Origin of Chemical Shifts BCMB/CHEM 8190
advertisement

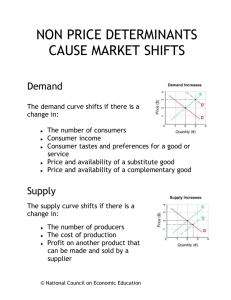
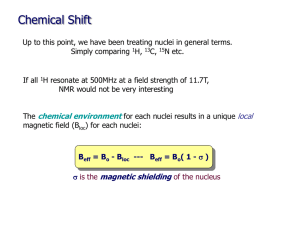
Origin of Chemical Shifts BCMB/CHEM 8190 Empirical p Properties p of Chemical Shift υi (Hz) = γB0 (1-σi) /2π σi, shielding constant dependent on electronic structure, is ~ 10-6. Measurements are made relative to a reference peak (TMS). Offsets given in terms of δ in parts per million, ppm, + downfield. δi = ((σref - σi ) x 106 or δi = (( υi - υref )/υref) x 106 Ranges: 1H, 2H, 10 ppm; 13C, 15N, 31P, 300 ppm; 19F, 1000 ppm Ramsey’s Equation for Chemical Shift • Additional Reference: G.A. Webb, in “Nuclear Magnetic Shielding and Molecular Structure”, J.A. Tossel, ed. Nato Adv. Sci. Series (1993) 1-25 • Physical origin: moving charges experience a f force perpendicular di l tto th the ttrajectory. j t H Hence electrons precess. • Circulating Ci l ti currentt gives i an opposing i field. fi ld B0 F r e- v F = - (e/c) v x B0 B’ = -(e/c) (r x v) / r3 = -(e/cm) (r x p) / r3 • But, we actually need to treat electrons at QM level Some Quantum Mechanics Fundamentals Expectation values correspond to observables: O = <ψ| O |ψ> = ∫ ψ* O ψ dτ O - an operator, ψ - a wave function (electronic or spin) Examples, wave functions: ψ = 1s, 2s, 2p1, 2p0, 2p-1…… (electronic wave functions) ψ = α, β (one ( spin i ½ )), αα, αβ, β βα, β ββ (two (t spins i ½) All are solutions to Schrodinger’s equation: H ψ = E ψ They are normalized: <ψ|ψ> = ∫ ψ*ψ dτ = 1 Examples, Operators: Hamiltonian operator p is special: p <ψ| ψ| H |ψ |ψ> = E Zeeman Hamiltonian for nuclei in a magnetic field: Hz = - μ•B0, Ez = < α|- μ•B0 |α> Begin with classical expression: substitute QM operators μz = γ Iz (h/2π) (magnetic moment) Ez = < α|-(γh/2π)IzB0 |α> = -½ γ(h/2π) B0<α*|α> = -½ γ(h/2π)B0 Quantum Expression for B’ B • Have QM operator for linear momentum: p0 = i(h/(2 i(h/(2π))(∂/∂x ))(∂/∂x + ∂/∂y +∂/∂z) • But momentum in magnetic field has a “curl” p = p0 + e(B (B x r)) / (2c) (2 ) = p0 + ((e/c) / )A A is the vector potential; A = (B x r)/2 B’ = -(e/cm) (r x p0) / r3 - e2 (r x A) / (r3c2m) • Quantum mechanically: B’ = <ψ0| -e (r x p0)/r3(cm) - e2(r x A)/(r3c2 m) |ψ0> paramagnetic diamagnetic Diamagnetic Shifts • Note: only the second term is proportional to B0 at first order theory; this is the diamagnetic term; Lamb term B’D = <ψ0| - e2(r x B0 x r)/(2r3c2 m) |ψ0> Only interested in the z component: k (x ⋅ (B0 x r)y – y ⋅ (B0 x r)x) i j k x ( Bxr ) x y ( Bxr ) y z ( Bxr ) z B’D = - (e2/(2c2 m)) ∫ψ0*| (x2 + y2)/r3) |ψ0 dτ B Predictions: depends on electron density near to nucleus opposes magnetic field (shields) Examples: He 2 1s eσ = 59.93 x 10-6 Ne 10 eσ = 547 x 10-6 H ~2 1s eσ = ~60 x 10-6 HO- O withdraws ~10% ~6 ppm downfield Paramagnetic Contribution to Shifts • This comes from the first term – there was no explicit B0 dependence – so carry to second order electronic wave function is changed by field • B0 can be in H H’ ψ = ψ0 + Σn (<ψn| H’ |ψ0> / (En-E0)) ψn = ψ0 + ψ’ H0 = ((1/(2m)) ( )) p02 + V … in absence of field H = (1/(2m)) (p0 + (e/c)A) 2 + V … in presence ) …. This introduces field dependence p A = ((B0 x r)/2 H’ = (e/(2mc)) A⋅p0 … keeping most important term Paramagnetic term continued A⋅p0 = ((B0 x r)/2)⋅p0 ≡ B0⋅(r x p0)/2 = B0⋅(Lh/(2π))/2 = B0Lzh/(2π))/2 h/(2 ))/2 H’ = B0eh/(8πmc) Lz Hence ψ’ ∝ Σn (<ψn| LZ |ψ0> /ΔE) B’P = <ψ0 + ψ’| (e/(cm))(r x p0)/r3 |ψ0 + ψ’> = <ψ < 0 + ψ’|’| (e/(cm))(Lz)/r3 |ψ | 0 + ψ’> ’> Substituting ψ’ and saving only terms linear in B0 B’P ∝ Σn[(<ψ0|Lz|ψn><ψn|Lz/r3|ψ0>)/(En-E0) + (<ψ0|Lz/r3|ψn><ψn|Lz|ψ0>)/(En-E E0)] Implications for Paramagnetic Term • σP is negative (B’ (B P ∝ - σP) … opposite to σD • σP is zero unless LZ |ψ> is finite hence, if only “s” orbitals populated, LZ |”s”> = 0 hence,, small shift range g for 1H • 13C has “p” orbitals (Lz |p1> = 1 p1) and finite σP • Electron distribution must also be assymetric otherwise, Σ Lz |p> = 0 hence, CH4 shift is small and resonance far upfield 13C Example: Ethane vs Ethylene • CH3-CH CH3 6 ppm, CH2= CH2 123 ppm, Why? • σD is about the same for both, ~200 x 10-6 • σP ∝ Σn[(<ψ0|Lz|ψn><ψn|Lz/r3|ψ0>)/(En-E0) + .. • Examine ψn = ΣicinφI, φI = 1sc, 2sc, 2pc0, 2pc+/-1, 1sH • Only ps count, ΔE small is most important • Consider first excited state: π* = (1/√2)(piA-piB) Consider Field Parallel to C-C Bond B0 A B π* = (1/√2)(pxA-pxB) π* = (1/√2)((p1A+p-1A)-(p1B+p-1B)) • Lz| π*> = (ih√2/π)((p1A-p-1A)-(p1B-p-1B))/(2i) = (ih√2/π)(L √ z| π*> ) • <ψ ψ0|Lz| π*> is finite if pyA, pyB are populated in ψ0 • ψ0 must also be assymetric – look at MOs Molecular Orbitals for Ethylene ψ3, 3 nodes, 1 bond ψ2, 2 nodes nodes, 4 bonds E ψ1, 1 node, 5 bonds • Fill with electrons: 2x6 for C, 4 for H = 16 • 4 in 1sC, 2 in π0 (⊥ to plane), 2 in C-C σ, 4 in C-H σ • Implies 4 maximum in above Calculating Paramagnetic Contribution • Only ψ1, ψ2, contribute • ψ1, is symmetric, implies <ψ1|Lz| π*> is zero • ψ2, is asymmetric and counts • σP = -(eh/(2πmc))2<(1/r3)>2p c22 ≅ -200 x 10-66 -6 = 0 x 10-6 • σc-c = σ + σ = (200-200) (200 200) x 10 cc D P What about Field Perpendicular to Plane? B0 π* = (1/√2)(pzA-pzB) π* = (1/√2)(p0A-p0B) A B • Lz| p0> = 0; 0 th therefore, f σP = 0 • σ⊥ = σD + σP = (200 +0) x 10-6 • Si Similar il ffor iin plane, l perpendicular di l tto pπs • σ (predict) = ⎡0 ⎤ (observe) ⎡− 20 ⎢ ⎢ ⎢⎣ 0 ⎥ ⎥ 200⎥⎦ ⎢ ⎢ ⎢⎣ ⎤ ⎥ 120 ⎥ 200⎥⎦ • Isotopic shift = 1/3 Tr σ = 70-100 ppm below Me • Waugh, Griffin, Wolff, JCP, 67 2387 (1977) – solids NMR 13C Chemical Shift Calculations on Peptides α helix, -57, -47; β sheet, -139, 135; Oldfield and Dios, JACS, 116, 5307 (1994) 13C • • • • shifts and Peptide Geometry Shifts relative to random coil with same amino acid Spera p and Bax,, JACS,, 113,, 5490 (1991) ( ) See also: Case, http://www.scripps.edu/mb/case (Shifts) See also: Wishart, http://redpoll.pharmacy.ualberta.ca/shiftz Remote Group Effects B0 C O H deshielded B’ B’ H shielded benzene does the same thing • σ’remote = Δχ/r3 (1-3cos2θ) • Benzene protons are 2 ppm further downfield • Johnson and Bovey, JCP, 29, 1012 (1962) Shielding from a Benzene Ring Recent Applications pp of Chemical Shift to Protein Structure Determination • Shen Y, Bax A, Protein backbone chemical shifts predicted from searching a database for torsion angle and sequence q homology gy JOURNAL OF BIOMOLECULAR NMR 38: 289-302,2007 • Cavalli A, Salvatella X, Dobson CM, et al. Protein structure determination from NMR chemical shifts PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA 104: 9615-9620, 2007 • Shen Sh Y Y, L Lange O O, D Delaglio l li F F, ett al. l C Consistent i t t bli blind d protein structure generation from NMR chemical shift data, PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA 105: 4685-4690, 2008 Other data can be combined with Chemical Shifts – Protein Targets now up to 25 kDa Comparison of traditional NMR structure and predicted structure t t with ith chemical h i l shift hift and d RDC d data. t Srivatsan, Lange, Rossi, et al. Science, 327:1014-1018, 2010