Organic Chemistry I Concepts
advertisement

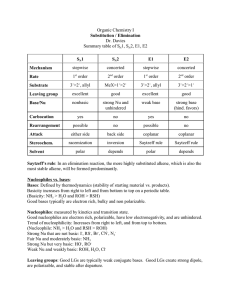
Organic Chemistry I Concepts Common Functional Groups Functional Group Name F, Cl, Br, or I Example Alkane CH3CH2CH3 (propane) Alkene CH3CH=CH2 (propene) Alkyne CH3C CH (propyne) Alkyl halide CH3Br (methyl bromide) Alcohol CH3CH2OH (ethanol) Ether CH3OCH3 (dimethyl ether) Amine CH3NH2 (methyl amine) Functional Groups That Contain a Carbonyl Functional Group Name Example Aldehyde CH3CHO (acetaldehyde) Ketone CH3COCH3 (acetone) Acyl chloride CH3COCl (acetyl chloride) Carboxylic acid CH3CO2H (acetic acid) CH3CO2CH3 (methyl acetate) Ester Typical Oxidation Numbers of Carbon Functional Group Example Oxidation Number of Carbon in the Example Alkane CH4 -4 Alkyllithium CH3Li -4 Alkene H2C=CH2 -2 Alcohol CH3OH Ether CH3OCH3 -2 Alkyl halide CH3Cl -2 Amine CH3NH2 -2 Carboxylic acid HCO2H 2 -2 Other Terms: α-carbon - The carbon adjacent to the leaving group . Backside Attack - The SN2 mechanism in which the nucleophile attacks the α-carbon from a direction directly opposite to the C-LG bond. Results in inversion of stereochemical configuration. Bimolecular - Involving two molecules. In this SparkNote, the term bimolecular is used to refer to SN2 and E2 reactions. The rate-limiting transition states of both reactions involve two molecules. Carbocation - A carbon that carries a positive charge. Carbocations are highly unstable and are prone to rearrangement. SN1 and E1 reactions proceed through a common carbocation intermediate. Frontside attack - A disproved SN2 mechanism in which the nucleophile attacks the α- carbon from the same side as the C-LG bond. If this mechanism was valid, it would result in retention of stereochemical configuration. See backside attack. Rate law - A mathematical equation that relates the rate of a reaction to the concentration of its reactants. For a generic reaction: X+Y→Z The rate law is expressed as: rate = k [X]a [Y]b Rate-limiting step - The slowest step in a reaction that determines the overall rate. Reaction order - In the rate law, the value of "a" and "b" for reactants "X" and "Y," respectively. The overall order of a reaction is the sum of "a" and "b" and tells how many molecules of reactant are involved in the transition state of the rate-limiting step. Reaction intermediate - An intermediary molecule that accumulates in negligible quantities during a reaction. SN2 - A SN2 reaction is a bimolecular (2 molecules) reaction involving the Substitution of a Nucleophile for a leaving group. SN1 - A reaction in which a Nucleophile Substitutes for a leaving group. SN1. It goes through a carbocation intermediate. Secondary - A secondary carbon has bonds to two other carbons. Tertiary - A tertiary carbon has bonds to three other carbons. Transition state - An extremely unstable molecule that forms between the reactants and their intermediates/products. A transition state exists at the peaks of an energy diagram, in contrast to intermediates and products, which form in the troughs. Practice Problems: #1. Identify the common functional groups in each compound. #2 #3 Which compound is not a member of the alkene series? (1) benzene; (2) acetylene; (3) toluene; (4) ethane #4 Which is the correct molecular formula of pentene? (1) C5H8; (2) C5H10; (3) C5H12; (4) C5H14 #5 C2H4 + H2 <======> C2H6 The above reaction is an example of (1) addition; (2) substitution; (3) saponification; (4) esterification. #6 3 Answers: #1. All three compounds are aromatic. Aspirin is also a carboxylic acid ( CO2H) and an ester ( CO2CH3). Tylenol is also an alcohol ( OH) and an amide ( CONH ). Ibuprofen contains alkane substituents and a carboxylic acid functional group. #2 #3 ethane #4 C5 H10 #5 addition #6 1) Electron donatin major product: para, minor product: ortho 2) Electron withdrawing Major product: meta 3) –CH2CH3 Electron donating -NO2 Electron withdrawing Major product: ortho position to –CH2CH3