The Maryland Cardiovascular Health Promotion Program MVP Score! Fadia T. Shaya, PhD, MPH
advertisement

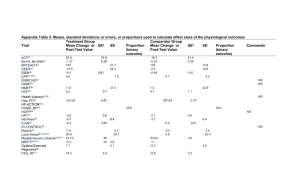
The Maryland Cardiovascular Health Promotion Program MVP Score! Fadia T. Shaya, PhD, MPH Associate Professor f fshaya@rx.umaryland.edu @ May 29th, 2010 MVP Score! Problem: African Americans at high risk CVD greatest morbidity, mortality burden Prevention is available Prior success of intervention and education Background The leading cause of death in males: Cardiovascular disease (CVD) African American males disproportionately affected by CVD (435 vs. 323 per 100,000 in Caucasian men)1 The prevalence of hypertension: 42 42.6% 6% in African American males vs. 32.5% in Caucasian males Minority males usually under-diagnosed under diagnosed, diagnosed late and under-treated even after being diagnosed 1. National Vital Statistics Reports (NVRS). 2008, 24;56:1-120 3 Current Study Goals Engage patients and community Significant impact of direct to patient approach Impact of pharmacists Hypothesis: H th i P Patients ti t who h are empowered d th through h information and monitoring by pharmacists, and encouraged to reach out to peers peers, achieve better outcomes Community Engagement Full-fledged outreach, education, intervention and research evaluation program Based on “train the trainer” b pharmacist by h i t approach h Aim: improve awareness awareness, health-seeking behavior and outcomes of CVD Build on Community Engagement Spread information, empower patients Information tree cascade Starting with positive deviants* E Empower patients ti t Build teams Economic incentives for teams * Marsh DR. The Power of Positive Deviance. BMJ;329:1177-1179 (2004) Place in Translation Research Phase 1 translation (T1) research seeks to move a basic discovery into a candidate health application. Phase 2 translation (T2) research assesses the value of T1 application for health practice leading to the development of evidence based guidelines evidence-based guidelines. Phase 3 translation (T3) research attempts to move evidence-based guidelines id li iinto t h health lth practice, ti th through hd delivery, li di dissemination, i ti and d diffusion research. Phase 4 translation (T4) research seeks to evaluate the "real world" health outcomes of a T1 application in practice. 7 Risk Factors for HTN Smoking Overweight/Obesity (BMI > 25 kg/m2) Low physical activity (<30 minutes 3 to 4 ti times per week) k) High g Total Cholesterol ((> 190 mg/dl) g ) Diabetes Age Men > 55 yo and Women > 65 yo The Rationale for Pharmacists in Community Engagement Access and cultural fluency Knowledge of patients and environments Central drug information source for patient and d prescriber ib Full access to p patient medication p profile Monitoring of therapeutic outcomes MTM Study Design A longitudinal cohort study Patients randomly assigned to either an intervention or control group Inclusion Criteria Age 18 and older Uncontrolled high BP 10 Scheme Built on team culture Align financial incentives and recognition Sustainability http://content.nejm.org/content/vol358/issue21/imag es/data/2249/DC1/NEJM_Christakis_2249a1.shtml? sid=ST2008052600601 id ST2008052600601 12 Intervention Intervention: Hypertension education sessions, offered once a month Three-part education module: education, prevention, and treatment of hypertension H Hypertension t i education d ti manuall 13 Methods Patients in the intervention group Patients reach out and invite relatives or friends to participate in the program Each patient with other people in social network forms a small cluster People in each cluster take part in the hypertension education sessions Patients in the control group Receive usual care 14 Baseline Information BP measurement: every 3 months, total 2.5 yrs Medical history Risk factors such as smoking and drinking alcohol Comorbidities F il Hi Family History Hospitalization and surgeries Patient Knowledge in Hypertension Instrument used: 12-item validated survey To evaluate the impact of the intervention on the improvement of patient knowledge of hypertension 15 Patients’ BP by Group Control (n=236) MVP (n=236) P Value Mean Std Mean Std Age (years) 55 0.95 48 0.86 <0.0001 Baseline DBP2 (mm Hg) 90 0.77 88 0.54 0.0202 Baseline SBP2 (mm Hg) 150 1.12 146 0.69 0.0030 1st Follow-up DBP3(mm Hg) 88 0.85 86 0.58 0.0166 1st Follow-up p SBP3((mm Hg) 150 1.18 144 0.75 <0.0001 1st Follow-up days 200 6.47 145 6.64 <0.0001 Note: 1. DBP and SBP represent diastolic and systolic blood pressure respectively. 16 Patients’ Profiles by Group Control ((n=236)) MVP ((n=236)) P value Gender Female Male N 117 119 Race Black Other 18 39 18-39 40-64 >=65 213 23 31 142 63 90.25 9.75 13 14 13.14 60.17 26.69 234 2 65 150 19 99.15 0.85 27 54 27.54 63.56 8.05 <0.0001 Yes No Yes No Yes No Yes No 69 167 52 184 20 216 24 212 29.24 70.76 22.03 77.97 8.47 91.53 10 17 10.17 89.83 62 174 58 178 13 223 26 149 26.27 73.73 24.58 75.42 5.51 94.49 11 02 11.02 63.14 0.4718 A group Age Diabetes Smoking status Achieve goal1 (Baseline) Achieve goal1 (1st Follow-up) % 49.58 50.42 N 57 178 % 24.15 75.42 <0.0001 <0 0001 <0.0001 0.5136 0.2064 <0 0001 <0.0001 17 Goal Achievement Survival analysis for time to achieve goal - Cox proportional Hazard model (N=410) Variable Estimate P Value Hazard Ratio 95% Hazard Ratio Confidence Intervals Group 0 68 0.68 0.0493 1 98 1.98 1 00 1.00 3 92 3.92 Male 0.05 0.8819 1.05 0.56 1.98 Black -0.73 0.1602 0.48 0.17 1.34 Age 40 40-64 64 vs vs. 18 18-39 39 -0 0.53 53 0 1395 0.1395 0 59 0.59 0 29 0.29 1 19 1.19 Age >=65 vs. 18-39 -0.73 0.1342 0.48 0.19 1.25 Diabetes -1.63 0.0101 0.20 0.06 0.68 Smoking -0.25 0 25 0 4762 0.4762 0 78 0.78 0 39 0.39 1 55 1.55 Baseline SBP2 -0.03 0.0430 0.97 0.94 1.00 Baseline DBP2 -0.03 0.0895 0.97 0.93 1.01 Notes:1 Goal is defined as: For patients without diabetes (systolic blood pressure<140 mmHg) Notes:1. mmHg), for patients with diabetes (systolic blood pressure<130 mmHg) 2. SBP is for systolic blood pressure; DBP is for diastolic blood pressure. 18 Conclusions The social networking intervention doubled the rate of BP control Diabetes was significantly and negatively associated with BP decrease 19