SN1 and SN2 reactions
advertisement

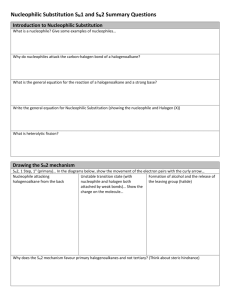
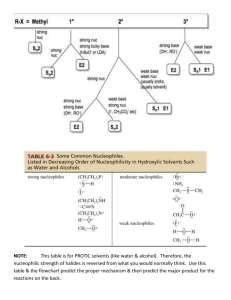
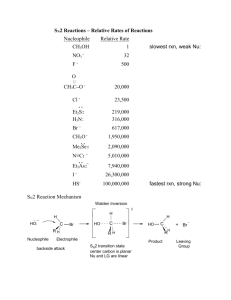
Chemistry (AS) Mechanisms, SN1 and SN2. Halogenoalkane + hydroxide ions acting as a base (when ethanolic NaOH, i.e. NaOH is dissolved in ethanol solvent) halogenoalkane alkene 3 H H H H C C C Cl H H 2 H H H H H C C C H 2 _ .. i.e. .. Cl .. .. _ + Cl (+ Na+) H + H .. OH .. 1 Note: This reaction is also happens for 1-bromopropane and 2-bromopropane _ .. .. OH + .. (dissolved in ethanol, or water ethanol mix) (+ Na ) The H is +in charge explaining the interaction between the negative hydroxide ion and the H. The OH attacks, C-H breaks and Cl is expelled (as a chloride ion, Cl-) all at the same time. It's a concerted process (think of 'concert' - where all the instruments play together at the same time). This way any 'exotic' species are not formed. This is an elimination reaction. When NaOH is dissolves in H2O then nucleophilic substitution takes place instead. There are two ways this reaction can possibly happen. Either by an SN1 mechanism or an SN2 mechanism. The mechanisms are shown below and instead of using a Cl, I've used a Br only because C-Br bonds are easier to break than C-Cl bonds. SN2 reaction: _ H Example: .. HO....... C ....... Br .. H H 1 _ .. .. OH + .. H transition state 2 C .. HO .. Br H H H + C H .. _ .. Br .. .. H The energy changes involved as the reaction is taking place can be shown on what's called an ENERGY PROFILE DIAGRAM. Energy transition state Ea Reactants Hrxn products products Reaction coordinate (i.e. progress of reaction) An SN2 reaction profile has ONE 'hump' Important SN2 points. 1) This what's called an SN2 reaction. S means substitution, N means nucleophile, 2 means "second order". i.e. the reaction involves substitution of a nucleophile and the slowest step of the reaction involves two (second = two) species (the hydroxide anion and the halogenoalkane) 2) The reaction takes place in only ONE STEP. 3) For SN2, the nucleophile must be shown attacking from behind the group that leaves (in this case the halogen). It is because as the Br breaks away it develops a negative charge on itself. Of the incoming -OH was coming in from the same direction as the increasingly negative Br was leaving from, the growing negative charge on the Br would repel the -OH, hence Br4) You must show the 'transition state' and it must look like it has trigonal bipyramidal geometry. Transition means 'change'. It shows the molecule changing from the halogenoalkane to the alcohol at a half-way stage. 5) If the nucleophile initially had a -'ve charge, the transition state will have a -'ve charge also and it must be shown. (It's placed on the outside of the 'square brackets') Important SN2 points (continued). 6) In the transition state you must show two 'partial' bonds, one showing the beginning of a bond between the incoming nucleophile and the functional group carbon, and the other partial bond showing the breaking of the bond from the carbon carrying the functional group and the outgoing species (which incidentally produce a new weaker nucleophile. 7) You must show the 3-dimensional bonding around (coming from) the functional group carbon. This is because this reaction has implications to the 3D configuration. 8) Related to point 6, you need to show the molecules bonds undergoing "3D inversion". There are many ways to draw this molecule. It can be hard to show the mechanism well, so I really recommend that you follow the exact way I've sketched the molecule. Look in particular at the C-H bond going away from you; how it starts out on the left and ends up on the right. And also the outgoing functional group was on the left in the initial molecule but the new incoming nucleophile ends up on the left. SN1 reaction: Example: H5C2 _ .. .. OH .. C Br H5C2 H5C2 2 1 H5C2 H5C2 C+ + H5C2 C HO H5C2 reactant H5C2 H5C2 product Intermediate (a carbocation) .. _ + .. Br .. .. Energy profile diagram for SN1 intermediate Energy Ea Reactants small, second energy barrier Hrxn products Reaction coordinate (i.e. progress of reaction) Important SN1 points. 1) The 1 in SN1 means "first order" i.e. only one molecule is involved in the slow step, (first = one) and this molecule is the bromoalkane. The slow step of reactions are the ones in which bonds break, The slowest of these steps is called the slow step (sometimes called the rate determining step, or RDS)., and any other slow steps then become ignored and is considered to be 'fast' in comparison. The slowest step is the most important one. The C-halogen bond is shown breaking all by itself (even though solvation effects actually aid the process). 2) The reaction takes place in a (minimum of) TWO STEPS. (as mentioned, only one of them is 'slow', the other is considered 'fast') 3) A short lives species called a carbocation is formed. This species is slightly stabilized by the solvent. The intermediate must be shown.!! 4) The intermediate has a FLAT geometry about the carbon carrying the +'ve charge 5) Once formed, the carbocation can be attacked from either face of the flat (trigonal planar) carbocation. This MAY cause a mixture of isomers called "enantiomers" to form if the product has a chiral carbon where the carbocation was. And each attack can occur with almost equal chance, so an almost equal number of each enantiomers may form. In the example the direction of attack by the -OH ion (and inversion of the carbon being attacked) just for the sake of consistency. But could come from any side and inversion will not happen if the incoming nucleophile attacked from to same side where the halogen used to be. Note: point 4 is more towards A2 Chemistry understanding. General notes on SN1 and SN2 a) Primary (1o) halogenoalkanes tend undergo SN2 reactions. The carbon carrying the halogen is 'exposed' i.e. open to attack. b) Tertiary (3o) halogenoalkanes tend undergo SN1 reactions. The carbon carrying the functional group is usually sterically hindered from attack by the bulky surrounding groups. The carbocation is reasonably stabilized by the e- releasing alkyl groups bonded to it. c) Secondary (2o) halogenoalkanes can undergo both mechanisms, probably favouring one mecahanism rather than the other.