Heterochrony, generic distinction and phylogeny in the family Hydractiniidae (Hydrozoa: Cnidaria)
advertisement

Commemorative volume for the 80th birthday of Willem Vervoort in 1997
Heterochrony, generic distinction and phylogeny in the family
Hydractiniidae (Hydrozoa: Cnidaria)
F. Boero, J. Bouillon & S. Piraino
Boero, F., J. Bouillon & S. Piraino. Heterochrony, generic distinction and phylogeny in the family
Hydractiniidae (Hydrozoa: Cnidaria).
Zool. Verh. Leiden 323, 31.xii.1998: 25-36, figs 1-4.— ISSN 0024-1652/ISBN 90-73239-68-0.
F. Boero, Dipartimento di Biologia, Stazione di Biologia Marina, Università di Lecce, 73100 Lecce,
Italy.
J. Bouillon, Laboratoire de Biologie Marine, Université Libre de Bruxelles, 50 Ave F.D. Roosevelt, 1050
Bruxelles, Belgium.
S. Piraino, Istituto Sperimentale Talassografico del CNR, Via Roma 3, 74100 Taranto, Italy.
Key words: Cnidaria; Hydrozoa; Anthomedusae; phylogeny; life cycle; classification; heterochrony;
paedomorphosis.
The taxonomy of Hydractinia, Stylactaria and Podocoryna is discussed and the three genera are merged
into Hydractinia since their diagnostic characters are liable to lead to polyphyly and paraphyly, due to
repeated episodes of medusa reduction via heterochrony (paedomorphosis). The phylogeny of the
Hydractiniidae is reconstructed by using two outgroups, Clava and Cytaeis, both having some characters in common with the Hydractiniidae. The resulting phylogenetic trees agree in identifying affinities among Hydractinia, Kinetocodium and Hydrocorella, all with polymorphic colonies with gastrozooids having oral tentacles. The position of Clavactinia (characterized by gastrozooids with widely
scattered tentacles) is at the root of the tree if Clava is the outgroup, whereas it becomes apical when
the outgroup is Cytaeis. The pattern of medusa suppression is different in the two cladograms, since
the presence of a medusa is a plesiomorphic feature when Cytaeis is the outgroup, whereas it becomes
apomorphic when the outgroup is Clava. These inconveniences are difficult to accommodate, since
medusa suppression has occurred many times in the evolution of the hydroidomedusae, and Recent
species do not witness past paedomorphic events of medusa reduction properly, so that many intermediate states are probably missing.
Introduction
T w o genera of the f a m i l y H y d r a c t i n i i d a e are w i d e l y u s e d i n e x p e r i m e n t a l b i o l o g y , n a m e l y Hydractinia
a n d Podocoryna.
A t h i r d a l l i e d genus, m u c h less u s e d f o r
e x p e r i m e n t a l research, i s Stylactaria.
P r e v i o u s studies of species of these genera h a v e concentrated o n r e g e n e r a t i o n (e.
g., A c h e r m a n n , 1980), e m b r y o l o g y (e.g., B o d o & B o u i l l o n , 1968; K r o i h e r & P l i c k e r t ,
1992), m y o g e n e s i s , gametogenesis a n d cnidogenesis (e.g., B o e l s t e r l i , 1977), i n d u c t i o n
of s e x u a l i t y (e.g., B r a v e r m a n , 1962), g r o w t h (e.g., B e r r i l l , 1953; B r a v e r m a n & Schrandt,
1969), t u m o r p r o m o t i o n (e.g., K u r t z & S c h m i d , 1991), e x p r e s s i o n of h o m e o b o x genes
(e.g., A e r n e et a l . , 1995, 1996), t r a n s d i f f e r e n t i a t i o n (e.g., S c h m i d , 1988; S c h m i d et a l ,
1988;
Reber-Müller
et a l , 1994), the o r g a n i z a t i o n of the extracellular m a t r i x (e.g.,
W e b e r & S c h m i d , 1985; Reber-Müller et a l . , 1996), the c o u p l i n g of o n t o g e n y a n d p h y l o g e n y (e.g., Blackstone & Buss, 1992), the c o u p l i n g of g e o l o g i c a l a n d m o l e c u l a r data
i n the r e c o n s t r u c t i o n of s y m b i o t i c r e l a t i o n s h i p s (e.g., C u n n i n g h a m et a l . , 1991), a n d
p a e d o m o r p h o s i s (e.g., C u n n i n g h a m & Buss, 1993; Blackstone & Buss, 1993).
I n spite of these c o n t r i b u t i o n s to e x p e r i m e n t a l b i o l o g y , a n d of b e i n g a classical
26
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
e x a m p l e of p o l y m o r p h i s m i n most textbooks o n C n i d a r i a , the H y d r a c t i n i i d a e still d o
not have a settled generic t a x o n o m y . C u n n i n g h a m & Buss (1993) inferred relationships f r o m m o l e c u l a r data of species of Hydractinia,
Podocoryna a n d Stylactaria to test
the phylogenetic m e a n i n g of m e d u s a r e d u c t i o n i n a l l h y d r o i d o m e d u s a e .
They
s h o w e d that the three genera of the f a m i l y are p r o b a b l y not m o n o p h y l e t i c , a n o p i n i o n
shared also b y Boero et al. (1996) f r o m a p r e l i m i n a r y comparative analysis of m o r p h o l o g i c a l characters. These f i n d i n g s s u p p o r t Petersen's (1990) c r i t i c i s m of the use of
m e d u s a s u p p r e s s i o n as a generic character.
Species, furthermore, are not easily i d e n t i f i e d a n d this is the first g r o u p of h y d r o zoans i n w h i c h s i b l i n g species have been r e c o g n i z e d (Buss a n d Y u n d , 1989), so that
m a n y e x p e r i m e n t a l studies m i g h t have been m a d e o n m a t e r i a l of d u b i o u s identity.
The characters u s e d to d i s t i n g u i s h Hydractinia,
Podocoryna a n d Stylactaria w i l l be
r e v i e w e d to attempt a f o r m a l i s a t i o n of shortcomings of current t a x o n o m y , a n d to
suggest e v o l u t i o n a r y hypotheses that w i l l be testable i n future w o r k w i t h the a i m of
constructing a phylogenetic classification.
The morphological and phylogenetic basis for generic boundaries in
Hydractinia,
Podocoryna and Stylactaria
The genera Hydractinia, Podocoryna a n d Stylactaria are d i s t i n g u i s h e d b y the states
of t w o m a i n m o r p h o l o g i c a l characters:
1 - the structure of the h y d r o r h i z a i n f u l l y g r o w n colonies (fig. 1):
• reticular, f o r m e d b y anastomosed stolons w h i c h r e m a i n separated (Stylactaria a n d
some Podocoryna),
•
encrusting, f o r m e d b y anastomosed stolons covered b y a c o m m o n perisarc (some
Podocoryna),
•
encrusting, f o r m e d b y anastomosed stolons covered b y n a k e d coenosarc after
degeneration of the u p p e r part of the stolonal perisarc sheath (Hydractinia a n d
some species of Podocoryna).
2 - the structure of the gonosome:
•
•
free medusae (Podocoryna).
f i x e d or libérable e u m e d u s o i d s (some species of Hydractinia
and
Stylactaria).
•
f i x e d sporosacs (some species Hydractinia a n d Stylactaria).
The structure of the h y d r o r h i z a , reticular or encrusting, has been generally accepte d b y recent authors as a generic character i n the H y d r a c t i n o i d e a ( M i l l a r d , 1975;
B o u i l l o n , 1985; 1995; C a l d e r , 1988; H i r o h i t o , 1988; Schuchert, 1996). T h i s character,
h o w e v e r , seems to d e p e n d sometimes o n the nature of the substrate (see E d w a r d s ,
1972; Jarms, 1987; H i r o h i t o , 1988). In m a n y y o u n g or regenerating colonies of Hydractinia the h y d r o r h i z a e are reticulate but, nevertheless, the potential for the p r o d u c t i o n
of a layer of n a k e d coenosarc is retained. For Blackstone & Buss (1991; 1992) reticulate
f o r m s are p a e d o m o r p h i c (progenetic) c o m p a r e d w i t h encrusting ones. The g r o w t h of
a h y d r a c t i n i i d colony, i n fact, a l w a y s starts w i t h a reticulate h y d r o r h i z a (thus, a juvenile character) w h i c h then becomes encrusting b y anastomosing.
A c c o r d i n g to 'classic' v i e w s o n h y d r o i d o m e d u s a n phylogenetic characters ( r e v i e w e d
b y C u n n i n g h a m & Buss, 1993), the presence of free medusae is a p l e s i o m o r p h i c feature, whereas m e d u s a r e d u c t i o n represents a d e r i v e d state. In this f r a m e w o r k , Podoco-
27
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
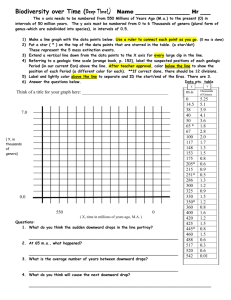
Podocoryna - Stylactaria
non-encrusting, reticular
hydrorhiza formed by
anastomosing stolonal tubes
surrounded by perisarc
Podocoryna
encrusting hydrorhiza formed
by coalescent stolons leading to
a perisarc-covered mat
Podocoryna - Hydractinia
encrusting hydrorhiza formed
by coalescent stolons whose
perisarc degenerates in the upper
portion leading to a coenosarccovered mat
Fig. 1. Schematic representation (in section) of hydrorhizal features of the three main genera of the
Hydractiniidae. Black: perisarc; dark grey: surface of transverse hydrorhizal tube connecting two
main hydrorhizal tubes; light grey: coenosarc.
ryna s h o u l d h a v e p l e s i o m o r p h i c features l i n k e d to the presence of free m e d u s a e a n d ,
also, to the possession of h y d r o r h i z a l character states c o v e r i n g a l l the possibilities liste d above, b e i n g either reticular, perisarc- or coenosarc-covered m a t - l i k e . Stylactaria
s h o u l d represent a m o n o p h y l e t i c clade w i t h m e d u s a e r e d u c e d to e u m e d u s o i d s , a n d a
reticular h y d r o r h i z a (both features as a result of p a e d o m o r p h o s i s ) . Hydractinia
should
be a m o n o p h y l e t i c clade w i t h m e d u s a e r e d u c e d to p a e d o m o r p h i c e u m e d u s o i d s o r to
sporosacs, a n d w i t h stolonal differentiation i n t o a n elaborated m a t of coenosarc, a
p l e s i o m o r p h i c feature present also i n some Podocoryna.
The m o r p h o l o g i c a l o v e r l a p s i n generic characters a m o n g the three taxa, a l o n g
w i t h the incongruences stressed b y C u n n i n g h a m & Buss (1993) u s i n g m o l e c u l a r data,
call for reconsideration of the v a l u e g i v e n to generic characters i n reconstructing p h y logenies.
Sibling species and sibling genera, or: are ancestors real?
A s s i g n m e n t of species to separate genera o n the basis of d i f f e r i n g m e d u s a express i o n (due to p a e d o m o r p h o s i s ) has been criticised b y Petersen (1979; 1990), since paed o m o r p h i c patterns h a v e been s h o w n i n a f e w genera to h a v e arisen m o r e t h a n once.
T h i s v i e w has been s u p p o r t e d w i t h i n the H y d r a c t i n i i d a e b y m o l e c u l a r evidence p r o v i d e d b y C u n n i n g h a m & Buss (1993). T h e same a r g u m e n t m i g h t be u s e d for the type
28
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
of h y d r o r h i z a l organisation, so that colonies w i t h p a e d o m o r p h i c features (such as
those assigned to Stylactaria) m i g h t be the result of i n d e p e n d e n t p h e n o m e n a of heter o c h r o n y affecting the expression of b o t h m e d u s a a n d h y d r o r h i z a .
It m i g h t be possible that a p a e d o m o r p h i c species d e r i v i n g f r o m a Podocoryna-like
ancestor o r i g i n a t e d a m o n o p h y l e t i c clade w h i c h c o u l d be recognised as a s o u n d
genus. B u t p a e d o m o r p h i c species d e r i v i n g f r o m other Podocoryna-like
ancestors
m i g h t e v o l v e the same m o r p h o l o g y i n d e p e n d e n t l y , so that one c o u l d consider t h e m
as b e l o n g i n g to ' s i b l i n g genera' (fig. 2).
U n d e r s u c h circumstances, the m o n o p h y l e t i c clade m i g h t still be considered as a
genus, b u t the isolated p a e d o m o r p h s s h o u l d r e m a i n connected to the 'ancestral'
genus. Petersen (1979, 1990) a r g u e d that i n a f e w genera m e d u s a loss has p r o b a b l y
o c c u r r e d m o r e t h a n once w i t h i n the same clade, thus l e a d i n g to p o l y p h y l e t i c taxa as
based o n a generic character not correlated w i t h other a u t a p o m o r p h i e s . F o r this reason the p a e d o m o r p h s are to be assigned to the same genus of the n o n - p a e d o m o r p h i c
species, if n o other characters d i v e r g e f r o m the ancestral state. H o w e v e r , if, as s u g gested above, a n event of m e d u s a r e d u c t i o n were f o l l o w e d b y r a d i a t i o n of species
r e t a i n i n g that character, the result m i g h t be a m o n o p h y l e t i c clade of possible generic
rank.
In a l l e v o l u t i o n a r y studies based o n l i v i n g species, it is almost i m p o s s i b l e to
recognise sister species f r o m ' m o t h e r ' species. C l a d i s m , furthermore, considers ancestors as i d e a l i s e d entities w i t h p l e s i o m o r p h i c features, whereas it is u n d e n i a b l e that
ancestors are not ideas b u t species, at least as m u c h as their descendants (Rasnitsyn,
1996). It is not inconceivable that a still l i v i n g species o r i g i n a t e d a g r o u p of species b y
cladogenesis, w h i l e r e m a i n i n g u n m o d i f i e d or g o i n g t h r o u g h anagenesis i n its core
population(s). These events, i n b o t h m o r p h o l o g i c a l a n d m o l e c u l a r studies, m i g h t l e a d
to i d e n t i f i c a t i o n of ' s i s t e r h o o d ' a n d not of ' m o t h e r h o o d ' . Palaeontology m i g h t h e l p i n
s o l v i n g the p r o b l e m , b u t the fossil r e c o r d of h y d r a c t i n i i d s is too scarce to be of use.
The lack of consistency f o u n d b y C u n n i n g h a m & Buss (1993) w h i l e c o m p a r i n g
genome fractions of Hydractinia, Podocoryna a n d Stylactaria m i g h t suggest that single
species assigned either to Stylactaria a n d Hydractinia o r i g i n a t e d i n d e p e n d e n t l y f r o m
Podocoryna-like
ancestors, l e a d i n g to p o l y p h y l e t i c {Stylactaria a n d Hydractinia) a n d
p a r a p h y l e t i c (Podocoryna) taxa, as suggested b y Boero et al. (1996) (fig. 2).
B e i n g ' p l e s i o m o r p h i c ' , the Podocoryna set of characters m i g h t lead to g r o u p i n g
species into a compact clade, also f r o m a m o l e c u l a r p o i n t of v i e w . The compactness of
the clade, h o w e v e r , s h o u l d not be necessarily a n i n d i c a t i o n of m o n o p h y l y , b u t o n l y of
lack of d e v i a t i o n f r o m a n ancestral state.
The c l a d o g r a m p r o p o s e d i n f i g . 2 is rather u n o r t h o d o x , since it represents ancestors as real taxa. F u r t h e r m o r e , it is characterised b y several p a r a l l e l events of e v o l u t i o n a r y change. H o w e v e r , if a change is d u e to a d d i t i o n of n e w characters (e.g., the
a c q u i s i t i o n of a m e d u s a stage), it is u n p a r s i m o n i o u s to p r o p o s e i n d e p e n d e n t origins
of the same character, b u t if the change is d u e character s i m p l i f i c a t i o n (e.g., the loss of
a m e d u s a stage v i a p a e d o m o r p h o s i s ) , the p o s s i b i l i t y of p a r a l l e l events is not to be
considered as u n p a r s i m o n i o u s as i n the f o r m e r case. M e d u s a s u p p r e s s i o n has
o c c u r r e d i n the H y d r o i d o m e d u s a e i n d e p e n d e n t l y so m a n y times (see B o u i l l o n , 1985;
Petersen, 1990; C o r n e l i u s , 1992) that is currently considered as a v e r y probable event.
C u r r e n t l y , the o n l y w a y to a v o i d the u n n a t u r a l taxa d e r i v i n g f r o m the situation
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
29
Fig. 2. Hypothetical phylogenetic scenario showing the possibility of the polyphyly of the currently
recognised hydractiniid genera with reduced medusae (Stylactaria and Hydractinia), and of the paraphyly of the genus comprising species with medusae (Podocoryne).
d e p i c t e d i n f i g . 2 is to merge b o t h p o l y p h y l e t i c a n d p a r a p h y l e t i c genera, so as to h a v e
a m o n o p h y l e t i c clade, e v e n if of v e r y remote ancestry. So w e p r o p o s e here to merge
the m a i n three genera of h y d r a c t i n i i d s (Hydractinia
Sars, 1846; Stylactaria
v a n Beneden, 1841;
Stechow, 1921) into the oldest one: Hydractinia
Podocoryna
v a n Beneden,
1841. S i m i l a r positions have been taken already b y several authors: M o t z - K o s s o w s k a
(1905) m e r g e d Hydractinia,
Podocoryna a n d Stylactaria
(= Stylactis) into
Hydractinia;
30
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
B r o c h (1914) considered that Stylactaria a n d Hydractinia
c o u l d not be kept apart; a n d
N a u m o v (1960/1969) m e r g e d Podocoryna into Hydractinia.
ryna a n d Stylactaria subgeneric r a n k w i t h i n Hydractinia,
K r a m p (1932) gave Podoco-
b u t this is not p h y l o g e n e t i c a l -
l y s o u n d . If Podocoryna a n d Stylactaria were m o n o p h y l e t i c , they c o u l d w e l l be r a n k e d
as genera; if they are not m o n o p h y l e t i c (as it is suggested here), they are u n s o u n d
e v e n as subgenera.
These inconveniences cannot be a v o i d e d i n m o r p h o l o g i c a l studies; m o l e c u l a r
approaches m i g h t h e l p i n d i s t i n c t i o n of m o n o p h y l e t i c clades f r o m 'sister clades' shari n g m e d u s a r e d u c t i o n , thus i d e n t i f y i n g w h a t c o u l d be p o s s i b l y called ' s i b l i n g genera'.
T h e d i a g n o s i s of Hydractinia v a n B e n e d e n ,
1841
C o l o n i e s w i t h a stolonal reticular h y d r o r h i z a f o r m e d b y tubes covered w i t h perisarc, or w i t h a n encrusting h y d r o r h i z a covered w i t h perisarc or w i t h n a k e d coenosarc;
t y p i c a l l y w i t h s i m p l e , canaliculated or b r a n c h e d spines. H y d r a n t h s sessile, n a k e d ,
p o l y m o r p h i c : gastrozooids, g o n o z o o i d s , a n d occasionally d a c t y l o z o o i d s . Gastroz o o i d s w i t h one or m o r e close w h o r l s of tentacles e n c i r c l i n g the h y p o s t o m e ; gonoz o o i d s w i t h one or m o r e close w h o r l s of tentacles, or w i t h o u t tentacles a n d / o r h y p o stome, b e i n g r e d u c e d to blastostyles; d a c t y l o z o o i d s w i t h o u t tentacles. G o n o p h o r e s :
either f i x e d sporosacs, libérable or retained e u m e d u s o i d s , or free medusae, a r i s i n g
f r o m v a r y i n g l y d e v e l o p e d g o n o z o o i d s or directly f r o m the h y d r o r h i z a . M e d u s a e
m o r e or less bell-shaped. F o u r r a d i a l canals; f o u r or m o r e s o l i d a n d s i m p l e m a r g i n a l
tentacles, w i t h or w i t h o u t ocelli. M a n u b r i u m w i t h or w i t h o u t p e d u n c l e , t u b u l a r or
sac-shaped, w i t h f o u r or eight s i m p l e or s l i g h t l y b r a n c h e d o r a l arms (dilatations of
the m o u t h r i m ) a r m e d w i t h clusters of cnidocysts. G o n a d s o n m a n u b r i u m , generally
i n t e r r a d i a l b u t sometimes e x t e n d i n g to the p r o x i m a l p o r t i o n of r a d i a l canals. Sometimes asexual r e p r o d u c t i o n b y m e d u s a b u d d i n g o n m a n u b r i u m .
T h e p h y l o g e n y of the H y d r a c t i n i i d a e
B o u i l l o n (1995) assigned the f o l l o w i n g l i v i n g genera to the H y d r a c t i n i i d a e : Clavactinia T h o r n e l y , 1904; Hansiella B o u i l l o n , 1980; Hydractinia v a n Beneden, 1841; Hydrocorella Stechow, 1921; Janaria Stechow, 1921; Kinetocodium K r a m p , 1921; Podocoryna
Sars, 1846; Stylactaria Stechow, 1921; a n d Tregoubovia P i c a r d , 1958. B o u i l l o n (1985;
1995) also listed a series of fossil genera d o u b t f u l l y assigned to this f a m i l y because of
skeletal remains, b u t the p a u c i t y of diagnostic features makes t h e m useless for a p h y logenetic reconstruction of the f a m i l y a n d they w i l l not be considered here.
B o t h Hansiella a n d Tregoubovia are medusa-based genera, their h y d r o i d s b e i n g
u n k n o w n . The m e d u s a of Thecocodium quadratum (Werner, 1965) (family P t i l o c o d i idae) described b y Jarms (1987), h o w e v e r , shares some k e y features w i t h the medusae
of the t w o above m e n t i o n e d genera, n a m e l y a m a r g i n a l nematocyst r i n g a n d d i d e r m i c e x u m b r e l l a r processes, a n d this w i l l a l l o w assignment of b o t h Hansiella a n d Tregoubovia to the P t i l o c o d i i d a e ( B o u i l l o n et al., 1997). Hydractinia, Podocoryna a n d Stylactaria are here m e r g e d into Hydractinia (see above). Hydrocorella a n d Janaria are characterized b y a calcareous skeleton. G e n e r i c differences are m i n o r for the p u r p o s e of this
r e v i s i o n a n d the t w o genera w i l l be treated together u n d e r Hydrocorella e v e n if they
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
31
are to be considered as separate (see C a i r n s & B a r n a r d , 1984 for a treatment
of
Janaria). Kinetocodium comprises o n l y one species w h i c h has been f o u n d a f e w times,
e p i z o i c o n p t e r o p o d s ( K r a m p , 1921; 1957). O n l y the y o u n g m e d u s a is k n o w n a n d its
p o s i t i o n is still uncertain, p e n d i n g k n o w l e d g e of its complete life cycle. T h u s , for ease
of analysis, the H y d r a c t i n i i d a e is considered here to comprise Clavactinia,
Kinetocodium a n d
Hydractinia,
Hydrocorella.
The o u t g r o u p s of the H y d r a c t i n i i d a e
The k e y features of the H y d r a c t i n i i d a e (see above for details) are the presence of
n a k e d h y d r a n t h s d e p r i v e d of stem, directly connected to a h y d r o r h i z a l system w h i c h
can be a m o r e or less elaborated n e t w o r k . The h y d r a n t h s are p o l y m o r p h i c , b e i n g d i s t i n g u i s h e d i n tentacled gastrozooids, a n d g o n o z o o i d s . In m a n y species b o t h dactyloz o o i d s a n d spines are present. The medusae, w h e n present, have o r a l l i p s a r m e d w i t h
nematocysts w h i c h can be arranged i n clusters (Podocoryna) or i n a single c o n t i n u o u s
row
(Kinetocodium).
A s u r v e y of a l l other A n t h o m e d u s a e (from B o u i l l o n , 1985) for the choice of a n
o u t g r o u p indicates t w o genera as best candidates:
•
Clava (family C l a v i d a e ) shares w i t h the H y d r a c t i n i i d a e the presence of n a k e d
h y d r a n t h s d e p r i v e d of stem, directly connected to a h y d r o r h i z a l system w h i c h
can range f r o m s i m p l e stolons to a n e t w o r k of t i g h t l y anastomosed stolons sometimes b e c o m i n g covered, as r e p o r t e d b y H i n c k s (1868) a n d M o t z - K o s s o w s k a
(1905), b y a layer of perisarc. C o n t r a r y to the H y d r a c t i n i i d a e , Clava is m o n o m o r phe
a n d its gonophores, as f i x e d sporosacs, originate o n undifferentiated h y -
dranths. Fraser (1946: 138) considered that Stylactaria
"must have come directly
f r o m the C l a v i d a e " .
•
Cytaeis (family Cytaeidae) shares w i t h the H y d r a c t i n i i d a e the presence of n a k e d
h y d r a n t h s d e p r i v e d of stem, directly connected to a reticular h y d r o r h i z a , b u t is
m o n o m o r p h i c , a n d the gonophores arise f r o m the h y d r o r h i z a . The medusae have
o r a l tentacles inserted above the m o u t h .
The p o l a r i s a t i o n of the H y d r a c t i n i i d a e
Clava as a n o u t g r o u p . — The h y d r a c t i n i i d genus w i t h characters closest to Clava is
Clavactinia.
I n b o t h genera the h y d r a n t h s are n a k e d a n d have scattered tentacles, b u t
Clavactinia is p o l y m o r p h i c a n d its h y d r o r h i z a is covered b y n a k e d coenosarc (see M i l l a r d & B o u i l l o n , 1973 for a detailed description). B o t h genera lack a m e d u s a stage. A l l
the other genera of the H y d r a c t i n i i d a e have tentacles concentrated a r o u n d the m o u t h ,
i n one or m u l t i p l e b u t closely set w h o r l s (for this reason w e i n c l u d e Clavactinia
tentaculata M i l l a r d , 1975, i n Hydractinia).
Hydractinia,
multi-
w i d e l y discussed above, is char-
acterised b y p o l y m o r p h i s m , gastrozooids w i t h o r a l tentacles, a n d b y the presence of
medusae w i t h o r a l l i p s a r m e d w i t h nematocyst clusters. Kinetocodium is k e p t separate
f r o m Hydractinia
because g o n o z o o i d s are absent, a n d its m e d u s a e originate directly
f r o m the h y d r o r h i z a a n d have a c o n t i n u o u s r i n g of nematocysts a r o u n d the m o u t h ,
instead of nematocyst
clusters. Hydrocorella
has p o l y m o r p h i c colonies w i t h f i x e d
gonophores a n d is separated because its h y d r o r h i z a is calcareous, r e s e m b l i n g that of
32
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
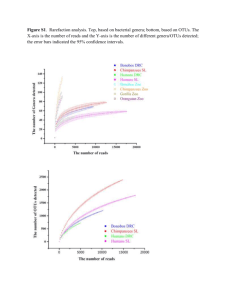
Fig. 3. Cladogram of the Hydractiniidae with Clava as an outgroup.
a h a r d coral. The r e s u l t i n g c l a d o g r a m is presented i n f i g . 3.
Cytaeis as a n o u t g r o u p . — B o t h Cytaeis a n d some species of Hydractinia (those form e r l y assigned to Podocoryna) share the presence of a m e d u s a . The m a i n difference is
that i n Cytaeis the o r a l z o n e of the m a n u b r i u m is a r m e d w i t h o r a l tentacles inserted
above the m o u t h , whereas i n Hydractinia the m o u t h is a r m e d w i t h m o r e or less d e v e l o p e d nematocyst clusters borne directly o n the l i p s . Besides this feature, the medusae
of the t w o families are quite s i m i l a r . T h e h y d r o r h i z a of Cytaeis is reticular, as is that of
some species of Hydractinia. The h y d r a n t h s , furthermore, are s i m i l a r , e v e n t h o u g h
Cytaeis is m o n o m o r p h i c . A s i n the c l a d o g r a m r e s u l t i n g f r o m the choice of Clava as a n
o u t g r o u p (fig. 3), the genera Kinetocodium a n d Hydrocorella are close to Hydractinia,
whereas Clavactinia is separated f r o m the rest of the clade b y the presence of scattered
tentacles. The r e s u l t i n g c l a d o g r a m is g i v e n i n f i g . 4.
The c o m p a r i s o n of the cladograms d e r i v i n g f r o m the t w o chosen o u t g r o u p s (figs.
3 a n d 4) s h o w that w h e n Clava is the o u t g r o u p , Clavactinia has p l e s i o m o r p h i c features, whereas its scattered tentacles become a p o m o r p h i c if Cytaeis is the o u t g r o u p .
The presence of a m e d u s a stage, w h e n Clava is the o u t g r o u p , is not connected w i t h
ancestral features, a n d this leads to the hypothesis that ancestral species w i t h
medusae a n d w i d e l y scattered tentacles became extinct or are still to be d i s c o v e r e d .
The hypothesis is s u p p o r t e d b y the presence of t w o genera w i t h medusae (Turritopsis
a n d Oceania) i n the C l a v i d a e . T h e i r h y d r o i d s have erect, b r a n c h e d stems, b e i n g
noticeably different f r o m those of the H y d r a c t i n i i d a e , b u t their medusae are v e r y s i m ilar to those of the H y d r a c t i n i i d a e , a n d there m i g h t be n o justification for k e e p i n g
t h e m i n separate families, if their h y d r o i d s w e r e not so different.
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
33
Fig. 4. Cladogram of the Hydractiniidae with Cytaeis as an outgroup.
W h e n Cytaeis is the o u t g r o u p , there is n o n e e d for the e x t i n c t i o n of ancestors w i t h
medusae, since b o t h Cytaeis a n d some Hydractinia
have medusae and naked feeding
h y d r a n t h s w i t h a single r o w of tentacles, o r i g i n a t i n g f r o m a reticular h y d r o r h i z a . The
m a i n difference i n the m e d u s a e of Cytaeis a n d Hydractinia
tacles i n the former. T h e passage f r o m Cytaeis to Hydractinia
is the presence of o r a l tenleads one to hypothesise
the loss of the o r a l tentacles (reduced to nematocyst clusters) a n d the e v o l u t i o n of
polymorphism.
Conclusion
T h e scope of Hydractinia still r e m a i n s d o u b t f u l , d u e to lack of i n f o r m a t i o n about
the genera here m e r g e d w i t h it: Podocoryna a n d Stylactaria. It m i g h t be possible that
m o n o p h y l e t i c clades of species f a l l i n g w i t h i n the scope of Hydractinia a n d Stylactaria
really exist, b u t that repeated heterochronic events i n Podocoryna-like
ancestors l e d to
' s i b l i n g genera'. The H y d r a c t i n i i d a e h a v e strict affinities w i t h b o t h the C l a v i d a e a n d
the C y t a e i d a e .
T h e p r o b l e m of m e d u s a loss raises some p r o b l e m s i n p h y l o g e n e t i c analysis. It is
conceivable, that a l l families w i t h m e d u s a e o r i g i n a t e d f r o m ancestors w i t h medusae,
a n d that m e d u s a e w e r e lost i n d e p e n d e n t l y i n the v a r i o u s families. The h y p o t h e s i s
that s u c h elaborate m o r p h s as the m e d u s a e e v o l v e d i n d e p e n d e n t l y i n m a n y clades
w i t h n o m e d u s a requires far too u n l i k e l y p a r a l l e l events. Some families, furthermore,
have n o species w i t h m e d u s a e . M e d u s a r e d u c t i o n or loss b y p a e d o m o r p h o s i s has
o c c u r r e d repeatedly a n d i n d e p e n d e n t l y d u r i n g h y d r o i d o m e d u s a n e v o l u t i o n r e s u l t i n g
34
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
i n s i m i l a r i t y i n m o r p h o l o g y perhaps b e i n g unreliable as a p o i n t of reference i n some
genera. The g r o u p i n g of a l l spec ies w i t h r e d u c e d medusae i n clades separate f r o m
those of s i m i l a r spec ies w i t h medusae, i n fac t, is m o r e p a r s i m o n i o u s t h a n c o n s i d e r i n g
t h e m as o r i g i n a t e d b y possible p a r a l l e l events, b u t this is the case i n a f e w k n o w n
examples (as co n v i n ci n g l y a r g u e d b y Petersen, 1990), i n spite of b e i n g less p a r s i m o ­
nious.
C o m p a r i s o n of the t w o c ladograms d e r i v e d u s i n g different o u t g r o u p s (figs 3 a n d
4), i n this f r a m e w o r k , makes Cytaeis a better o u t g r o u p t h a n Clava, bec ause i n this c ase
b o t h the o u t g r o u p a n d the stem spec ies of the f a m i l y have the p l e s i o m o r p h i c feature
of a m e d u s a stage. W h e n Clava is the o u t g r o u p , the m e d u s a appears as a n e w l y ­
acquired
feature,
a less p a r s i m o n i o u s c onc lusion,
as h y p o t h e s i z e d
above (but
medusae c o u l d bec ome suppressed i n some c lades w h i l e h a v i n g the p o s s i b i l i t y of
b e i n g re­expressed i n the c ourse of e v o l u t i o n , as a r g u e d b y Boero et al., 1996). Clava,
furthermore, is referred to the distant f a m i l y C l a v i d a e solely o n the basis of its sc at­
tered tentac les, b u t s u c h a feature is p r o v i n g to be shared b y m a n y spec ies referred to
different families a n d is p o s s i b l y not a g o o d f a m i l y c harac ter. The h y d r o r h i z a l system
of Clava c losely resembles that of some Hydractinia
spec ies a n d future m o l e c u l a r s t u d ­
ies w i l l p o s s i b l y better define the affinities of these taxa a n d the w h o l e p h y l o g e n y of
the H y d r a c t i n i d a e .
Acknowledgements
T h i s s t u d y w a s s u p p o r t e d b y c ontributions f r o m M . U . R . S . T . (40 a n d 60% p r o ­
grams), the M a r i n e Reserve of U s t i ca (Italy), P r o v i n c e of Lecce, a n d of the F . N . R . S . d e
Belgique. L e o Buss a n d C l i f f C u n n i n g h a m read a n d c o m m e n t e d o n the m a n u s c r i p t ,
s h a r i n g w i t h us their prec ious ' m o l e c u l a r ' insights.
T h i s paper is dedicated to o u r f r i e n d a n d c olleague W i m V e r v o o r t , w h o s e w o r k
o n h y d r o z o a n s w i l l r e m a i n an i n v a l u a b l e e x p l o r a t i o n of the d i v e r s i t y of the g r o u p .
References
Achermann, J., 1980. The fate and regeneration capacity of isolated ecto­ and endoderm in polyps of
Podocoryne carnea M . Sars (Hydrozoa, Athecata). In: P. Tardent & R. Tardent (eds). Developmental
and cellular biology of coelenterates.— Elsevier/North­Holland Biomedical Press, Amsterdam:
273­279.
Aerne, B.L., C D . Baader & V. Schmid, 1995. Life stage and tissue­specific expression of the homeobox
gene cnoxl­Pc of the hydrozoan Podocoryne carnea.— Dev. Biol. 169: 547­556.
Aerne, B., H . Gròger, P. Schuchert, J. Spring & V. Schmid, 1996. The polyp and its medusa: a molecu­
lar approach.— Sei. Mar. 60 (1): 7­16.
Berrill, N .J. 1953. Growth and form in gymnoblastic hydroids. VI. Polymorphism within the Hydrac­
tiniidae.—J. Morph. 92: 241­272.
Blackstone, N .W. & L. Buss, 1991. Shape variation in hydractiniid hydroids.— Biol. Bull. (Woods
Hole) 180 (3): 394­405.
Blackstone, Ν. W. & L. Buss, 1992. Treatment with 2, 4­dinitrophenil mimics ontogenetic and phylo­
genetic changes in a hydractiniid hydroid.— Proc. N at. Acad. Sei. U.S.A. 89: 4057­4061.
Blackstone, Ν. W. & L. W. Buss, 1993. Experimental heterochrony in hydractiniid hydroids: why
mechanisms matter.— J. Evol. Biol. 6 (3): 307­327.
Bodo, F. & J. Bouillon, 1968. Étude histologique du développement
embryonnaire de quelques
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
35
hydroméduses de Roseoff: Phialidium hemisphaericum (L.), Obelia sp. Péron et Lesueur, Sarsia
eximia (Allman), Podocoryne carnea (Sars), Gonionemus vertens Agassiz.— Cah. Biol. Mar. 9: 69-104.
Boelsterli, U . 1977. A n electron microscopic study of early developmental stages, myogenesis, oogenesis and cnidogenesis in the Anthomedusa, Podocoryne carnea M . Sars.— J. Morph. 154: 259-290.
Boero, F., J. Bouillon & S. Piraino, 1996. Classification and phylogeny in the Hydroidomedusae
(Hydrozoa, Cnidaria).— Sei. Mar. 60 (1): 17-33.
Bouillon, J. 1985. Essai de classification des Hydropolypes - Hydroméduses (Hydrozoa-Cnidaria).—
Indo-Malayan Zool. 2: 29-243.
Bouillon, J. 1995. Classe des Hydrozoaires. In: P.P. Grasse & D . Doumenc (eds). Traité de Zoologie
3.— Masson, Paris: 29-416.
Bouillon, J., D. Medel & A . L . Pena Cantero, 1997. The taxonomie status of the genus Stylactaria Stechow, 1921 (Hydroidomedusae, Anthomedusae, Hydractiniidae) with the description of a new
species.— Sei. Mar. 61 (4): 471-486.
Braverman, M . H . 1962. Studies in hydroid differentiation. I. Podocoryne carnea culture methods and
carbon dioxide induced sexuality.— Exp. Cell Res. 26: 301-306.
Braverman, M . H . & R. Schrandt, 1969. Studies on hydroid differentiation. V. The control of growth in
young colonies of Podocoryne carnea.— Growth 33: 241-254.
Broch, H . 1914. Hydrozoa benthonica. In: W. Michaelsen (ed.). Beiträge zur Kenntnis der Meeresfauna
westafrikas. 1.— Friederichsen, Hamburg: 19-50.
Buss, L. & P. Yund, 1989. A sibling species group of Hydractinia in the north-eastern United States.— J.
Mar. Biol. Ass. U . K. 69: 857-874.
Cairns, S.D. & J.L. Barnard, 1984. Redescription of Janaria mirabilis, a calcified hydroid from the eastern Pacific— Bull. S. Calif. Acad. Sei. 83:1-11.
Calder, D. 1988. Shallow water hydroids of Bermuda. The Athecatae.— Life Sei. Contr. R. Ontario
Mus. 148:1-107.
Cornelius, P.F.S., 1992. Medusa loss in leptolid Hydrozoa (Cnidaria), hydroid rafting, and abbreviated
life-cycles among their remote-island faunae: an interim review.— Sei. Mar. 56 (2-3): 245-261.
Cunningham, C , L.W. Buss & C. Anderson, 1991. Molecular and geologic evidence of shared history
between hermit crabs and the symbiotic genus Hydractinia. — Evolution 45:1301-1315.
Cunningham, C. & L.W. Buss, 1993. Molecular evidence for multiple episodes of paedomorphosis in
the family Hydractiniidae.— Biochem. Syst. Ecol. 21: 57-69.
Edwards, C. 1972. The hydroids and the medusae Podocoryne areolata, P. borealis and P. carnea.— J.
Mar. Biol. Ass. U . K. 52: 97-144.
Fraser, C M . 1946. Distribution and relationship in American hydroids.— The Univ. Toronto Press,
Toronto: 1-464.
Hincks, T. 1868. The History of the British hydroid zoophytes.— John Van Voorst, London, Vol. 1: 1338, Vol. 2: 67 plates.
Hirohito, Emperor of Japan, 1988. The Hydroids of Sagami Bay. Part 1. Athecata.— Pubis Biol. Lab.,
Imp. Household, Tokyo: 1-179.
Jarms, G . 1987. Thecocodium quadratum (Werner, 1965) redescribed, T. penicillatum sp. nov., and a
method for rearing hydrozoans. In: J. Bouillon, F. Boero, F. Cicogna, & P.F.S. Cornelius (eds).
Modern trends in the Systematics, Ecology and Evolution of Hydroids and Hydromedusae.—
Clarendon Press, Oxford: 57-66.
Kramp, P.L. 1921. Kinetocodium danae, n. g., n. sp. a new gymnoblastic hydroid, parasitic on a pteropod.— Vidensk. Meddr dansk naturh. Foren 74:1-21.
Kramp, P.L. 1932. Hydroids. In: The Godthaab Expedition 1928.— Meddr Grönland 79:1-86.
Kramp, P.L. 1957. Notes on a living specimen of the hydroid Kinetocodium danae Kramp, parasitic on a
pteropod.— Vidensk. Meddr dansk naturh. Foren. 119: 47-54.
Kroiher, M . & G . Plickert, 1992. Analysis of pattern formation during embryonic development of
Hydractinia echinata.— Wilhelm Roux's Arch. Dev. Biol. 201: 95-104.
Kurtz, E. & V. Schmid, 1991. Effect of tumor promotors and diacylglycerol on the transdifferentiation
of striated muscle cells on the medusa Podocoryne carnea to RF-amide positive nerve cells.—
Hydrobiologia 216-217:11-17.
36
Boero et al. Heterochrony and phylogeny of Hydractiniidae. Zool. Verh. Leiden 323 (1998)
Millard, N . A . H . 1975. Monograph on the Hydroida of southern Africa.— Ann. S. Afr. Mus. 68:1-513.
Millard, N . A . H . & J. Bouillon, 1973. Hydroids from the Seychelles (Coelenterata).— Ann. Mus. R. Afr.
Centr., Série in 8°, Sei. Zool. 206:1-106.
Motz-Kossowska, S., 1905. Contribution à la connaissance des hydraires de la Méditerranée occidentale. I.- Hydraires Gymnoblastiques.— Archs Zool. exp. Gén. 4: 39-88.
Naumov, D.V. 1960-1969. Gidroidi i gidromedusy morskikh, solonovatovodnykh i presnovodnykh
basseinov
SSSR.— Opredeleteli po faune
SSSR, Izdavaemye Zoologicheskim
Institutom
Akademii Nauk SSSR. 70 :1-626 (English translation by Israel Program for Scientific Translations,
cat. no. 5108, as "Hydroids and Hydromedusae of the USSR").
Petersen, K.W. 1979. Development of coloniality in Hydrozoa. In: G. Larwood & B. Rosen (eds). Biology and systematics of colonial organisms.— Symp. Syst. Assoc. 11:105-139.
Petersen, K. 1990. Evolution and taxonomy in capitate hydroids and medusae.— Zool. J. Linn. Soc.
100:101-231.
Rasnitsyn, A.P. 1996. Conceptual issues in phylogeny, taxonomy, and nomenclature.— Contr. Zool.
66: 3-41.
Reber-Müller, S., S.-I. Ono, M . Wehrle-Haller & V. Schmid, 1994. Transdifferentiation of striated muscle of jellyfish to smooth muscle and nerve cells: the role of cell-ECM interactions and carbohydrates revealed by a monoclonal antibody.— Differentiation 57: 77-87.
Reber-Müller, S., S.-I. Ono, P. Schuchert, J. Spring & V. Schmid, 1996. Fibrillin in the extracellular
matrix of cnidarians: A n immunohistochemical approach.— Sei. Mar. 60: 55-68.
Schmid, V. 1988. The potential for transdifferentiation and regeneration of isolated striated muscle of
medusae in vitro.— Cell Differ. 22:173-182.
Schmid, V., H . Alder, G . Plickert & C. Weber, 1988. Transdifferentiation from striated muscle of
medusae in vitro. In: G. Eguchi, T.S. Okada & L. Saxen (eds.). Regulatory mechanisms in developmental processes.— Elsevier Scientific Publishers, Dordrecht, Netherlands: 137-146.
Schuchert, P. 1996. Athecate hydroids and their medusae (Cnidaria: Hydrozoa).— N.Z. Oceanogr.
Inst. Mem. 106:1-159.
Weber, C. & V. Schmid, 1985. The fibrous system in the extracellular matrix of Hydromedusae.— Tissue Cell. 17 (6): 811-822.