. Determining Molecular formula given l d %
advertisement

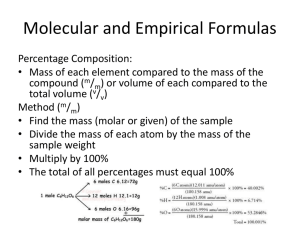
Determining Molecular formula given molecular l l mass andd % composition iti . Of the three ways of determining a formula from composition information, this is the easiest. Let’s try a typical problem. I have a compound with a molecular mass of 60.05g, and it is 6.71% H, 40.00% C and 53.29% O, what is the molecular formula of this compound? Determining Molecular formula given molecular l l mass andd % composition iti . I have a compound with a molecular mass of 60.05g, and it is 6.71% H, 40.00% C and 53.29% O, what is the molecular formula of this compound? STEP 1. Since we know the total molecular mass of the compound, and the % of each element in the compound, the first step is to take the total molar mass and multiply it by the % composition for each element to find out the mass of each element. The onlyy trick you y have to remember is to divide the % number by 100% to get rid of the % unit. Let’s try this on the next page. Determining Molecular formula given molecular l l mass andd % composition iti . I have a compound with a molecular mass of 60.05g, and it is 6.71% H, 40.00% C and 53.29% O, what is the molecular formula of this compound? Molecular mass x (% element/100%) = mass of element H 60.05gg x ((6.71%/100%)) = 4.029gg C 60.05g x (40.00%/100%) = 24.02g O 60.05g x (53.29%/100%) = 32.00g Determining Molecular formula given molecular l l mass andd % composition iti . STEP 2. Since step one gives us the mass of each element in one mole of the compound. All we do now is to divide that mass by the mass of a single mole of that atom to find out the total number of moles of the element in the compound. H 4.029g of H in compound/1.008g in 1 mole of H = 4 moles H C 24.02g of C in compound /12.01g in 1 mole of C = 2 moles of C O 32 00g of O in compound/16 32.00g compound/16.00g 00g in 1 mole of O = 2 moles of O Determining Molecular formula given molecular l l mass andd % composition iti . STEP 3. Combine the known number of moles into a formula. H: 4 moles of H C: 2 moles of C O: 2 moles of O molecular formula = H4C2O2 For proper form, there are rules for the order that the atoms should occur in the formula. That is a picky point for a more advanced y list yyour atoms in,, jjust as longg class,, so I don’t care what order you as you have the numbers right. Determining Molecular formula given molecular l l mass andd % composition iti . Practice Problem: A compound has a molecular mass of 96.094g, and it is composed of 88.39% 39% H H, 29 29.16% 16% N N, 12 12.50% 50% C and 49.95% 49 95% O. O What is the molecular formula of the compound? Determining Molecular formula given molecular l l mass andd % composition iti . A compound has a molecular mass of 96.094g, and it is composed of 8.39% H, 29.16% N, 12.50% C and 49.95% O. What is the molecular formula of the compound? Step 1: 96.094g x (8.39%/100%) = 8.06 g H 96.094g x (29.16%/100%) = 28.02 g N 96.094g x (12.50%/100%) = 12.01 g C 96.094g x (49.95%/100%) = 48.00 g O Step 2: 8.06 g H / 1.008g in 1 mole = 8 mole H 28 02 g N/ 14.01g 28.02 14 01 iin 1 mole l = 2 mole l N 12.01 g C/ 12.01g in 1 mole = 1 mole C 48.00 g O/ 16.00g in 1 mole = 3 mole O Final formula: H8N2CO3, or the more chemically correct formula (NH4)2CO3