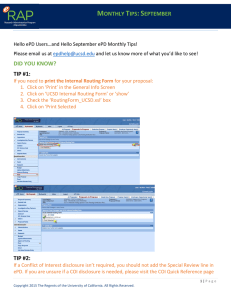
DID YOU KNOW? TIP #1:
advertisement

Hello ePD Users! Where is the summer going? It’s already time for ePD Monthly Tips – August! As always, we value your feedback and suggestions. Please email epdhelp@ucsd.edu and let us know more of what you’d like to see! DID YOU KNOW? TIP #1: When creating a proposal for a Clinical Trial the Activity Type will always be ‘Clinical Research’. However, when selecting the ‘Anticipated Award Type’ of either “Clinical Trial (CTO)” or “PI Initiated Clinical Trial (OCGA), you will need to consider the following criteria: ** More information can be found at OCTA or OCGA TIP #2: When entering the Conflict of Interest line on the Special Review Screen, don’t forget to enter the Last Name, First Name & Middle Initial of the investigator as the Protocol number. TIP #3: If your proposal has Participating Units, remember that the aggregator will need to approve the proposal as the Lead Unit and as the aggregator for each of the Participating Units added to the record. *Note: The order of the routing map is determined numerically by Unit Number.