Document 12099047
advertisement
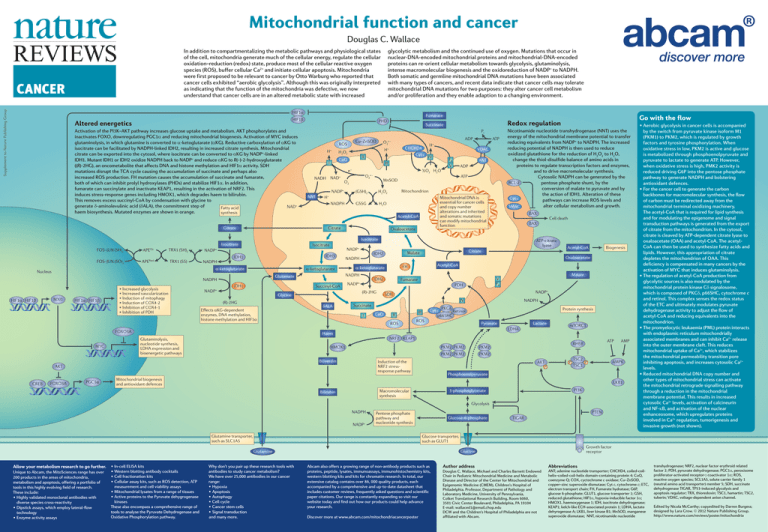
Mitochondrial function and cancer Douglas C. Wallace In addition to compartmentalizing the metabolic pathways and physiological states of the cell, mitochondria generate much of the cellular energy, regulate the cellular oxidation–reduction (redox) state, produce most of the cellular reactive oxygen species (ROS), buffer cellular Ca2+ and initiate cellular apoptosis. Mitochondria were first proposed to be relevant to cancer by Otto Warburg who reported that cancer cells exhibited “aerobic glycolysis”. Although this was originally interpreted as indicating that the function of the mitochondria was defective, we now understand that cancer cells are in an altered metabolic state with increased Supplement to Nature Publishing Group CANCER glycolytic metabolism and the continued use of oxygen. Mutations that occur in nuclear-DNA-encoded mitochondrial proteins and mitochondrial-DNA-encoded proteins can re-orient cellular metabolism towards glycolysis, glutaminolysis, intense macromolecular biogenesis and the oxidoreduction of NADP+ to NADPH. Both somatic and germline mitochondrial DNA mutations have been associated with many types of cancers, and recent data indicate that cancer cells may tolerate mitochondrial DNA mutations for two purposes: they alter cancer cell metabolism and/or proliferation and they enable adaption to a changing environment. HIF1α Fumarate HIF1β Altered energetics Activation of the PI3K–AKT pathway increases glucose uptake and metabolism. AKT phosphorylates and inactivates FOXO, downregulating PGC1α and reducing mitochondrial biogenesis. Activation of MYC induces glutaminolysis, in which glutamine is converted to α-ketoglutarate (αKG). Reductive carboxylation of αKG to isocitrate can be facilitated by NADPH-linked IDH2, resulting in increased citrate synthesis. Mitochondrial citrate can be exported into the cytosol, where isocitrate can be converted to αKG by NADP+-linked IDH1. Mutant IDH1 or IDH2 oxidize NADPH back to NADP+ and reduce αKG to R(–)-2-hydroxyglutarate ((R)-2HG), an oncometabolite that affects DNA and histone methylation and HIF1α activity. SDH mutations disrupt the TCA cycle causing the accumulation of succinate and perhaps also increased ROS production. FH mutation causes the accumulation of succinate and fumarate, both of which can inhibit prolyl hydroxylases (PHDs) and stabilize HIF1α. In addition, fumarate can succinylate and inactivate KEAP1, resulting in the activation of NRF2. This induces stress-response genes including HMOX1, which degrades haem to bilirubin. This removes excess succinyl-CoA by condensation with glycine to generate δ-aminolevulinic acid (δALA), the commitment step of NAD+ Fatty acid haem biosynthesis. Mutated enzymes are shown in orange. synthesis Citrate PHD Succinate Pi H+ II CoQ I NAD+ NNT IV O2· – F0 V ANT ADP ATP MnSOD Mitochondrion H2O2 (GSH)2 H+ NADPH VDAC ½O2 H2O O2 NADP+ H + III e– NADH CHCHD4 Cyt c H+ H2O2 GSSG Mitochondrial DNA is essential for cancer cells and copy number alterations and inherited and somatic mutations can modify mitochondrial function H 2O Acetyl-CoA Citrate ATP ADP O2· – Cu–ZnSOD ROS Oxaloacetate Nicotinamide nucleotide transhydrogenase (NNT) uses the energy of the mitochondrial membrane potential to transfer reducing equivalents from NADP+ to NADPH. The increased reducing potential of NADPH is then used to reduce oxidized glutathione for the reduction of H2O2 to H2O, change the thiol-disulfide balance of amino acids in proteins to regulate transcription factors and enzymes, and to drive macromolecular synthesis. Cytosolic NADPH can be generated by the BCL-2 pentose phosphate shunt, by the conversion of malate to pyruvate and by the action of IDH1. Alteration of these Cyt c pathways can increase ROS levels and alter cellular metabolism and growth. SMAC BAX FOS–JUN (SH)2 FOS–JUN (SO)2 APEOx APERed TRX1 (SH)2 TRX1 (SS) Isocitrate NADPH IDH3 IDH1 α-ketoglutarate Nucleus α-ketoglutarate HIF1α HIF1β MXI1 HIF1α HIF1β IDH1 Succinyl-CoA NADP+ NADPH Effects αKG-dependent enzymes, DNA methylation, histone methylation and HIF1α δALA I PGC1α NADPH PKCδ Cyt c Retinol p66SHC III Protein synthesis ROS Lactate Pyruvate LDHA Haem Glutaminolysis, nucleotide synthesis, LDHA expression and bioenergetic pathways II ROS mTORC1 NRF2 KEAP1 HMOX1 Induction of the NRF2 stressresponse pathway AKT FOXO3A NADP+ SDH Succinate Biliverdin CREB Malate V F0 Fumarate PDH CoQ Biogenesis Acetyl-CoA IV (R)-2HG Acetyl-CoA Oxaloacetate FH IDH2 NADP+ Citrate Malate α-ketoglutarate (R)-2HG Glycine FOXO3A MYC ATP-citrate lyase IDH2 NADPH Glutamate NADPH • Increased glycolysis • Increased vascularization • Induction of mitophagy • Induction of COX4-2 • Inhibition of COX4-1 • Inhibition of PDH NADP+ NADP+ Cell death BAX Isocitrate Isocitrate Go with the flow Redox regulation PKM2 PKM2 PKM2 PKM2 PKM2 PKM2 ATP RHEB AKT P P TSC2 P TSC1 AMPK Phosphoenolpyruvate Mitochondrial biogenesis and antioxidant defences LKB1 PI3K 3-phosphoglycerate Macromolecular synthesis Bilirubin Glycolysis NADPH NADP+ PTEN Pentose phosphate pathway and nucleotide synthesis Glutamine transporter, such as SLC1A5 Glucose-6-phosphate Unique to Abcam, the MitoSciences range has over 200 products in the areas of mitochondria, metabolism and apoptosis, offering a portfolio of tools in this highly evolving field of research. These include: •Highly validated monoclonal antibodies with diverse species cross-reactivity •Dipstick assays, which employ lateral-flow technology •Enzyme activity assays •In-cell ELISA kits •Western blotting antibody cocktails •Cell fractionation kits •Cellular assay kits, such as ROS detection, ATP measurement and cell viability assays •Mitochondrial lysates from a range of tissues •Active proteins to the Pyruvate dehygrogenase kinases These also encompass a comprehensive range of tools to analyse the Pyruvate Dehydrogenase and Oxidative Phosphorylation pathway. TIGAR Glucose transporter, such as GLUT1 Why don’t you pair up these research tools with antibodies to study cancer metabolism? We have over 25,000 antibodies in our cancer range: •Hypoxia •Apoptosis •Autophagy •Cell cycle •Cancer stem cells •Signal transduction and many more. Growth factor receptor Glucose Glutamine Allow your metabolism research to go further. AMP •Aerobic glycolysis in cancer cells is accompanied by the switch from pyruvate kinase isoform M1 (PKM1) to PKM2, which is regulated by growth factors and tyrosine phosphorylation. When oxidative stress in low, PKM2 is active and glucose is metabolized through phosphoenolpyruvate and pyruvate to lactate to generate ATP. However, when oxidative stress is high, PMK2 activity is reduced driving G6P into the pentose phosphate pathway to generate NADPH and bolstering antioxidant defences. •For the cancer cell to generate the carbon backbones for macromolecular synthesis, the flow of carbon must be redirected away from the mitochondrial terminal oxidizing machinery. The acetyl-CoA that is required for lipid synthesis and for modulating the epigenome and signal transduction pathways is generated from the export of citrate from the mitochondrion. In the cytosol, citrate is cleaved by ATP-dependent citrate lyase to oxaloacetate (OAA) and acetyl-CoA. The acetylCoA can then be used to synthesize fatty acids and lipids. However, this appropriation of citrate depletes the mitochondrion of OAA. This deficiency is compensated in many cancers by the activation of MYC that induces glutaminolysis. •The regulation of acetyl-CoA production from glycolytic sources is also modulated by the mitochondrial protein kinase Cδ signalosome, which is composed of PKCδ, p66SHC, cytochrome c and retinol. This complex senses the redox status of the ETC and ultimately modulates pyruvate dehydrogenase activity to adjust the flow of acetyl-CoA and reducing equivalents into the mitochondrion. •The promyelocytic leukaemia (PML) protein interacts with endoplasmic reticulum mitochondrially associated membranes and can inhibit Ca2+ release into the outer membrane cleft. This reduces mitochondrial uptake of Ca2+, which stabilizes the mitochondrial permeability transition pore inhibiting apoptosis, and increases cytosolic Ca2+ levels. •Reduced mitochondrial DNA copy number and other types of mitochondrial stress can activate the mitochondrial retrograde signalling pathway through a reduction in the mitochondrial membrane potential. This results in increased cytosolic Ca2+ levels, activation of calcineurin and NF-κB, and activation of the nuclear enhanceosome, which upregulates proteins involved in Ca2+ regulation, tumorigenesis and invasive growth (not shown). Abcam also offers a growing range of non-antibody products such as proteins, peptide, lysates, immunoassays, immunohistochemistry kits, western blotting kits and kits for chromatin research. In total, our extensive catalog contains over 86, 000 quality products, each accompanied by a comprehensive and up-to-date datasheet that includes customer reviews, frequently asked questions and scientific paper citations. Our range is constantly expanding so visit our website today and find out how our products could help advance your research. Discover more at www.abcam.com/mitochondriacancerposter Author address Douglas C. Wallace, Michael and Charles Barnett Endowed Chair in Pediatric Mitochondrial Medicine and Metabolic Disease and Director of the Center for Mitochondrial and Epigenomic Medicine (CMEM), Children's Hospital of Philadelphia. Professor, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Colket Translational Research Building, Room 6060, 3501 Civic Center Boulevard, Philadelphia, PA 19104 E-mail: wallaced1@email.chop.edu DCW and the Children's Hospital of Philadelphia are not affiliated with Abcam. Abbreviations ANT; adenine nucleotide transporter; CHCHD4, coiled-coilhelix–coiled-coil-helix domain-containing protein 4; CoQ, coenzyme Q; COX, cyctochrome c oxidase; Cu–ZnSOD, copper–zinc superoxide dismutase; Cyt c, cytochrome c; ETC, electron transport chain; FH, Fumarate hydratase; G6P, glucose 6-phosphate; GLUT1, glucose transporter 1; GSH, reduced glutathione; HIF1α, hypoxia-inducible factor 1α; HMOX1, haemoxygenase 1; IDH, isocitrate dehydrogenase; KEAP1; kelch-like ECH-associated protein 1; LDHA, lactate dehyrogenase A; LKB1, liver kinase B1; MnSOD, manganese superoxide dismutase; NNT, nicotinamide nucleotide transhydrogenase; NRF2, nuclear factor erythroid related factor 2; PDH, pyruvate dehydrogenase; PGC1α, peroxisome proliferator-activated receptor-γ coactivator 1α; ROS, reactive oxygen species; SCL1A5, solute carrier family 1 (neutral amino acid transporter) member 5; SDH, succinate dehydrogenase TIGAR, TP53-induced glycolysis and apoptosis regulator; TRX, thioredoxin; TSC1, hamartin; TSC2, tuberin; VDAC, voltage-dependent anion channel. Edited by Nicola McCarthy; copyedited by Darren Burgess; designed by Lara Crow. © 2012 Nature Publishing Group. http://www.nature.com/reviews/poster/mitochondria