5-32 removed and they are allowed to mix until equilibrium is... 5-42
advertisement

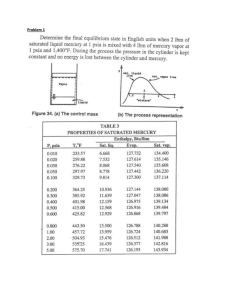
5-32 5-42 Two tanks initially separated by a partition contain steam at different states. Now the partition is removed and they are allowed to mix until equilibrium is established. The temperature and quality of the steam at the final state and the amount of heat lost from the tanks are to be determined. Assumptions 1 The tank is stationary and thus the kinetic and potential energy changes are zero. 2 There are no work interactions. Analysis (a) We take the contents of both tanks as the system. This is a closed system since no mass enters or leaves. Noting that the volume of the system is constant and thus there is no boundary work, the energy balance for this stationary closed system can be expressed as E E in out Net energy transfer by heat, work, and mass Qout TANK B 3 kg 150qC x=0.5 TANK A 2 kg 1 MPa 300qC 'Esystem Q Change in internal, kinetic, potential, etc. energies >m(u 2 u1 )@A >m(u 2 u1 )@B 'U A 'U B (since W KE = PE = 0) The properties of steam in both tanks at the initial state are (Tables A-4 through A-6) P1, A 1000 kPa ½°v 1, A ¾ 300qC °¿u1, A T1, A 0.25799 m 3 /kg 2793.7 kJ/kg 150qC ½ v f 0.001091, v g 0.39248 m 3 /kg ¾ u fg 1927.4 kJ/kg 0.50 ¿ u f 631.66, T1, B x1 v 1, B v f x1v fg u1, B u f x1u fg 0.001091 >0.50 u 0.39248 0.001091@ 0.19679 m 3 /kg 631.66 0.50 u 1927.4 1595.4 kJ/kg The total volume and total mass of the system are V V A V B m m A mB m Av 1, A m Bv 1, B 3 2 (2 kg)(0.25799 m 3 /kg) (3 kg)(0.19679 m 3 /kg) 1.106 m 3 5 kg Now, the specific volume at the final state may be determined v2 V m 1.106 m 3 5 kg 0.22127 m 3 /kg which fixes the final state and we can determine other properties P2 v2 T2 ½° ¾ x2 0.22127 m3/kg °¿ u2 300 kPa Tsat @ 300 kPa 133.5 qC v2 v f 0.22127 0.001073 vg v f u f x2u fg 0.3641 0.60582 0.001073 561.11 0.3641 u 1982.1 1282.8 kJ/kg (b) Substituting, Qout 'U A 'U B >m(u 2 u1 )@A >m(u 2 u1 )@B (2 kg)(1282.8 2793.7)kJ/kg (3 kg)(1282.8 1595.4)kJ/kg or Qout 3959 kJ 3959 kJ PROPRIETARY MATERIAL. © 2008 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.