Name: Chem 114 Hour Exam I
advertisement
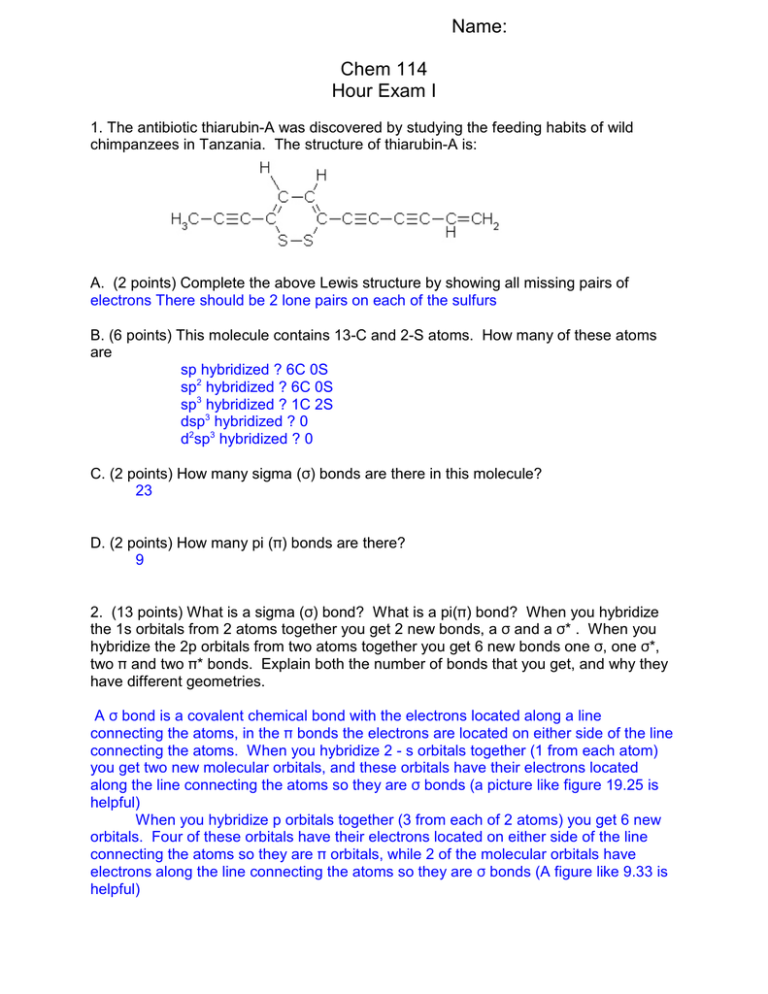
Name: Chem 114 Hour Exam I 1. The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in Tanzania. The structure of thiarubin-A is: A. (2 points) Complete the above Lewis structure by showing all missing pairs of electrons There should be 2 lone pairs on each of the sulfurs B. (6 points) This molecule contains 13-C and 2-S atoms. How many of these atoms are sp hybridized ? 6C 0S sp2 hybridized ? 6C 0S sp3 hybridized ? 1C 2S dsp3 hybridized ? 0 d2sp3 hybridized ? 0 C. (2 points) How many sigma (F) bonds are there in this molecule? 23 D. (2 points) How many pi (B) bonds are there? 9 2. (13 points) What is a sigma (F) bond? What is a pi(B) bond? When you hybridize the 1s orbitals from 2 atoms together you get 2 new bonds, a F and a F* . When you hybridize the 2p orbitals from two atoms together you get 6 new bonds one F, one F*, two B and two B* bonds. Explain both the number of bonds that you get, and why they have different geometries. A F bond is a covalent chemical bond with the electrons located along a line connecting the atoms, in the B bonds the electrons are located on either side of the line connecting the atoms. When you hybridize 2 - s orbitals together (1 from each atom) you get two new molecular orbitals, and these orbitals have their electrons located along the line connecting the atoms so they are F bonds (a picture like figure 19.25 is helpful) When you hybridize p orbitals together (3 from each of 2 atoms) you get 6 new orbitals. Four of these orbitals have their electrons located on either side of the line connecting the atoms so they are B orbitals, while 2 of the molecular orbitals have electrons along the line connecting the atoms so they are F bonds (A figure like 9.33 is helpful) 2 3. (13 points) Use the molecular orbital model to write the electron configuration of the following diatomic species: C2, C2+, and C2-. What is the bond order of each of these species? Which is the most stable? The least stable? Which has the longest bonds length and the shortest bond length? Which species are diamagnetic, which are paramagnetic? C2 C2+ C2F*2p __ __ __ B*2p __ __ __ __ __ __ F2p __ __ 8_ B2p 8988 899 9889 F*2s 98 89 98 F2s 98 89 98 Bond Order (6-2)/2=2 (5-2)/2= 1.5 (7-2)/2=2.5 diamaganetic Paramagnetic Paramagnetic Stability medium low high Length medium longest shortest 4. (12 points) Below are the names of four organic compounds. Write a reasonable structure for each for these compounds. One or more of these compounds may be incorrectly named. Identify and incorrect name and give the correct name for this compound 2-chloro-3-ethylbutane 5-methylcyclohexanol 3-methyl-2-pentanone Cis-1,2-dimethylnonene 3 5. (12 points) Below are structures of four different organic molecules. Give the correct name for each compound. 1,2,4-trimethylcyclopentane 3,5-diehtyl-2-methylheptane 2-chlorohexanol 5-methyl-3-heptyne 6. (13 points) In class we learned that proteins were amino acids connected together to from a polymer. The interesting thing about this polymer is that because there are 20 different amino acids, and each amino acids is build differently, we can make a polymer with lots of different functional groups to do chemistry with. Below is a short, 4 amino acid protein. Identify as many different functional groups as possible on this molecule (Note some C’s are not drawn in to make picture clearer) 4 7. (13 points) In class I talked about six different recyclable plastics. Name at least four of these plastics, and give both their monomer and polymer structure. (+1 extra credit point if you can name their recycle number) #1 PETE Polyethylene terephthalate #2 HDPE High density polyethylene #4 LDPE Low density polyethylene #3 PVC Polyvinylchloride #5 PPE Polypropylene #6 PS Polystyrene 8. (12 points) I want to synthesize 1-chloroethane. How would I do this if my starting material was ethane? How would I do this if my starting material was ethene? If the starting material was ethyne? CH3-CH3 +Cl2 CH2=CH2 + HCl 6(substitution with light) CH2CLCH3 + HCl 6(addition with catalyst CH2CLCH3 CH/CH + H2 6(addition with catalyst) CH2=CH2 + HCl 6 (Second addition with catalyst) CH2CLCH3






