COMMENTARY LOBOTOMY OF GENES: USE OF RNA INTERFERENCE IN NEUROSCIENCE
advertisement

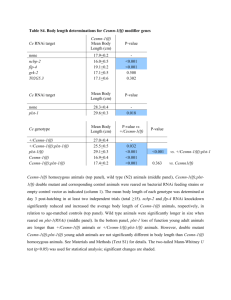
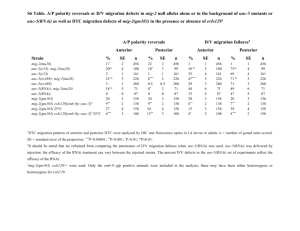
Neuroscience 126 (2004) 1–7 COMMENTARY LOBOTOMY OF GENES: USE OF RNA INTERFERENCE IN NEUROSCIENCE T. HOLENa* AND C. V. MOBBSb Although only discovered within the last decade, RNAi has been used to reduce mRNA levels in hundreds of studies in different species. In this commentary we highlight the great potential, but also the possible pitfalls, for the use of RNAi in neuroscience research. The rapid exploration of RNAi the last 5 years has left many issues unaddressed, and those who decide to use this technique may encounter difficulties of a technical nature. Thus this commentary focuses on experimental design using RNAi, but also aims to give a starting point for further studies of RNAi and the RNAi literature when the inevitable technical difficulties arise with this potent, but yet still developing tool. a The National Hospital and University of Oslo, Center for Molecular Biology and Neuroscience, P.b. 1105 Blindern, 0317 Oslo, Norway b Box 1639, Mt. Sinai School of Medicine, One Gustave Levy Pl., New York, NY 10029, USA Abstract—Galen of Pergamon studied nerve function by shearing nerves in various species including monkeys, dogs, bulls and even elephants (humans being off limits to researchers; Sarton, 1954). An analogous strategy to determine gene function by ablating gene expression has recently been developed. RNA interference (RNAi) is a cellular response to double-stranded RNA (dsRNA) apparently as a defense against viral or transposon activity (Denli and Hannon, 2003; Dykxhoorn et al., 2003; Plasterk, 2002; Zamore, 2002). By activating this ancient defense mechanism through the introduction of artificial dsRNA, it is now possible to inhibit expression of almost any gene in almost any cell type, among them neuronal cells. In mammalian cells the active RNAi species must be short, approximately 21 nucleotide RNAs; these 21-bp species are called short interfering RNA (siRNA; Fig. 1). © 2004 IBRO. Published by Elsevier Ltd. All rights reserved. RNAi reduces gene expression in neurons: importance of delivery In early studies in C. elegans there was evidence that RNAi, while reducing gene expression in neurons, was not as effective in neuronal tissue as in other tissues (Timmons et al., 2001; Timmons and Fire, 1998). However, with the development of C. elegans strains that were more sensitive to RNAi (Simmer et al., 2002), it became clear that RNAi could indeed be used to reduce gene expression in neurons (Bianchi et al., 2003). RNAi has now been used to reduce gene expression and to study gene function in neuronal tissues of several invertebrates, including Drosophila (Dearborn Jr. et al., 2002; Dzitoyeva et al., 2003; Eaton et al., 2002; Kalidas and Smith, 2002; Schindelholz et al., 2001; Yang et al., 2003) planaria (Cebria et al., 2002; Gehring, 2002), and Aplysia (Korneev et al., 2002). A major technical obstacle initially prevented effective use of RNAi in mammals: in contrast to invertebrates, in mammals long (greater than 30 bp) dsRNA produces a PKR/interferon/response causing non-specific effects including general reduction in protein synthesis and even cell death (Caplen et al., 2001; Williams, 1999). However, dsRNA if shorter than 30 bp, can activate the RNAi response and reduce gene expression (Brummelkamp et al., 2002; Castanotto et al., 2002; Jacque et al., 2002; Lee et al., 2002; Miyagishi and Taira, 2002; Paddison et al., 2002; Paul et al., 2002; Sui et al., 2002; Yang et al., 2002; Yu et al., 2002). Therefore in mammals a common way to activate RNAi is to simply infuse synthetic siRNA (21–23 bp) dsRNA species. An alternative approach is to infuse a plasmid whose transcript produces a short dsRNA species. For a plasmid to produce short RNA species, transcription is driven by promoters that use RNA polymerase III, which terminates transcription of a transcript after 5 Key words: siRNA, RNA interference, RNAi, neuroscience. Contents RNAi reduces gene expression in neurons: importance of delivery RNAi versus antisense oligonucleotides A strategy for developing effective RNAi constructs Plasmid-produced shRNAs SiRNA Designing RNAi constructs Testing efficacy and specificity of RNAi Verification References 1 3 3 3 4 4 5 5 6 *Corresponding author. Tel: ⫹47-2285-1018; fax: ⫹47-952-777-36. E-mail address: torgeir.holen@basalmed.uio.no (T. Holen). Abbreviations: AGRP, agouti-related protein; Dicer, a central protein in RNAi: produces siRNA from dsRNA and shRNA, or microRNA from longer transcripts from micro genes; dsRNA, double-stranded RNA, mainly occurs due to viral infection and therefore produces an anti-viral response, generally cleaved into short interfering RNA by Dicer; GFP, green fluorescent protein; Nt, nucleotide(s); RNAi, RNA interference, an immune system of the genome that recognizes and destroys target sequences; shRNA, short hairpin RNA: a short RNA transcribed inside cells, that can be processed to an active siRNA; siRNA, short interfering RNA: the active agent of RNAi, consists of approximately 21 nt RNA strands, in duplex or single-stranded form. 0306-4522/04$30.00⫹0.00 © 2004 IBRO. Published by Elsevier Ltd. All rights reserved. doi:10.1016/j.neuroscience.2004.03.008 1 2 T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 Fig. 2. Putative pathway for shRNA. Transcription of the selfcomplimentary hairpin-transcript is performed by DNA polymerase III, which release the shRNA-transcript after five uridines, cleaving after the second uridine. The hairpin transcript is then processed into siRNA, possibly by the nuclease Dicer, losing the loop and possibly superfluous nucleotides at the 5⬘ and 3⬘ ends. Fig. 1. A simplified schematic showing a cell with parts of the RNAi and siRNA mechanism. When dsRNA appear, e.g. due to virus or transposon activity, a cellular immune mechanism is activated and siRNA are produced. These siRNA are incorporated the protein complex RISC (RNA induced silencing complex: an RNA:protein complex that recognizes and cleaves the mRNA target) and RNAs containing sequences corresponding to the siRNAs sequence, such as mRNA or virus genomes, are depleted. This is exploited by introducing synthetic siRNA to deplete a specific gene of interest, e.g. by transfection. successive thymidines. The transcript is then cleaved after the second uridine (Brummelkamp et al., 2002; Castanotto et al., 2002; Jacque et al., 2002; Lee et al., 2002; Miyagishi and Taira, 2002; Paddison et al., 2002; Paul et al., 2002; Sui et al., 2002; Yang et al., 2002; Yu et al., 2002). To make a short dsRNA species, the plasmid is designed with complementary strands of the target sequence separated by short non-target sequence (see below). The transcript will therefore form a short hairpin loop, so the construct is called shRNA (Lee et al., 2002; Miyagishi and Taira, 2002); eventually the shRNA is cleaved to form a final siRNA product (Fig. 2). siRNA has now been used to reduce gene expression in cell lines, including cells derived from neuroblastoma (Fukumoto et al., 2002; Torocsik et al., 2002; Zhang et al., 2003) and glioblastoma cells (Asai et al., 2003), that are similar to those found in the nervous system. Some studies have also used siRNA to reduce gene expression and study gene function in primary cultures of mammalian neurons (Gaudilliere et al., 2002; Krichevsky and Kosik, 2002) and glial cells (Nicchia et al., 2003). Whereas RNAi in some invertebrates is so robust that even systemic application, for example by feeding, reduces gene expression throughout the organism, this widespread reduction does not occur in mammals. Therefore the use of RNAi in the whole organism, as opposed to in vitro, requires direct delivery of the RNAi reagent to the area of interest. The technical feasibility of using RNAi to reduce expression of transfected genes in neuronal tissue was first demonstrated in mouse embryos using electroporation of siRNA (Calegari et al., 2002), and using adenoviral-mediated transfer to produce shRNA (Xia et al., 2002), though in these studies it was not demonstrated if the effect was observed in neurons in vivo. However, RNAi has also now been shown to be effective in reducing expression of neuronal genes in vivo in adult mammals (Makimura et al., 2002; Hommel et al., 2003; Matsuda and Cepko, 2004). In the first such demonstration, liposome-mediated delivery to the hypothalamus of plasmid-based shRNA, complimentary to the agouti-related protein (AGRP) gene, reduced hypothalamic AGRP mRNA and protein by about 50%, and led to the expected reduction in body weight and increase in metabolic rate (though interestingly not the expected decrease in food intake; Makimura et al., 2002). Since AGRP is only expressed in neurons, this study demonstrated that RNAi can be used to study the physiological function of a gene expressed in neurons in adult mammals. The same study also indicated the siRNA could be similarly effective in reducing neuronal gene expression (Makimura et al., 2002). In a subsequent study an shRNA construct directed against tyrosine hydroxylase (expressed in neurons) was delivered to substantia nigra by an adeno-associated viral vector (Hommel et al., 2003). As with the AGRP study, the shRNA construct directed against tyrosine hydroxylase reduced expression of the targeted gene and produced the expected phenotype (Hommel et al., 2003). Finally, shRNA was delivered to rodent photoreceptor cells by electroporation (Matsuda and Cepko, 2004), and again expression of the targeted genes, neuronal transcription factors, was reduced. On the other hand, in a recent report, siRNA constructs that were effective in vitro were not effective when infused into rat brain without transfection reagents (Isacson et al., 2003). Since a previous report suggested that even without a transfection reagent siRNA could be effective (Makimura et al., 2002), the efficacy of siRNA in mammalian brain probably depends on the specific construct, which vary widely in efficacy and even if effective in vitro may not be effective at the lower cellular concentration obtained in vivo. Precise method of delivery and location in the brain may also influence the efficacy of siRNA. The importance of delivery has been examined in more detail in the liver. A single (high pressure) tail vein injection of a plasmid which produces a dsRNA complimentary to green fluorescent protein (GFP) reduces GFP in liver of GFP-transgenic mice by about 50% (Makimura et al., unpublished observations). Detailed analysis demonstrated T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 3 that before tail vein injection, nearly 100% of hepatocytes of these GFP-transgenic mice express GFP. After tail vein injection only about 50% of the hepatocytes express GFP, but of those that do express GFP, the signal intensity is about the same as in mice injected with a control plasmid. These data suggest that the limiting factor in inhibition of at least hepatic gene expression by RNAi is delivery: in cells in which the plasmid is actually taken up, the target RNA appears to be almost completely eliminated. Similar results have been reported, in which a similar protocol for RNAi delivery by tail vein injection was used to reduce transgenic luciferase gene expression in vivo in liver of adult mice (McCaffrey et al., 2002). As a practical matter, because non-viral methods of transduction in vivo are not as efficient as viral methods, reduction of gene expression by much greater than 50% may be unrealistic for most experiments, unless viral transfection methods are used. cantly reduce gene expression is greater than for antisense oligonucleotides. However, it remains to be demonstrated that this is true in mammalian systems, since the 21-base-pair constructs used for mammalian systems are less robust than the full-length constructs used in nonmammalian systems. However, a clear advantage of RNAi is that it is possible to use plasmid-based systems to produce long-term, even permanent, expression of the active construct, whereas anti-sense constructs must be delivered as oligonucleotides whose activity is relatively transient (on the order of hours). Therefore RNAi can be implemented in a plasmid-based system to study long-term phenotypes after gene transfer of the RNAi construct. This strategy has now been successfully used in mammalian brain (Hommel et al., 2003). RNAi versus antisense oligonucleotides Based on these considerations, the following strategy can be recommended for using RNAi to study gene function in the mammalian nervous system. Although in principle the same issues apply to using RNAi in invertebrate systems, the relative lack of robustness of RNAi in vertebrate systems necessitates more stringent controls. First, the investigator must decide if synthetic double-stranded siRNA or plasmid-based shRNA will be used. While siRNA may be somewhat more robust than anti-sense oligonucleotides, this has yet to be convincingly demonstrated in neurons in adult mammalian brain (Isacson et al., 2003). A comparison of the two technologies performed by Vickers et al. (2003), working at ISIS Pharmaceuticals, a company developing anti-sense technology, indicated that the most developed forms of antisense technology might be as robust as siRNA in reducing mammalian gene expression in vitro. Furthermore, like anti-sense oligonucleotides dsRNAs must be produced and delivered anew for every experiment, and the effects are quite transient, thus for longer term studies may require chronic infusion, a possibly prohibitively expensive proposition. Thus for initial phases of a study (e.g. screening for sequences that produce effective down-regulation, see below) siRNA might save time, especially for those with little experience in cloning, but RNAi in mammalian neurons, especially in vivo, is probably most fully exploited using a plasmid-based shRNA. shRNA may require somewhat more effort in the first phase, to produce the plasmid, but thereafter more construct can be made very easily and cheaply, and the plasmid can be designed to produce much more sustained down-regulation. Furthermore, since delivery of the construct may ultimately constitute the limiting factor in the robustness of the down-regulation (Isacson et al., 2003). Ultimately plasmid delivery can be enhanced by a number of protocols, including packaging into viral vectors. Most investigators are interested in RNAi mainly as a method to specifically reduce expression of a gene of interest in order to assess the physiological function of that gene, similar to the use of targeted gene ablation. For many years, a similar strategy, using anti-sense oligonucleotides, has been used to study gene function in neurons and other tissues (Abraham et al., 1997; Agrawal, 1999; Lewis et al., 2000; McCarthy et al., 2000; Schobitz et al., 1997; Van Oekelen et al., 2003). Such ablation techniques are essentially pharmacological manipulations, the value of which depends on efficacy and specificity. For example, as with any pharmacological intervention, anti-sense oligonucleotides can produce effects that are independent of reduction in expression of the target gene (Abraham et al., 1997; Schobitz et al., 1997). Therefore the use of antisense oligonucleotides requires the use of controls that address the issue of specificity, usually involving the use of oligonucleotides with similar base composition but altered sequence (McCarthy et al., 2000). Another key element of studies using anti-sense oligonucleotides is the demonstration that expression of the targeted gene (especially the protein encoded by the gene) is actually reduced, and that the effect is specific to the targeted gene. Similar issues must be addressed in studies using RNAi (see below). While the use of RNAi and the use anti-sense oligonucleotides entail strategically similar considerations, the two approaches entail different advantages. A key advantage of using anti-sense oligonucleotides is that the technology is more mature than the technology of RNAi, and therefore the essential reagents are currently less expensive and time-consuming to produce. A potential advantage of using RNAi rather than anti-sense oligonucleotides is that RNAi may provide more robust inhibition than anti-sense oligonucleotides, in the sense that RNAi may produce inhibition at lower concentrations and may therefore result in fewer non-specific effects at effective doses. In invertebrates RNAi is also probably more robust in the sense that when plasmid-based delivery systems covering the entire gene are used, the likelihood that a given construct will signifi- A strategy for developing effective RNAi constructs Plasmid-produced shRNAs While all shRNAs are roughly similar, entailing two complementary strands separated by a non-coding loop, they differ in some details, including the number of bases in the 4 T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 linking loop (Fig. 2). Although Brummelkamp et al. (2002) found that a nine nucleotide (nt) linking loop between the two siRNA strands is more effective than a five nt or seven nt loop, corroborated by Castanotto et al. (2002), a wide range of loops connecting the two strands can also be effective: Paul et al. (2002) and Paddison et al. used four loops (UUCG and UUAA, respectively), while Yu et al. (2002) and Yang et al. (2002) used three loops (AUG and UCU, respectively). Sui et al. (2002) report that a six-loop can be effective, while Jacque et al. (2002) found no difference between three, five and seven loops. The shRNA seems to be processed by a nuclease to siRNA, whether longer mimics of let-7 hairpins, or simple, 29 bp hairpins (Paddison et al., 2002). At the moment for standard applications a nine nucleotide linking loop can be recommended. An additional variation of shRNA has been the development of plasmids that allow expression to be regulated. For example, Ohkawa and Taira (2000) constructed a RNA polymerase III promoter that is regulatable by fusing this promoter with a tetracycline response element. Others versions has been developed by Agami and coworkers (van de Wetering et al., 2003) and Matsukura et al. (2003). This system will obviously find many applications for tissue and time-point specific induction of RNAi silencing. A variation of shRNA may be the production of transgenic mice with RNAi constructs (Carmell et al., 2003). Similarly, an improved delivery system for shRNA, developed for in vivo targeting of viral sequences (Lee et al., 2002), involves delivery by lentivirus, a virus suitable for neuronal cells (An et al., 2003; Stewart et al., 2003). SiRNA Since plasmid-based shRNA production requires transcription to produce dsRNA species, siRNA may be preferable if an immediate reduction of mRNA is necessary. On the other hand, even with siRNA, there is a time-lag for establishing silencing (Holen et al., 2002, 2003; McManus et al., 2002). As indicated above, delivery of siRNA in brain in vivo may also be problematic (Isacson et al., 2003); efficient delivery of siRNA will possibly require extensive chemical modification. Fortunately, despite siRNAs having to interact with the effector proteins in RISC (Fig. 1), they show surprisingly high tolerance for chemical modifications. For example, the 3⬘ ends of siRNA can be modified to DNA without losing significant activity (Elbashir et al., 2001a,b). A systematic study to find the limit of the tolerance to chemical modifications in various parts of the two 21-nt strands found a surprisingly high tolerance of modifications (Amarzguioui et al., 2003). Most of the chemically modified versions tested had initial activity near the activity of the unmodified form, while the modification enhanced long-term activity of certain siRNA species. When more extensive 2⬘-O-methylation was introduced, a gradual decline of activity was observed and ultimately destroying activity of even the most robust candidate siRNA. Other studies have also found tolerance for chemical modifications (Chiu and Rana, 2003; Czauderna et al., 2003; Harborth et al., 2003). Tolerance to modification has important implications for siRNA design, in particular in development of siRNA as a therapeutic agent, where modifications of siRNA might be necessary for stabilization of siRNA in tissues, for avoidance of side effects and for delivery to the tissues of interest, among them neuronal tissues. Designing RNAi constructs A key consideration in the use of RNAi is that siRNAs vary extremely widely in their abilities to activate the RNAi mechanism, and therefore in their ability to inhibit RNA expression. Therefore regardless of whether siRNA or shRNA is used, RNAi constructs must be designed that either are or will produce 21-base-pair dsRNAs corresponding to the targeted gene, and will efficiently activate the RNAi mechanism. Several companies now synthesize, and even design, candidate dsRNAs. Constructs with base-pair mismatches, preferably at least three, constitute crucial controls. As with anti-sense, however, a “standard” control, such as an RNAi constructs directed toward GFP, is also a valuable addition to RNAi experimental designs. Although several investigators and companies have developed rules that are claimed to optimize the efficacy of RNAi constructs, these rules have not been clearly validated in the published literature and in our experience do not substantially increase the frequency of successful down-regulation. Initially, it was predicted that it would be possible to cleave a target RNA at almost any position, and that RNAi differed from other types of antisense molecules by not having to search for the optimal target sites (Elbashir et al., 2001b; Stein, 2001). However, clear RNAi target position effects were found when siRNA constructs were systematically tested against multiple targets in tissue factor mRNA, from the start to the stop codon (Holen et al., 2002). Several siRNA constructs in this screen were found to be inactive, and the effect verified in four different assays and four different cell-lines, showing that identification of accessible target sites is critical. This natural variation in siRNA activity has also subsequently been demonstrated by systematic screens by other groups (Kawasaki et al., 2003; Vickers et al., 2003). Mechanisms mediating this remarkable variability are speculative, but may involve internal stability profiles of the siRNA (Khvorova et al., 2003; Schwarz et al., 2003). The degree of specificity of RNAi remains unclear. A recent review of RNAi claims that one of the unique features of RNAi is its exquisite sequence specificity (Shi, 2003), and a recent editorial in Nature Cell Biology, on a Horizon RNAi symposium, proposes that a single nucleotide mismatch between the target RNA and the central region of the RNAi construct can be used as an inactive control (Editorial, 2003). On the other hand, this might not be valid for all RNAi sequences, in particular for highly active RNAi constructs. For example, a mismatch in the central portion of the RNAi construct does not necessarily completely ablate RNAi activity (Boutla et al., 2001), a toleration effect also found by our group (Holen et al., 2002). A systematic study introducing a series of G:C mutations from one end to the other of the siRNA, found a general tolerance to mutations, but with less tolerance for T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 mutations at the 3⬘ end of the siRNA (Amarzguioui et al., 2003). This last point possibly facilitates rational design siRNAs sensitive to single-nucleotide polymorphisms. A similar mutation study was recently performed on an shRNA-construct against the cell-cycle regulator p53 (Pusch et al., 2003). Reports on tolerance of single mutations are now available from other groups (Jacque et al., 2002; Saxena et al., 2003; Vickers et al., 2003; Yu et al., 2002; Zeng and Cullen, 2003). Recent microarray investigations examining RNAi specificity come to different conclusions about the specificity of RNAi, with two papers finding a very high degree of specificity of siRNA (Chi et al., 2003; Semizarov et al., 2003), while two other studies found surprisingly abundant non-specific effects (Jackson et al., 2003; Persengiev et al., 2004). Furthermore, another DNA microarray study concluded that shRNA entailed relatively specific downregulation of the targeted gene (van de Wetering et al., 2003), whereas Nicchia et al. (2003)reported that constructs targeted to the Aquaporin-4 gene in astrocytes also produced significant alterations in expression of five genes also differentially expressed in ischemia-induced brain edema. Since the specificity of RNAi constructs is a subject of continuing debate, a key step in developing candidate RNAi constructs is to ensure by computer search of databases that the candidate sequence appears only in the target gene. A review of over 300 published and commonly used siRNAs shows that 75% of these siRNAs exhibit more than 18 of 21 positions in common with mRNAs other than the intended target mRNA, and 25% of the siRNAs have two or less mismatches (Snøve and Holen, unpublished observations). On the other hand, regardless of the rules used, between 10% and 50% of RNAi constructs tested can be expected to reduce expression of the targeted gene by more than 50%, assuming greater than 50% transfection rate. Therefore, as with anti-sense oligonucleotides, control constructs should also be chosen with care. Testing efficacy and specificity of RNAi In general, it is best to first test each construct in vitro to assess if it reduces expression of the target gene because fewer than half of all 21-base-pair RNAi constructs produce significant decreases in gene expression in mammalian cells, regardless of the efficiency of the gene transfer. Since transfection efficiency in most cell types of interest in neuroscience research using non-viral methods is relatively low, it is best to assess the efficacy of the construct in vitro under conditions of relatively reliable transfection efficiency (which applies especially to dsRNA constructs, whose transfection efficiency and reliability in vivo in mammals remains unclear (Isacson et al., 2003). A generally reliable method to test construct efficacy is to transfect the target gene into a cell type that is easily transfected, such as 293-T cells, then assess if the RNAi construct reliably reduces the expression of the transfected gene. In contrast to anti-sense oligonucleotides, whose mechanism of action may entail attenuation of translation, RNAi works exclusively by reducing mRNA levels; therefore efficacy can 5 be assessed by examining RNA levels, either by Northern blot, RNAse protection assay, or real-time PCR. Protein levels can of course also be measured, but generally mRNA levels are easier to quantify and are a more accurate an indicator of efficacy of the construct (since, for example, an mRNA can be down-regulated without an immediate effect on protein levels, depending on the stability and abundance of the protein). Of course eventually the goal is to reduce protein levels, but this question can be addressed more thoroughly once the optimum RNAi construct has been obtained. As with any study involving pharmacological intervention, a failure to observe an effect with RNAi will not strongly support that the gene does not control the phenotype under study: observing an effect is much more informative. Therefore interpretation of the result will always necessitate demonstration of significant down-regulation of the targeted gene. Should downregulation in the tissue under observation be insufficient to produce a phenotype, packaging the plasmid-based construct into a viral, especially an adenoviral vector (Xia et al., 2002) would provide a more rigorous assessment of the importance of the targeted gene in mediating the target phenotype. As with production of the plasmidbased system, although the initial effort to produce such a vehicle is considerable, once an effective RNAi vehicle has been produced, a viral-based RNAi construct can be reproduced quite easily. This may be a useful strategy if extensive studies of the specific gene are planned, but may not be practical if the experimental design is to screen the effect of numerous genes. Verification Even should the RNAi construct reduce expression of the targeted gene (compared with expression observed in the presence of the control construct) and produce the expected phenotype, it cannot be definitively concluded that the effect of the RNAi construct is due to down-regulation of the targeted gene; it is still conceivable that the RNAi construct has reduced expression of genes not targeted. Such a concern, of course, applies to any study involving pharmacological inhibition, including in particular those using anti-sense oligonucleotides. One way to assess nonspecific effects is to measure expression of non-target genes. Nevertheless, demonstrating that the expression of any given non-target gene is not reduced does not demonstrate that the observed phenotype is due to the downregulation of the target gene. On the other hand, RNAi offers a unique opportunity to address this problem definitively. Since RNAi is less effective with increasing mismatches, the targeted gene, engineered with neutral mutations that cause RNAi mismatches but no alteration in the coded protein, can be transfected along with the RNAi construct. If the RNAi construct reduces expression of the targeted gene and produces the expected phenotype, whereas transfection of the mismatched construct reverses the effect, such a result would constitute extremely strong evidence that the observed phenotype is in fact due to down-regulation of the targeted gene. 6 T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 References Abraham WC, Logan B, Thompson VL, Williams JM, Tate WP (1997) Sequence-independent effects of phosphorothiolated oligonucleotides on synaptic transmission and excitability in the hippocampus in vivo. Neuropharmacology 36:345–352. Agrawal S (1999) Importance of nucleotide sequence and chemical modifications of antisense oligonucleotides. Biochim Biophys Acta 1489:53–68. Amarzguioui M, Holen T, Babaie E, Prydz H (2003) Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Res 31:589 –595. An DS, Xie Y, Mao SH, Morizono K, Kung SK, Chen IS (2003) Efficient lentiviral vectors for short hairpin RNA delivery into human cells. Hum Gene Ther 14:1207–1212. Asai M, Hattori C, Szabo B, Sasagawa N, Maruyama K, Tanuma S, Ishiura S (2003) Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem Biophys Res Commun 301:231–235. Bianchi L, Kwok SM, Driscoll M, Sesti F (2003) A potassium channelMiRP complex controls neurosensory function in Caenorhabditis elegans. J Biol Chem 278:12415–12424. Boutla A, Delidakis C, Livadaras I, Tsagris M, Tabler M (2001) Short 5⬘-phosphorylated double-stranded RNAs induce RNA interference in Drosophila. Curr Biol 11:1776 –1780. Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296:550 –553. Calegari F, Haubensak W, Yang D, Huttner WB, Buchholz F (2002) Tissue-specific RNA interference in postimplantation mouse embryos with endoribonuclease-prepared short interfering RNA. Proc Natl Acad Sci USA 99:14236 –14240. Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA (2001) Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA 98:9742–9747. Carmell MA, Zhang L, Conklin DS, Hannon GJ, Rosenquist TA (2003) Germline transmission of RNAi in mice. Nat Struct Biol 10:91–92. Castanotto D, Li H, Rossi JJ (2002) Functional siRNA expression from transfected PCR products. RNA 8:1454 –1460. Cebria F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sanchez AA, Agata K (2002) FGFRrelated gene nou-darake restricts brain tissues to the head region of planarians. Nature 419:620 –624. Chi JT, Chang HY, Wang NN, Chang DS, Dunphy N, Brown PO (2003) Genomewide view of gene silencing by small interfering RNAs. Proc Natl Acad Sci USA 100:6343–6346. Chiu YL, Rana TM (2003) siRNA function in RNAi: a chemical modification analysis. RNA 9:1034 –1048. Czauderna F, Fechtner M, Dames S, Aygun H, Klippel A, Pronk GJ, Giese K, Kaufmann J (2003) Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res 31:2705–2716. Dearborn R Jr, He Q, Kunes S, Dai Y (2002) Eph receptor tyrosine kinase-mediated formation of a topographic map in the Drosophila visual system. J Neurosci 22:1338 –1349. Denli AM, Hannon GJ (2003) RNAi: an ever-growing puzzle. Trends Biochem Sci 28:196 –201. Dykxhoorn DM, Novina CD, Sharp PA (2003) Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol 4:457–467. Dzitoyeva S, Dimitrijevic N, Manev H (2003) Identification of a novel Drosophila gene, beltless, using injectable embryonic and adult RNA interference (RNAi). BMC Genomics 4:33. Eaton BA, Fetter RD, Davis GW (2002) Dynactin is necessary for synapse stabilization. Neuron 34:729 –741. Editorial (2003) Whither RNAi? Nat Cell Biol 5:489 – 490. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001a) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494 –498. Elbashir SM, Lendeckel W, Tuschl T (2001b) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15:188 –200. Fukumoto H, Deng A, Irizarry MC, Fitzgerald ML, Rebeck GW (2002) Induction of the cholesterol transporter ABCA1 in central nervous system cells by liver X receptor agonists increases secreted Abeta levels. J Biol Chem 277:48508 –48513. Gaudilliere B, Shi Y, Bonni A (2002) RNA interference reveals a requirement for myocyte enhancer factor 2A in activity-dependent neuronal survival. J Biol Chem 277:46442–46446. Gehring WJ (2002) The genetic control of eye development and its implications for the evolution of the various eye-types. Int J Dev Biol 46:65–73. Harborth J, Elbashir SM, Vandenburgh K, Manninga H, Scaringe SA, Weber K, Tuschl T (2003) sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev 13:83–105. Holen T, Amarzguioui M, Babaie E, Prydz H (2003) Similar behaviour of single-strand and double-strand siRNAs suggests they act through a common RNAi pathway. Nucleic Acids Res 31:2401– 2407. Holen T, Amarzguioui M, Wiiger MT, Babaie E, Prydz H (2002) Positional effects of short interfering RNAs targeting the human coagulation trigger Tissue Factor. Nucleic Acids Res 30:1757–1766. Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ (2003) Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 9:1539 –1544. Isacson R, Kull B, Salmi P, Wahlestedt C (2003) Lack of efficacy of ‘naked’ small interfering RNA applied directly to rat brain. Acta Physiol Scand 179:173–177. Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS (2003) Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 21:635–637. Jacque JM, Triques K, Stevenson M (2002) Modulation of HIV-1 replication by RNA interference. Nature 418:435–438. Kalidas S, Smith DP (2002) Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron 33:177– 184. Kawasaki H, Suyama E, Iyo M, Taira K (2003) siRNAs generated by recombinant human Dicer induce specific and significant but target site-independent gene silencing in human cells. Nucleic Acids Res 31:981–987. Khvorova A, Reynolds A, Jayasena SD (2003) Functional siRNAs and miRNAs exhibit strand bias. Cell 115:209 –216. Korneev SA, Kemenes I, Straub V, Staras K, Korneeva EI, Kemenes G, Benjamin PR, O’Shea M (2002) Suppression of nitric oxide (NO)-dependent behavior by double-stranded RNA-mediated silencing of a neuronal NO synthase gene. J Neurosci 22:RC227. Krichevsky AM, Kosik KS (2002) RNAi functions in cultured mammalian neurons. Proc Natl Acad Sci USA 99:11926 –11929. Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A, Salvaterra P, Rossi J (2002) Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 20: 500 –505. Lewis EJ, Agrawal S, Bishop J, Chadwick J, Cristensen ND, Cuthill S, Dunford P, Field AK, Francis J, Gibson V, Greenham AK, Kelly F, Kilkushie R, Kreider JW, Mills JS, Mulqueen M, Roberts NA, Roberts P, Szymkowski DE (2000) Non-specific antiviral activity of antisense molecules targeted to the E1 region of human papillomavirus. Antiviral Res 48:187–196. Makimura H, Mizuno TM, Mastaitis JW, Agami R, Mobbs CV (2002) Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci 3:18. Matsuda T, Cepko CL (2004) Inaugural article: electroporation and T. Holen and C. V. Mobbs / Neuroscience 126 (2004) 1–7 RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA 101:16 –22. Matsukura S, Jones PA, Takai D (2003) Establishment of conditional vectors for hairpin siRNA knockdowns. Nucleic Acids Res 31:e77. McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA (2002) RNA interference in adult mice. Nature 418:38 –39. McCarthy MM, Auger AP, Mong JA, Sickel MJ, Davis AM (2000) Antisense oligodeoxynucleotides as a tool in developmental neuroendocrinology. Methods 22:239 –248. McManus MT, Haines BB, Dillon CP, Whitehurst CE, Van Parijs L, Chen J, Sharp PA (2002) Small interfering RNA-mediated gene silencing in T lymphocytes. J Immunol 169:5754 –5760. Miyagishi M, Taira K (2002) U6 promoter-driven siRNAs with four uridine 3⬘ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 20:497–500. Nicchia GP, Frigeri A, Liuzzi GM, Svelto M (2003) Inhibition of aquaporin-4 expression in astrocytes by RNAi determines alteration in cell morphology, growth, and water transport and induces changes in ischemia-related genes. FASEB J 17:1508 –1510. Ohkawa J, Taira K (2000) Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum Gene Ther 11:577–585. Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 16:948 –958. Paul CP, Good PD, Winer I, Engelke DR (2002) Effective expression of small interfering RNA in human cells. Nat Biotechnol 20:505– 508. Persengiev SP, Zhu X, Green MR (2004) Nonspecific, concentrationdependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA 10:12–18. Plasterk RH (2002) RNA silencing: the genome’s immune system. Science 296:1263–1265. Pusch O, Boden D, Silbermann R, Lee F, Tucker L, Ramratnam B (2003) Nucleotide sequence homology requirements of HIV-1specific short hairpin RNA. Nucleic Acids Res 31:6444 –6449. Sarton G (1954) Galen of Pergamon. University of Kansas Press. Saxena S, Jonsson ZO, Dutta A (2003) Small RNAs with imperfect match to endogenous mRNA repress translation: implications for off-target activity of siRNA in mammalian cells. J Biol Chem 278: 44312–44319. Schindelholz B, Knirr M, Warrior R, Zinn K (2001) Regulation of CNS and motor axon guidance in Drosophila by the receptor tyrosine phosphatase DPTP52F. Development 128:4371–4382. Schobitz B, Pezeshki G, Probst JC, Reul JM, Skutella T, Stohr T, Holsboer F, Spanagel R (1997) Centrally administered oligodeoxynucleotides in rats: occurrence of non-specific effects. Eur J Pharmacol 331:97–107. Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199 –208. Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert DN, Fesik SW (2003) Specificity of short interfering RNA determined through gene expression signatures. Proc Natl Acad Sci USA 100:6347–6352. Shi Y (2003) Mammalian RNAi for the masses. Trends Genet 19:9 – 12. 7 Simmer F, Tijsterman M, Parrish S, Koushika SP, Nonet ML, Fire A, Ahringer J, Plasterk RH (2002) Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr Biol 12:1317–1319. Stein CA (2001) Antisense that comes naturally. Nat Biotechnol 19: 737–738. Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, Novina CD (2003) Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9:493–501. Sui G, Soohoo C, Affar EB, Gay F, Shi Y, Forrester WC, Shi Y (2002) A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 99:5515–5520. Timmons L, Court DL, Fire A (2001) Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263:103–112. Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395:854. Torocsik B, Angelastro JM, Greene LA (2002) The basic region and leucine zipper transcription factor MafK is a new nerve growth factor-responsive immediate early gene that regulates neurite outgrowth. J Neurosci 22:8971–8980. van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H (2003) Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4:609 – 615. Van Oekelen D, Luyten WH, Leysen JE (2003) Ten years of antisense inhibition of brain G-protein-coupled receptor function. Brain Res Brain Res Rev 42:123–142. Vickers TA, Koo S, Bennett CF, Crooke ST, Dean NM, Baker BF (2003) Efficient reduction of target RNAs by small interfering RNA and RNase H-dependent antisense agents: a comparative analysis. J Biol Chem 278:7108 –7118. Williams BR (1999) PKR; a sentinel kinase for cellular stress. Oncogene 18:6112–6120. Xia H, Mao Q, Paulson HL, Davidson BL (2002) siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol 20:1006 –1010. Yang D, Buchholz F, Huang Z, Goga A, Chen CY, Brodsky FM, Bishop JM (2002) Short RNA duplexes produced by hydrolysis with Escherichia coli RNase III mediate effective RNA interference in mammalian cells. Proc Natl Acad Sci USA 99:9942–9947. Yang Y, Nishimura I, Imai Y, Takahashi R, Lu B (2003) Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 37:911–924. Yu JY, DeRuiter SL, Turner DL (2002) RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 99:6047–6052. Zamore PD (2002) Ancient pathways programmed by small RNAs. Science 296:1265–1269. Zeng Y, Cullen BR (2003) Sequence requirements for micro RNA processing and function in human cells. RNA 9:112–123. Zhang WB, Zhang Z, Ni YX, Wu YL, Pei G (2003) A novel function of Goalpha: mediation of extracellular signal-regulated kinase activation by opioid receptors in neural cells. J Neurochem 86:1213–1222. (Accepted 8 March 2004)