advertisement
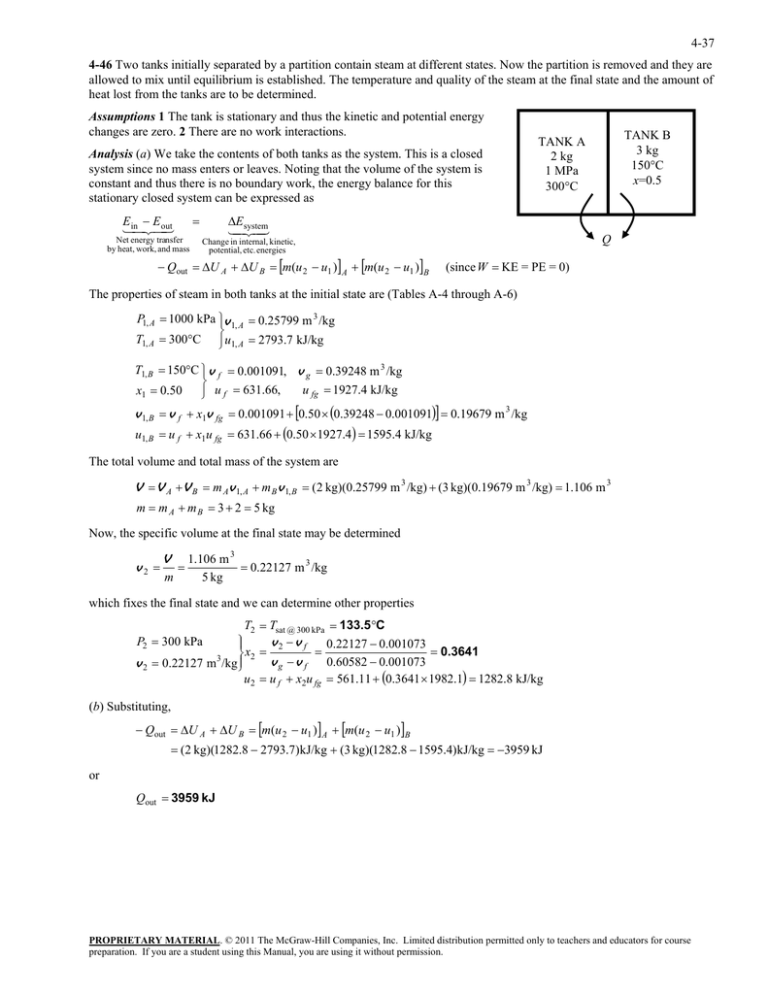
4-37 4-46 Two tanks initially separated by a partition contain steam at different states. Now the partition is removed and they are allowed to mix until equilibrium is established. The temperature and quality of the steam at the final state and the amount of heat lost from the tanks are to be determined. Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions. Analysis (a) We take the contents of both tanks as the system. This is a closed system since no mass enters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as E E inout Net energy transfer by heat, work, and mass E system Q Change in internal, kinetic, potential, etc. energies Qout U A U B m(u 2 u1 ) A m(u 2 u1 )B TANK B 3 kg 150C x=0.5 TANK A 2 kg 1 MPa 300C (since W KE = PE = 0) The properties of steam in both tanks at the initial state are (Tables A-4 through A-6) P1, A 1000 kPa v 1, A 0.25799 m 3 /kg T1, A 300C u1, A 2793.7 kJ/kg T1, B 150C v f 0.001091, v g 0.39248 m 3 /kg u fg 1927.4 kJ/kg x1 0.50 u f 631.66, v 1, B v f x1v fg 0.001091 0.50 0.39248 0.001091 0.19679 m 3 /kg u1, B u f x1u fg 631.66 0.50 1927.4 1595.4 kJ/kg The total volume and total mass of the system are V V A V B m Av 1, A m B v 1, B (2 kg)(0.25799 m 3 /kg) (3 kg)(0.19679 m 3 /kg) 1.106 m 3 m m A m B 3 2 5 kg Now, the specific volume at the final state may be determined v2 V m 1.106 m 3 0.22127 m 3 /kg 5 kg which fixes the final state and we can determine other properties T2 Tsat @ 300 kPa 133.5 C v2 v f 0.22127 0.001073 0.3641 x2 3 v g v f 0.60582 0.001073 v 2 0.22127 m /kg u2 u f x2u fg 561.11 0.3641 1982.1 1282.8 kJ/kg P2 300 kPa (b) Substituting, Qout U A U B m(u 2 u1 ) A m(u 2 u1 )B (2 kg)(1282.8 2793.7)kJ/kg (3 kg)(1282.8 1595.4)kJ/kg 3959 kJ or Qout 3959 kJ PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.