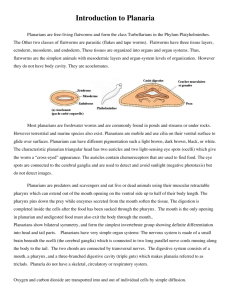
E 2 Regeneration and Maintenance of the Planarian ... LIBRARIES
advertisement

Regeneration and Maintenance of the Planarian Nervous System by Sylvain William Lapan B.A., Biochemistry (2005) Columbia University, New York, NY SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY MEi AT THE MASSACHUTS INSiME OF TECHNOLOGY MASSACHUSETTS INSTITUTE OF TECHNOLOGY E 2 0 7ii LIBRARIES SEPTEMBER 2012 © 2012 Sylvain William Lapan. All rights reserved. The author hereby grants to MIT the permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium known or hereafter created. Signature of Author: ________________ u oDepartment of Biology August 30, 2012 Certified by:- __________ -'eter Reddien Professor of Biology Thesis Supervisor Accepted by: Alan Grossman Professor of Biology Chair, Committee for Graduate Students 1 2 Regeneration and Maintenance of the Planarian Nervous System by Sylvain William Lapan SUBMITTED TO THE DEPARTMENT OF BIOLOGY ON AUGUST 31, 2012 IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN BIOLOGY ABSTRACT Planarians can regenerate all tissues, including the central nervous system and the eyes. This process depends on a population of cells in the adult, the neoblasts, that includes pluripotent stem cells. Whether the neoblast population also includes progenitors specialized for forming specific lineages has not been demonstrated. This thesis describes the identification of progenitors that contribute to eyes during regeneration. Expression and functional analyses identified the genes eyes absent, six1/2 and ovo as critical for the formation of all cells of the eye. otxA and soxB were specifically required for photoreceptor regeneration, and sp6-9 and dlx were required for regeneration of the optic pigment cup. Expression analysis of these transcription factors in situ revealed that eye progenitors were distributed in mesenchymal trails extending posteriorly from the regenerating eye. These progenitors originate in the neoblasts, and promixity to the eye primordium correlates with increased differentiation. The spatial and genetic identification of a progenitor population in planarians elucidates migratory and morphogenetic mechanisms underlying organ regeneration in these animals. RNA sequencing of eye tissue also identified hundreds of genes with highly enriched expression in the eye, including numerous orthologs of eye pathology-related gene as well as likely components of key visual processes such as phototransduction and optic pigment cell function. The planarian brain is composed of dozens of cell types with regionalized distribution. The function of the planarian hedgehog gene in the patterning of CNS regions was investigated. hedgehog was expressed in the medial planarian brain, flanked by nkx2 and nkx6, then pax6b, and finally dlx-1 and msx at the most distal positions. This 3 organization is similar to the expression domains of orthologous transcription factors in the vertebrate neural tube. However, in contrast to vertebrates, the expression of nkx2, nkx6, and pax6b in planarians was not affected by loss of hedgehog expression. RNA sequencing analysis identified a strong effect of Hedgehog signaling genes on a medially positioned cell with glia-like features. Therefore, Hedgehog signaling affects formation of at least one cell type in the planarian brain, but does not broadly regulate transcription factor expression domains and cell type identity. Thesis Supervisor: Peter Reddien Title: Professor of Biology 4 Acknowledgements I would like to thank Peter for inviting me to join his lab. Peter's lab is based on the premise that awe and respect for biological processes is the basis for good research. Peter also knows the importance of long hours and dedication to craft. He was involved in my work both during fun times (reviewing in situ screens, looking at live worms by DIC) but also on more mundane days, revising drafts and practicing talks. His influence on my development as a scientist will last a lifetime. I appreciate the many hours that my committee members, Troy and Hazel, have spent advising me. They have always been engaged, supportive, and helpful during meetings. I had a great experience rotating in Troy's lab during my first year. Hazel's course on development, which I took during my first semester of grad school, was a terrific introduction to a topic that I previously knew almost nothing about. My seniors, Danielle and Dan, are brilliant scientists and established a lab environment that valued hard work, high quality data, and thoughtfulness. Their discoveries and technical advances made possible many aspects of my work. My Juniors, Mike, Irving, and Jared, are also terrific scientists and have contributed to making the lab a vital, social, and collaborative work environment. I am also indebted to three postdocs - Chris, Mansi, and Josien - for their generosity and kindness, providing hours of guidance and deeply influencing my thinking. Above all, thanks to Mom, Dad, Pierre and Lena for their unwavering love and support. 5 6 Table of contents Chapter 1: Introduction......................................................... 1. Adult neurogenesis.................................................. 11. Injury-induced neurogenesis..................................... 111. Response to injury by glia and RPE cells...................... IV. Nervous system regeneration in planarians................... V. Introduction to work presented in this thesis................. 32 . . ..... F ig ures ................................................................ References................................................................. 9 12 20 27 29 34 40 Chapter 2: dlx and sp6-9 control optic cup regeneration in a prototypic eye................................................................. S u m m a ry ...................................................................... .. Introd uctio n .............................................................. Results and Discussion................................................... Materials and Methods.................................................... . ..... F ig u res .................................................................. R efe re n ce s .................................................................... 51 52 53 55 68 74 1 16 Chapter 3: Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration.......... S u m m a ry ..................................................................... In tro d u ctio n ................................................................... Results and Discussion................................................... Materials and Methods.................................................... F ig u re s ......................................................................... R efe re nce s ................................................................... 123 12 4 12 5 127 144 1 46 18 0 Chapter 4: Medially-expressed Hedgehog in the planarian CNS regulates formation of a glia-like cell type........................ S u m m a ry ..................................................................... In tro d u ctio n .................................................................. Results and Discussion................................................... Materials and Methods.................................................... F ig u res ........................................................................ Refe re nce s ................................................................... 187 18 8 18 9 191 204 2 06 2 26 Chapter 5: Discussion.............................................................. 1. Cross-species comparison of transcriptional regulation of eye formation................................................. 11. The cellular lineage of planarian eye cells.......................... Ill. Migration during planarian regeneration............................ IV. The progenitor response to injury.................................... R efe re nce s ..................................................................... 7 233 235 238 239 241 2 43 8 Chapter 1 Introduction 9 Animal development does not end with the dramatic events of embryogenesis, or even with birth or sexual maturation. Most animal species continue to incorporate new cells into tissue throughout life, as a means of replacing aged or damaged cells and coordinating tissue composition with environmental conditions. Many of the questions that can be asked about cellular specification in adults are similar to those asked of embryonic development. What is the source of new cells? How is the establishment of cellular identity regulated to generate the correct number of cells in the correct location? Certain aspects of adult developmental biology clearly differ from embryogenesis. In adults, proliferation is often restricted to particular microenvironments because much of the differentiated tissue is inhospitable to long-term maintenance of cycling cells (Fuchs et al., 2004). Furthermore, there are stark differences between the rates of new cell formation in mature tissues. Those with exposure to the environment intestine and skin, for instance - often involve rapid proliferation, whereas other organs may be largely post-mitotic even in long-lived animals (Hsu et al., 2012). Basic principles regarding the regulation of tissue maintenance in adults remain to be described. The two primary invertebrate model organisms, Drosophila melanogaster and Caenorhabditis elegans, exhibit new cell formation as adults in very few tissues (Joshi et al., 2011; Losick et al., 2011). Furthermore, adult development has been a difficult process to study because many of the genes involved are also required during embryogenesis. Therefore, embryonic lethality or developmental defects confound the ability to analyze later loss-of-function phenotypes. This hurdle is now being circumvented with sophisticated genetic tools that allow perturbation of gene function after embryogenesis. It is also possible to circumvent these issues by establishing new model organisms which have ample tissue turnover as adults, and in 10 which perturbation of gene function by RNA interference can be readily conducted in the adult state. These advantages are demonstrated by the use of planarians as a model system (Newmark and Sanchez Alvarado, 2002; Reddien and Sanchez Alvarado, 2004). This thesis is focused on homeostatic maintenance and regeneration of the nervous system in planarians. The nervous system is a particularly interesting context in which to investigate cellular turnover and regeneration. It is the tissue that possesses the greatest diversity of cell types and the greatest anatomical complexity in most animals. The nervous system responds in complex ways to a wide range of environmental stimuli. The ability of neural networks to change in response to environmental exposure - during memory formation and learning, for instance - has classically focused on the modulation of synaptic connections. However, evidence for the role of adult neurogenesis in such processes is accumulating (Deng et al., 2010; Lazarini and Lledo, 2011). In addition, the nervous system can have an instructive role in guiding regeneration of whole body regions, as demonstrated by the requirement of innervation for salamander limb regeneration (Singer, 1952). Below, I describe adult neurogenesis in vertebrates and arthopods during homeostasis and following injury, and then describe what is known in planarians regarding these processes. 11 I. Adult neurogenesis Neurogenesis in the mammalian subventricular zone (SVZ) Most vertebrates display adult neurogenesis in a subset of brain regions. In mammals, there are only two neurogenic brain regions - the subventricular zone (SVZ) of the lateral ventricles (Gage, 2000; Lois and Alvarez-Buylla, 1993), and the subgranular zone (SGZ) of the dentate gyrus (Altman and Das, 1965; Kuhn et al., 1996) (Figure 1A). The lateral ventricle SVZ is the source of new interneurons that populate the olfactory bulb (OB), a forebrain region that receives input from olfactory sensory neurons and relays information to the olfactory cortex. Periglomerular cells and granule cells are OB interneurons that modulate connections between sensory neurons and relay neurons. The formation of new interneurons is required to replace those lost during normal tissue turnover, and to sustain olfactory learning and memory (Lazarini and Lledo, 2011). The most upstream progenitors in the SVZ that give rise to OB interneurons have been identified as astrocyte-like cells, termed Type B cells (Doetsch et al., 1997). These cells express the astrocyte marker glial fibrillar acid protein (GFAP), and have structural features of astrocytes, including intermediate filament bundles, gap junction complexes and numerous processes that extend between neighboring cells (Doetsch et al., 1999). Astroglial cells are descended from embryonic radial glia, which are primary progenitors during cortical neurogenesis (Merkle and Alvarez-Buylla, 2006). Although Type B cells do not reside directly at the ventricular surface, they extend processes that pass through the ependymal cell layer to directly contact the CSF (Doetsch et al., 1999). Type B cells divide to give rise to transit amplifying cells, which in turn give rise to neuroblasts (Ihrie and Alvarez-Buylla, 2008). The exact pattern of divisions linking these categories of progenitors is unclear. Because studies have not been performed with clonal resolution, it is not known whether all Type B cells can divide both symmetrically and 12 asymmetrically, or whether some are multipotent with respect to producing both neurons and glia (Ming and Song, 2011). The OB is the most anterior part of the forebrain in rodents, and newborn neuroblasts in mouse undergo extensive tangential migration of 5-8 millimeters to reach this region (Doetsch and Alvarez-Buylla, 1996). During migration, closely associated neuroblasts pass through tunnels formed by astrocyte processes along a path termed the rostral migratory stream (RMS). Cell-cell adhesion is an important part of this type of "chain" migration, and is dependent upon the cadherin PSA-NCAM (Doetsch and Alvarez-Buylla, 1996). Several chemoattractive and repulsive cues have been proposed to orient migrating neuroblast chains. Glial cell-line derived neurotrophic factor (GDNF) is expressed in the OB and exists in a protein gradient along the RMS. In explants GDNF acts as an attractant for neuroblasts (Paratcha et al., 2006). Migrating neuroblasts express the Slit receptors Robo2 and Robo3 (Marillat et al., 2002), genes that also function axon guidance. In mice lacking Slitl/2, neuroblasts migrate caudally rather than rostrally (Nguyen-Ba-Charvet et al., 2004). Following RMS migration, neuroblasts lose contact with one another and migrate radially within the OB. The switch from tangential to radial migration depends on Reelin signaling through the ERK pathway (Hack et al., 2002), a mechanism that also regulates neuroblast migration during lamination in the embryo (D'Arcangelo et al., 1995). Substantial heterogeneity exists among the interneurons formed by the SVZ (Alvarez-Buylla et al., 2008). Periglomerular cells can be categorized as calbindin+ (CaIB+), calretinin+ (CaIR+), or tyrosine hydroxylase+ (TH+) (Parrish-Aungst et al., 2007). OB Granule cells can be distinguished as superficial, deep, or CaIR+, and can vary in the branch positions of dendritic arbors (Liu and Shipley, 1994; Mori and Shepherd, 1994). Therefore, the question arises whether specification of OB interneuron identity occurs via environmental cues, or whether type B cells are heterogeneous and 13 already committed to produce particular subtypes. Several lines of evidence indicate that specification mechanisms exist prior to arrival in the OB. First, heterogenous expression of transcription factors such as Pax6 and Sp8 is evident among migrating neuroblasts, and these genes are associated with formation of subclasses of OB interneurons (Hack et al., 2005; Waclaw et al., 2006). Second, viral fate mapping studies targeting various regions of the SVZ demonstrate that distinct regions give rise to interneuron subtypes in different proportions. For instance, most periglomerular cells originating from the pallial SVZ are TH+, whereas most arising from the ventrolateral SVZ are CaIB+ (Merkle et al., 2007; Ventura and Goldman, 2007). These observations do not preclude the possibility that progenitors derived from distinct regions are exposed to different signals during migration. Consequently, explant experiments have been used to examine whether type B cells are specified to produce particular progeny types. In culture, astroglial cells from distinct SVZ regions produce neuronal subtypes consistent with the subtypes produced in vivo according to fatemapping studies (Alvarez-Buylla et al., 2008; Scheffler et al., 2005). In addition, transplantation of Type B cells from one region to another has not been observed to cause a change in subtype identity of progeny produced (Kelsch et al., 2007; Merkle et al., 2007). Therefore, stem cells of distinct regions of the SVZ appear to be specified and committed to form distinct lineages of interneurons. Neurogenesis in the mammalian dentate gyrus (DG) The hippocampus is a region of the forebrain required for episodic and spatial memory, and for the regulation of emotional behavior (Squire, 1992). The dentate gyrus (DG), a region of the hippocampus receiving imput from the entorhinal cortex, exhibits high rates of neurogenesis during adulthood (Gage, 2000). Newly formed cells in the hippocampus are primarily dentate granule cells (DGCs) (Cameron et al., 1993), which 14 project to the CA3 region of the hippocampus. Neurogenesis has been associated with hippocampal activity. Learning by hippocampus-based mechanisms increases neurogenesis (Dupret et al., 2007; Gould et al., 1999), as does environmental enrichment (Kempermann et al., 1997) or stimulation of the DG via experimental manipulation of entorhinal cortex inputs (Bruel-Jungerman et al., 2006). Some studies have reported that ablation of new DGC cell formation yields defects in fear conditioning and long-term retention of memories, although experimental evidence in this regard is conflicting (Deng et al., 2010). Adult-born DGCs arise from the subgranular zone, located below the granule cell layer of the DG (Ming and Song, 2011). The primary progenitors giving rise to new cells, Type 1 cells, are similar to Type B cells of the SVZ with respect to astroglial properties, such as expression of GFAP and nestin (Seri et al., 2004). Type 1 cells make extensive contact with the vasculature, but do not contact the CSF or the ventricular surface, unlike Type B cells. The subgranular zone is extensively innervated by synaptic termini, which likely mediate experience-based regulation of neurogenesis (Mu et al., 2009). Type 1 cells yield proliferative progenitors and neuroblasts. The progeny of Type 1 cells do not undergo significant tangential or radial migration, and position themselves in the inner layers of the dentate gyrus just above the subgranular zone (Seri et al., 2004). Type 1 cells exhibit a plasticity that contrasts sharply with type B cells. For instance, transplantation of type 1 cells from the DGZ into the RMS causes differentiation into OB interneurons (Suhonen et al., 1996). Therefore, the two primary centers of neurogenesis in the adult rodent brain show similarity in some aspects of progenitor morphology, but display substantial differences with respect to progenitor commitment and migration. Adult neurogenesis in the brain and eye of non-mammalian verterbrates 15 Songbirds are a classic model for studying neurogenesis in adulthood, as new cells are added throughout the telencephalon, particularly in the vocal centers involved in song learning (Nottebohm, 2008). Unlike mammals, birds maintain radial glia in the brain throughout life (Kaslin et al., 2008). Newly formed cells undergo tangential migration, as in mammals, but also migrate radially from the ventricular zone, yielding a much larger distribution of newly born neurons in adulthood. Radial glia directly contact the ventricle, and are thought to be the primary precursors of new neurons, based on thymidine incorporation and viral infection studies (Alvarez-Buylla et al., 1998). In teleost fish, the generation of new neurons occurs at rates that are extremely high compared to other vertebrates (Zupanc and Horschke, 1995). Neurogenesis occurs in many brain regions that are normally quiescent in mammals or birds, including the thalamus, hypothalamus, and cerebellum (Grandel et al., 2006). The zebrafish, Danio rerio, has been developed as a powerful genetic model system with well-described embryonic development, and is emerging as a strong system for the study of adult neurogenesis in a non-mammalian vertebrate. As in birds, ventricles of the zebrafish CNS are lined directly with radial glia-like cells, rather than ependymal cells. Progenitors with radial glial properties in the ventricular zone are divided into slow cycling (Type 1) and fast cycling (Type 11). Neuroblasts (Type Ill) that lack glial markers and express PSA-NCAM have also been identified (Marz et al., 2010). Viral transfection at clonal levels has shown that type I cells are multipotent progenitors that undergo both symmetric and asymmetric divisions, whereas type Ill cells undergo amplification and symmetric divisions to yield neurons (Rothenaigner et al., 2011). The cerebellum experiences the most active neurogenesis in adult zebrafish, and new cerebellar granule cells are regularly generated (Kizil et al., 2011) (Figure 1 B). Interestingly, the source of new granule cells is a neuroepithelial layer with clear apicalbasal polarity, and which lacks radial glia marker expression or morphology (Kaslin et 16 al., 2009). These cells and their descendants, which migrate ventrally into the granule layer, express markers of embryonic granule cell progenitors such as Atoh1, Sic1, NeuroD1, Meis1, and Pax6 (Kani et al., 2010). The adult progenitor cells maintain contact with a ventricular zone via a small recess of the IVth ventricle, which disappears in mammalian and avian development, but is retained in fish (Kaslin et al., 2009). Fish also undergo persistent neurogenesis in the retina, which continues to grow well into adulthood (Stenkamp, 2007) (Figure 1C). Growth is fueled by cells generated from the ciliary marginal zone (CMZ), a progenitor region at the periphery of the retina. CMZ progenitors, which are not overtly similar to radial glia in morphology or gene expression, express transcription factors important during embryonic eye development, including Pax6A, Rx1 and Vsx1 (Raymond et al., 2006). CMZ progenitors are capable of differentiating into all cells of the retina with the exception of rod photoreceptors (although these may be derived indirectly from the CMZ) (Stenkamp, 2007). In contrast to CMZ progenitors, rod progenitors exist throughout the retina. These progenitors were identified by BrdU labeling studies and are defined by nuclear morphology and laminar position (Knight and Raymond, 1990; Mack and Fernald, 1997). Moller glia, a radial glia-like cell type in the retina, are proposed to be the source of rod progenitors. Experiments in which GFP expression is driven under a MOller glia-specific promoter show transient expression of GFP in newly born rod progenitors (Bernardos et al., 2007). Therefore, teleosts display a variety of neurogenic stem cell types in adulthood. Some of these depend on glia-like progenitors, while others have characteristics of developmental neuroepithelia. Adult neurogenesis in invertebrates Neurogenesis in adult animals has mostly been studied in the vertebrates. Drosophila and C. elegans do not produce new neurons in the adult state. There are, 17 however, numerous other invertebrates that are promising systems to study adult neurogenesis. Cnidarians, such as Nematostella vectensis and hydra experience tissue turnover throughout adulthood (Tanaka and Reddien, 2011), but little is known about the formation of new neuronal tissue specifically. Adult neurogenesis has been studied in several arthropod groups, particularly decapod crustaceans and crickets. Some decapods, such as lobsters, grow throughout life and continuously add neurons to regions of the brain associated with chemoreception and sensory information processing (Sandeman et al.). Similar to SVZ neurogenesis in vertebrates, migration from a niche of primary progenitors appears to be a feature of neurogenesis in these animals, although the identity of the primary progenitor is unclear (Schmidt, 2007). Adult neurogenesis also occurs in brains of numerous insects, including cockroach, cricket, and moth species. Neurogenesis in insects occurs in the mushroom bodies, which are sensory integration centers in the brain (Heisenberg, 1998). A cortex of interneurons termed Kenyon cells tops the mushroom bodies. In crickets, the insect in which adult neurogenesis is best studied, additional Kenyon cells are added throughout life by a population of -100 neuroblasts at the apex of the mushroom body (Cayre et al., 2007) (Figure 1D). Neuroblast descendants do not migrate, but are gradually pushed deeper as new cell layers are formed. Adult-born neurons make up substantial portion of the mushroom body, approximately 20 percent by age 40 days (Malaterre et al., 2002). These neurons project into the alpha and beta lobes, which have been associated with learning and memory processes in Drosophila (Pascual and Preat, 2001), and there is evidence associating adult neurogenesis with olfactory learning in crickets (ScottoLomassese et al., 2003). Conclusions pertaining to adult neurogenesis 18 1. Adult neurogenesis can be associated with growth in tissue (ciliary marginal zone of the fish retina, mushroom bodies of crickets), but it can also occur in tissues of stable size for balancing tissue turnover or reasons related to cognitive function (subventricular zone and dentate gyrus of mammals.) 2. Newly born neurons in adults can be highly migratory (olfactory bulb progenitors, cerebellum progenitors in teleosts) or remain local (dentate gyrus, ciliary marginal zone) 3. Adult neural stem cells may be already committed to produce neuronal subtypes (subventricular zone Type B cells) or may display plasticity (dentate gyrus Type I cells). 4. Many adult neural stem cells have glial properties (Type B cells, Type I cells, MOller glia) but others are more characteristic of embryonic neuroepithelial cells (ciliary marginal zone and cerebellum progenitors in teleosts). 19 1I. Injury-induced neurogenesis Recovery from injury can require adult neurogenesis. Successful regeneration often requires the existence of specialized cellular and molecular mechanisms. The rapid formation of large amounts of patterned tissue depends on proliferative bursts and dynamic changes in the tissue signaling environment (King and Newmark, 2012). Moreover, regenerative response must be regulated dependent upon the type of injury incurred, as injuries are by nature unpredictable in scale and location. Remarkable regenerative capabilities are widespread among animals including vertebrates, echinoderms, cnidarians, and annelids, to name a few (Sanchez Alvarado, 2000). However, these processes are generally poorly understood at the cellular and molecular level, particularly for the CNS. The mammalian brain, the primary system in which adult neurogenesis has been studied, has extremely limited capacity to regenerate (Kaslin et al., 2008). Fortunately, the study of CNS regeneration is benefiting from the introduction of new model organisms, particularly zebrafish, which exhibit a strong ability to regenerate patterned neuronal tissues. Injury induced neurogenesis in the SGZ and SVZ Despite being a site of abundant new neuron incorporation during adulthood, the OB does not regenerate following surgical injury (Slotnick et al., 2004). Following bulbectomy, rostral migratory stream progenitors continue to migrate to the bulb, but have difficulty leaving the RMS. This jam results in a large increase in RMS volume. Proliferation rates are not elevated in the stream, and therefore compensatory neurogenesis is not thought to contribute to RMS growth (Kirschenbaum et al., 1999). More precise lesions in the OB also do not stimulate progenitor proliferation, nor do they attract increased numbers of neural progenitors to compensate for tissue loss. However, lesioning does not prevent the eventual incorporation of some new neurons into the 20 bulb, and these can differentiate in lesioned areas (Liu and Guthrie, 2011). In these experiments, proliferation is examined across diverse progenitor types, and therefore response to injury in a subpopulation of progenitors, such as Type B cells, might not have been detectable. A focused examination of the effect of OB injury on more precisely defined progenitor populations may be worthwhile. Neurogenesis is stimulated in the dentate gyrus following ischemia or traumatic brain injury (Kernie and Parent, 2010). In this case, new neurons migrate and differentiate within outer layers of the DG, which typically does not experience incorporation of new neurons during adulthood (Dash et al., 2001; Ramaswamy et al., 2005). The functional relevance of this incorporation is unclear. Differentiation of new cells in this region may actually be detrimental, and potentially contributes to epileptic episodes (Kernie and Parent, 2010). CA1 pyramidal neurons of the hippocampus typically do not experience any tissue turnover in adulthood. However, an ischemic model targeting this region can lead to massive compensatory production of new CA1 neurons if growth factors are applied in situ (Nakatomi et al., 2002). In this model, the incorporation of new functional CA1 neurons into the hippocampus was accompanied by improvement in hippocampusbased spatial learning tasks. Injury induced neurogenesis in other regions of the mammalian brain Production of new neurons following injury has been reported in a variety of mammalian brain regions that undergo little or no neurogenesis under homeostatic conditions (Gould, 2007). Lesions in the striatum can stimulate proliferation in the SVZ and cause neuroblasts destined for the OB to be diverted to the site of injury (Arvidsson et al., 2002; Jin et al., 2003). Whereas RMS migration follows a path layed by astrocytes, this altered migratory route follows blood vessels (Ohab et al., 2006; Thored 21 et al., 2007). Most migrating neuroblasts ultimately die, but some neurons differentiate and persist at the site of injury (Arvidsson et al., 2002; Parent et al., 2002). The functional ramifications of this differentiation, however, remain unclear. It is not known whether normal synaptic connections are made nor whether neurogenesis in these regions facilitates recovery from injury (Kernie and Parent, 2010). One model of neocortex regeneration involves creation of focused lesions using chromophore targeted photoablation (Magavi et al., 2000). In this context, BrdU+ cells could be found in the lesioned area and in regions between the SVZ and the lesion. Using retrograde tracing, projections from the BrdU+ neocortical neurons could be found in the thalamus, indicating that new neurons differentiated and projected to proper brain regions (Magavi et al., 2000). The effect of this integration on functional recovery of neural processing was not examined. Therefore, compensatory neurogenesis can occur in a variety mammalian brain regions, but the physiological relevance of these processes is not evident. Regeneration in the brain and eye of teleost fish Teleosts have a high capacity to regenerate following brain injury. Large lesions can be repaired such that the brain is apparently normal by histology, with no long-term glial scar (Marz et al., 2011). The source for regenerative cells was originally localized to the ventricular (matrix) zone by experiments in carp, wherein the capacity for the optic tectum to regenerate was correlated with the amount of the matrix preserved following injury (Kaslin et al., 2008). More recently, a stab injury model in the telencephalon of zebrafish has been the focus of detailed molecular study (Kroehne et al., 2011; Marz et al., 2011). In this model a needle is driven through the skull and into the forebrain, resulting in a large increase in proliferation of type 1/Il (radial glia) and type Ill (neuroblast) progenitors. Lineage tracing using Cre driven by a radial glial marker, 22 her4. 1, showed that new neurons that formed following injury were derived at least partly from radial glia (Kroehne et al., 2011). Injury can induce expression of transcription factors that are required for cell fate determination in embryogenesis. Tbr2 is a transcription factor involved in formation of pallial glutamatergic neurons during embryonic development (Hevner et al., 2006), but is not normally expressed in the ventricular zone of adults. Injury was observed induced expression of Tbr2 in newly born descendants of radial glia at the ventricular surface near the injury site (Marz et al., 2011). At sites distant from the ventricular zone, injuryinduced Tbr+ progenitors also expressed Vglut2.2, a gene expressed in differentiated glutamatergic cells. Together, these data suggest that injury can induce proliferation of neuronal progenitors, followed by migration of differentiating neurons from the ventricular zone to the region of injury. Teleosts also display a high capacity to regenerate following retinal injury (Stenkamp, 2007). Surgical removal of retinal regions, as well as ablation of retinal neurons using the toxin ouabain, results in regeneration of a retina with laminar structure, all retinal cell types, and reformation of funcational synapses between the newly regenerated retina and pre-existing neurons (Cameron and Carney, 2000; Hitchcock, 1997; Stenkamp et al., 2001). The optic nerve grows following retinal regeneration, indicating that newly formed neurons project to the brain. Furthermore, retinal regeneration correlates with recovery of vision-based behavior (Mensinger and Powers, 1999). However, regenerated anatomy following chemical ablation and surgical ablation can be somewhat abnormal with respect to lamination, and the regular spacing of cone photoreceptors typical of uninjured retinas is absent (Raymond et al., 1988; Stenkamp et al., 2001). Regeneration following injury to the peripheral retina occurs more faithfully, likely owing to the neighboring population of multipotent CMZ progenitors that create patterned tissue during growth (Stenkamp, 2007). 23 MOller glia are thought to be the source of new neurons during repair of injury to the central retina in several injury paradigms. GFP driven by a MOller glia-specific promoter (GFAP) was used to observe progeny of these cells following injury (Bernardos et al., 2007). After ablation of rods and cones using light injury, GFP expression was observed in progenitors of regenerating cone cells, with decreasing expression associated with increased differentiation. This suggests perdurance of GFP from an upstream MOller glia progenitor source. Furthermore, injury could induce interkinetic nuclear migration and proliferation of the MOller glia. Similar methods with two other MOller glia-specific promoters were used to identify these cells as a likely progenitor source in regeneration following chemical ablation (Fimbel et al., 2007) and localized puncture injury (Kassen et al., 2008). The amount of injury required to induce retinal regeneration has been a longstanding topic of interest. Low doses of 6-hydroxydopamine selectively kills amacrine cells, but does not trigger a regenerative response (Braisted and Raymond, 1992). Treatment with tunicamycin, which specifically ablates rod photoreceptors, also does not induce repair (Braisted and Raymond, 1993). Because light-induced ablation of the photoreceptor layers, which kills both rods and cones, does induce regeneration (Vihtelic and Hyde, 2000), the source of the injury signal has been thought to be cone cells. However, more recent experiments have shown a robust regenerative response with ouabain treatments in which neurons of the ganglion cell layer and the inner nuclear layer, but not the photoreceptor layer, are destroyed (Fimbel et al., 2007). In conclusion, injury to brain and eye in zebrafish can induce neural stem cells to produce progenitors that are not otherwise normally made. However, the signals that trigger this response largely remain to be discovered. 24 Regeneration of nervous system structures in salamander Among vertebrates, Urodele amphibians have the greatest ability to regenerate following removal of large pieces of tissue such as the limb, spinal cord, lens and retina. The source of retinal cells during salamander retinal regeneration is not a neuronal or glial cell type, but rather the retinal pigment epithelium (RPE) (Stone, 1950), a layer of phagocytic cells adjacent to the photoreceptor neuron layer. Following removal of the retina, RPE cells become depigmented and undergo proliferation to form an apically located neuroepithelium (Del Rio-Tsonis and Tsonis, 2003; Raymond and Hitchcock, 2000). This epithelium continues to proliferate over the course of weeks, becomes stratified, and ultimately differentiates to form a largely normal neural retina. Meanwhile, cells lining Baruch's membrane at the base of the new neuroepithelium differentiate to re-establish RPE identity. Although many instances of apparent transdifferentiation during regeneration have been questioned (Sugimoto et al., 2011), RPE dedifferentiation during retinal repair remains uncontroversial. If true, this is one of the most remarkable feats of transdifferentiation known among animals, with no parallel outside of amphibians. In spinal cord regeneration following tail amputation, migration and proliferation of radial glia from the ventricular zone of the old tissue forms an ependymal tube, which subsequently yields progenitors that differentiate into a variety of spinal cord neurons (Tanaka and Ferretti, 2009). A classic experiment demonstrates that orientation of preexisting tissue governs polarity of regenerated tissue: if a portion of spinal cord is surgically inverted 180 degress, and a tail amputation is subsequently performed through the inverted graft, the new tail regenerates with inverted orientation (Detwiler and Holtzer, 1954). Therefore, patterning of new spinal cord tissue occurs by propagation of pattern from the intact spinal cord. 25 The vertebrate spinal cord develops with regionalized domains of interneuron subtypes and motoneurons distributed along the D-V axis. These regions are patterned by a gradient of Hedgehog signaling that establishes distinct transcription factor expression domains along the D-V axis (Dessaud et al., 2008). Interestingly, axolotls maintain expression of this patterning system in the adult spinal cord, with ongoing ventral Hh expression, intermediate Pax6 expression, and dorsal expression of Msx and Pax7 (Schnapp et al., 2005). How is this organization propagated in the new tissue? GFP fate-mapping showed that newly formed dorsal and ventral regions are derived primarily from preexisting (old-tissue) dorsal and ventral regions, respectively (McHedlishvili et al., 2007). However, when investigating the clonal descendants of single cells or grafts near the tip of the ependymal tubes, greater flexibility was observed. Cells could switch from dorsal to ventral domains, and vice-versa, but normal D-V patterning was ultimately maintained (McHedlishvili et al., 2007). Therefore, pattern in the regenerating axolotl spinal cord can be generated by extension of preexisting lineage-restricted domains or by establishment of new signaling systems that pattern multipotent progenitors. Conclusions pertaining to injury-induced neurogenesis 1. CNS Regeneration in vertebrate model systems is often imperfect (zebrafish central retina) or extremely limited, with questionable functional outcomes (mammalian brain). 2. Regenerating tissue is typically derived from cells that drive neurogenesis during homeostasis. 2. Regeneration can cause neurogenic systems to produce progeny not typically formed in growth or homeostasis (DG, SVZ, zebrafish MOller glia and radial glia in pallial injury model). 26 4. Proliferative responses can depend upon the type (or extent) of injury inflicted. For instance, the retinal regenerative response in fish is induced by photoreceptor ablation, but not amacrine cell ablation, although both cell types can be regenerated. Ill. Response to injury by glia and RPE cells Retinal pigment epithelium (RPE) Despite the fact that RPE degeneration is a major source of eye disease, the proliferative and regenerative capacity of RPE cells has not been thoroughly investigated. Laser induced lesions in the rat RPE (-100pm in diameter) can induce RPE depigmentation and proliferation, and the lesions are repaired with grossly normal histology after several months (von Leithner et al., 2010). In uninjured mammalian retinas, some RPE proliferation occurs at the periphery of the retina, a region that also displays enhanced ability to repair injury (Al-Hussaini et al., 2008; von Leithner et al., 2010). The neural retina might keep the RPE in a largely quiescent state, as removal of photoreceptor cell layer induces RPE proliferation in a mammalian model (Anderson et al., 1981). In frogs and fish, the CMZ drives growth of the RPE into adulthood, and two studies have proposed that new RPE and retinal neurons may be derived from a common upstream progenitor in these animals (Wehman et al., 2005; Wetts et al., 1989). This would be remarkable, given that growth of the RPE and neural retina proceed via independent lineages beginning early in development, and that RPE cells have such distinctive functions. However, a shared lineage relationship is consistent with the transdifferentiation cababilities observed in salamander regeneration. Glia 27 Glial cells, including oligodendrocytes and astrocytes, are an important part of responses to CNS injury (Robel et al., 2011). Mammalian NG2 glia, the precursors of myelinating oligodendrocytes, proliferate rapidly in the vicinity of injury (Levine et al., 2001). Subsequently, the astrocyte population reacts to injury in a process called reactive gliosis. Under these conditions, astrocytes express high levels of the radial glial markers GFAP and vimentin, become hypertrophic and proliferate (Pekny and Nilsson, 2005). The proliferation of astrocytes at wound sites has both negative and positive effects on regenerative ability (Robel et al., 2011). In the short term, gliosis helps to seal the injury site. In the long term, the glial scars formed by this process can inhibit axon regeneration, and limit the capacity of transplanted stem cells to form neurons (Silver and Miller, 2004). In vivo, astrocytes are only observed to give rise to other astrocytes (Ge et al., 2012). However, activated astrocytes have the in vitro potential to differentiate into other cell types, including neurons (Buffo et al., 2008). This observation, combined with the fact that neural stem cells in vivo have astroglial features, has generated interest in the possibility of driving astrocytes to transdifferentiate into neurons in vivo. Because of the abundance of astrocytes in the brain, this approach is thought to hold therapeutic potential for treatment of neurodegenerative disease (Robel et al., 2011). 28 IV. Nervous system regeneration in planarians Planarians are an ideal system for the study of nervous system regeneration. Planarians have a complex centralized nervous system, with at least several dozen distinct cell types (Collins et al., 2011; Umesono and Agata, 2009). Regeneration occurs with perfect repair from a wide variety of injuries, from large amputations to ablation of specific cell types (Nishimura et al., 2011; Reddien and Sanchez Alvarado, 2004). Planarians also have indeterminately long lifespans during which new cells are constantly integrated into the CNS (Wagner et al., 2011). New neurons are added in the presence of nutrient abundance, and neuronal populations decline dramatically, but reversibly, during starvation. Therefore, planarians allow for the study of a variety of neurogenic processes that occur only partially or imperfectly in other organisms. Composition of the planarian brain and eye The planarian central nervous system is composed of two ventral nerve cords, each connecting to one lobe of an anterior cephalic ganglion (Figure 2). A series of studies has revealed distinct distributions of dopaminergic, serotonergic, cholinergic, GABAergic, and octopaminergic neurons in the planarian CNS (Umesono and Agata, 2009). Furthermore, a survey of peptide hormones in planarians revealed a large assortment of distinct expression patterns in the planarian brain (Collins et al., 2011). The expression domains of several transcription factors have also been described in the brain. Three orthologs of orthodenticle homeobox transcription factors are expressed in the brain, with expression near the midline, the periphery of the brain, or intermediate regions (Umesono et al., 1999). These and other studies begin to describe a brain architecture in which cell types are organized approximately in a pattern of nested arcs along the medial-lateral axis. 29 Two cup eyes are present on the dorsal side of the animal, above the brain. The eyes are composed of pigmented non-neuronal cells that form a cup enclosing the lightsensing projections of photoreceptor neurons (Figure 3). Electron microscopy studies have shown that planarian photoreceptors are rhabdomeric, with microvilli that face the apical surface of the pigmented cup (Carpenter et al., 1974). Immunohistochemistry and dye-labeling studies have shown that photoreceptors connect to the brain via ipsilateral and contralateral axonal projections (Okamoto et al., 2005). The planarian eye has been studied for expression and function of widely conserved metazoan eye genes. The transcription factors six-1/2, eyes absent, and otx are expressed in the eye (Mannini et al., 2004; Pineda et al., 2000; Umesono et al., 1999), but expression of Pax6 orthologs in the eye has not been detected (Pineda et al., 2002). Regeneration of the planarian CNS and eyes Head regeneration following decapitation takes place over the course of approximately 2 weeks. Most differentiation occurs in an upigmented growth at the wound site, the blastema. Few molecular studies detailing the course of nervous system regeneration in the blastema exist. Many brain-expressed genes become re-expressed in small bilateral domains at the tips of preexisting nerve cords between days 2 and 3 (Cebria et al., 2002b; Takeda et al., 2009). At these time points the expression domains are already positioned in accordance with their later medial-lateral position. As the blastema grows outward, these domains expand and the boundaries between them are refined. Expression of some eye genes also appears in the blastema at days 2-3 of regeneration (Mannini et al., 2004), and eye pigment is visible by day 5. By day 7 animals can respond to light, but remain uncoordinated. Refinement in coordination, CNS proportions, and eye pigmentation continues in the following week. 30 Numerous genes have been identified that cause severe defects in brain regeneration. An Fgf pathway inhibitor, nou darake, is expressed in the brain and other anterior tissues (Cebria et al., 2002a). RNAi of nou darake results in a large expansion of brain and eye tissue, implicating Fgf signaling as pro-neural in planarians. A wnt ligand-econding gene, DjwntA, is expressed at the posterior border of the brain and functions to restrict the dimensions of the brain (Kobayashi et al., 2007). Correspondingly, RNAi of this Wnt gene causes expansion of the brain into more posterior body regions. Slit signaling is required for normal regeneration of the CNS midline (Cebria et al., 2007). All of these phenotypes can also manifest themselves in uninjured planarians, pointing to the ongoing patterning that occurs in the planarian nervous system even in the absence of injury. The source of new neurons during homeostasis and regeneration A detailed understanding of the function of genes such as nou darake in brain/eye formation is lacking because the cells directly impacted by signaling progenitors of the nervous system - have not been defined, either genetically or spatially. In general, the source of new tissue in planarians are the neoblasts (Reddien and Sanchez Alvarado, 2004). This term describes a population of proliferative mesenchymal cells that exist between the intestinal epithelium and the body wall. Neoblasts have large nuclei relative to cell size, no clear differentiated function, and are the only cycling somatic cells in the animal (Dubois, 1949). Several lines of evidence indicate the potency of this population. First, the ability to regenerate correlates with the presence of these cells. The pharynx and the tip of the head, two regions that lack neoblasts, are the only regions of the animal that cannot regenerate. Furthemore, irradiation doses that eliminate neoblasts also eliminate the ability to regenerate (Baguha et al., 1989). Conclusive evidence for pluripotency of neoblasts at the single31 cell level comes from neoblast transplantation experiments. A single neoblast transplanted into an irradiated host lacking all cycling cells is able to rescue the animal, contribute to all germ layers, and transform a sexual planarian strain into an asexual strain (Wagner et al., 2011). It is not known what percentage of the neoblast population consists of pluripotent stem cells. All cycling cells within the animal are characterized as neoblasts. Because determination of cellular identity is often coupled with proliferation (Morrison and Kimble, 2006; Orford and Scadden, 2008), it would be surprising if lineage-specified progenitors were not found among the neoblasts. However, prior to the work presented here, no progenitor population corresponding to a specific lineage had been identified in this population in vivo. Two non-cycling progenitor populations (neoblast descendants) have been described that exist peripheral to the neoblast population (Eisenhoffer et al., 2008). These cells are broadly distributed, and the identity of these progenitors with respect to the differentiated cell types they form is not described. Therefore, the localization and gene expression profile of nervous system progenitors in planarians has been completely unknown, and this is a major hurdle to understanding the cellular basis of nervous system regeneration. V. Overview of the work presented in this thesis In this thesis the eye is selected for targeted study of regenerative progenitors in the nervous system. The eye has several advantages as an experimental system, including its highly localized position, small number of cell types, and ease of targeting for surgery. Chapter 2 describes transcription factors required for generation of the two principal cell types of the eye. dlx and sp6-9 are found to be required for formation of optic pigment cells, and otxA is required specifically for the formation of photoreceptor neurons. These transcription factors serve as markers for identifying early progenitors of 32 eye cells. Progenitors of the pigment cells originate in the neoblast population separate from photoreceptor neurons, and migrate anteriorly in the blastema to supply new cells for eye regeneration. This is the first progenitor population in planarians defined by position and gene expression, and associated with a specific tissue type. In Chapter 3, quantitative RNA sequencing is used to analyze the profile of gene expression in purified eye tissue, greatly expanding the number of genes known to be expressed in the planarian eye. This dataset includes 10 newly described transcription factor-encoding genes, and a functional role in eye regeneration is identified for 5 of these. The transcription factor ovo is expressed in all eye cells and eye progenitors, and is required for eye regeneration and homeostasis. ovo expression is used to demonstrate that eye progenitors occur in the neoblast population in uninjured animals as well as in regenerating animals. The profile of eye genes also allows prediction of the molecular components of visual processes in planarians, such as phototransduction, photoreceptor morphology, and the function of pigment cells. In Chapter 4 the expression of orthologs of transcription factors that pattern the vertebrate CNS is described in the planarian brain. Based on regional expression of transcription factors and the distribution of neuronal cell types, medial expression of a Hedgehog ortholog in the planarian CNS is hypothesized to parallel Hedgehog expression in the ventral neural tube of vertebrates. Planarian Hedgehog signaling is required for maintenance of a medial, glia-like cell type in the brain, but does not generally influence expression of CNS patterning transcription factors. This finding has implications regarding the ancestral role for Hedgehog in the CNS, and provides a context for studying new cell formation in the planarian brain. 33 A D M13 DGO Cricket Brain Mouse Brain Ventricle Ependymal cells Granule ED cells 0 Type B cil Type Neuroblast stem celfl Later diferenating Earlier difheentlating Neuroblasts Neuroblastsf A Kenyon CellsO I CeNl Utrytes Intemnsuron DGS SVZ C B CMZ Zebrafish Brain Zebrafish Retina Ventricle Granule Cells stem CMZt e~l [] Neuroep helial Migrating progev-f Muler Gale Later Progentorsf Glal scaffold Cerebellum Rod progendtois ] Rods D 34 CMZ Central retina Figure 1. Neurogenic zones in diverse animals (A) Mouse brain, showing neurogenic zones of the subventricular zone (SVZ) and dentate gyrus of the hippocampus (DG). Type B stem cells in the SVZ are positioned below the ependymal cells that line the ventricle. Type B cells give rise to transit amplifying cells and neuroblasts, which migrate to the olfactory bulb (OB) via the rostral migratory stream (RMS). Type I stem cells in the SVZ give rise to transit ampiflying cells and neuroblasts that form new granule cell neurons after only short migrations. (B) Zebrafish brain, showing neurogenesis in the cerebellum. New cerebellar granule cell neurons are formed by a neuroepithelial stem cell population that lines a recess of the ventricle. Progenitors of this population migrate through the molecular layer before reaching the to the granule cell layer, where they terminally differentiate. (C) Zebrafish retina, showing neurogensis in the ciliary marginal zone (CMZ) and the central retina. Neuroepithelial stem cells in the CMZ produce progenitors for all retinal cell types except for rods. MOller glia in the central retina produce rod progenitors that migrate to the outer layer. (D) Cricket brain, showing the neurogenic region of the mushroom body (MB). Neuroblast stem cells at the apex of the mushroom body produce new Kenyon cells, which are pushed deeper in into the brain as neurogenesis continues. 35 OD -el 0 Figure 2. Planarian anatomy (A) Schematic illustration of tissue types in planarians (from Newmark and SsnchezAlvarado, 2002). (B) Image of a live Schmidtea mediterranea. (C) In situ hybridization is used to detect choline acetyl-transferase expression, which labels most neurons in the planarian. Panel shows optical sections through a single intact animal. Left image is most ventral, right image is most dorsal. Anterior is up in all images. The bilobed cephalic ganglion is evident by bright signal in the anterior. Photoreceptor neurons appear as bright crescents of signal on the dorsal side, adjacent to black pigment spots (arrow). 37 *planarian ascidian scallop *vertebrate cnidarian nautilus *1 ~47? hammu moth cephalopod * photoreceptors Flens N pigment cells/granules , 38 Figure 3. Schematic of various metazoan eye types (A) The red branch represents Ecdysozoa; green branch, Lophotrochozoa; blue branch, Deuterostomia; purple branch, Cnidaria. Asterisks indicate that planarians, Drosophila, and vertebrate eyes can currently be studied with a completed genome and loss of gene function tools. Illustration by Irving Wang. 39 REFERENCES Al-Hussaini, H., Kam, J.H., Vugler, A., Semo, M., and Jeffery, G. (2008). Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Molecular vision 14, 1784-1791. Altman, J., and Das, G.D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of comparative neurology 124, 319-335. Alvarez-Buylla, A., Garcia-Verdugo, J.M., Mateo, A.S., and Merchant-Larios, H. (1998). Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci 18, 1020-1037. Alvarez-Buylla, A., Kohwi, M., Nguyen, T.M., and Merkle, F.T. (2008). The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harbor symposia on quantitative biology 73, 357-365. Anderson, D.H., Stern, W.H., Fisher, S.K., Erickson, P.A., and Borgula, G.A. (1981). The onset of pigment epithelial proliferation after retinal detachment. Investigative ophthalmology & visual science 21, 10-16. Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z., and Lindvall, 0. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine 8, 963-970. Baguha, J., Sa16, E., and Auladell, C. (1989). Regeneration and pattern formation in planarians. Ill. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. . Development 107, 77-86. Bernardos, R.L., Barthel, L.K., Meyers, J.R., and Raymond, P.A. (2007). Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci 27, 7028-7040. Braisted, J.E., and Raymond, P.A. (1992). Regeneration of dopaminergic neurons in goldfish retina. Development 114, 913-919. Braisted, J.E., and Raymond, P.A. (1993). Continued search for the cellular signals that regulate regeneration of dopaminergic neurons in goldfish retina. Brain Res Dev Brain Res 76, 221-232. Bruel-Jungerman, E., Davis, S., Rampon, C., and Laroche, S. (2006). Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci 26, 58885893. 40 Buffo, A., Rite, I., Tripathi, P., Lepier, A., Colak, D., Horn, A.P., Mori, T., and Gotz, M. (2008). Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. PNAS 105, 3581-3586. Cameron, D.A., and Carney, L.H. (2000). Cell mosaic patterns in the native and regenerated inner retina of zebrafish: implications for retinal assembly. The Journal of comparative neurology 416, 356-367. Cameron, H.A., Woolley, C.S., McEwen, B.S., and Gould, E. (1993). Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337344. Carpenter, K.S., Morita, M., and Best, J.B. (1974). Ultrastructure of the photoreceptor of the planarian Dugesia dorotocephala. 1.Normal eye. Cell and tissue research 148, 143158. Cayre, M., Scotto-Lomassese, S., Malaterre, J., Strambi, C., and Strambi, A. (2007). Understanding the regulation and function of adult neurogenesis: contribution from an insect model, the house cricket. Chemical senses 32, 385-395. Cebria, F., Guo, T., Jopek, J., and Newmark, P.A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Developmental biology 307, 394-406. Cebria, F., Kobayashi, C., Umesono, Y., Nakazawa, M., Mineta, K., Ikeo, K., Gojobori, T., Itoh, M., Taira, M., Sanchez Alvarado, A., et al. (2002a). FGFR-related gene noudarake restricts brain tissues to the head region of planarians. Nature 419, 620-624. Cebria, F., Nakazawa, M., Mineta, K., Ikeo, K., Gojobori, T., and Agata, K. (2002b). Dissecting planarian central nervous system regeneration by the expression of neuralspecific genes. Development, growth & differentiation 44, 135-146. Collins, J.J., 3rd, Hou, X., Romanova, E.V., Lambrus, B.G., Miller, C.M., Saberi, A., Sweedler, J.V., and Newmark, P.A. (2011). Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS biology 8, e1000509. D'Arcangelo, G., Miao, G.G., Chen, S.C., Soares, H.D., Morgan, J.l., and Curran, T. (1995). A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719-723. Dash, P.K., Mach, S.A., and Moore, A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. Journal of neuroscience research 63, 313319. 41 Del Rio-Tsonis, K., and Tsonis, P.A. (2003). Eye regeneration at the molecular age. Dev Dyn 226, 211-224. Deng, W., Aimone, J.B., and Gage, F.H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews 11, 339-350. Dessaud, E., McMahon, A.P., and Briscoe, J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489-2503. Detwiler, S.R., and Holtzer, H. (1954). The inductive and formative influence of the spinal cord upon the vertebral column. Bulletin of the Hospital for Joint Diseases 15, 114-123. Doetsch, F., and Alvarez-Buylla, A. (1996). Network of tangential pathways for neuronal migration in adult mammalian brain. PNAS 93,14895-14900. Doetsch, F., Caille, I., Lim, D.A., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703-716. Doetsch, F., Garcia-Verdugo, J.M., and Alvarez-Buylla, A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17, 5046-5061. Dubois, F. (1949). Contribution 6 l'6tude de la migration de cellules de regeneration chez les Planaires dulcicoles. . Bull Biol Fr BeIg 83, 213-283. Dupret, D., Fabre, A., Dobrossy, M.D., Panatier, A., Rodriguez, J.J., Lamarque, S., Lemaire, V., Oliet, S.H., Piazza, P.V., and Abrous, D.N. (2007). Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS biology 5, e214. Eisenhoffer, G.T., Kang, H., and Sanchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell stem cell 3, 327-339. Fimbel, S.M., Montgomery, J.E., Burket, C.T., and Hyde, D.R. (2007). Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci 27, 1712-1724. Fuchs, E., Tumbar, T., and Guasch, G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116, 769-778. Gage, F.H. (2000). Mammalian neural stem cells. Science 287,1433-1438. 42 Ge, W.P., Miyawaki, A., Gage, F.H., Jan, Y.N., and Jan, L.Y. (2012). Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484, 376-380. Gould, E. (2007). How widespread is adult neurogenesis in mammals? Nature reviews 8, 481-488. Gould, E., Beylin, A., Tanapat, P., Reeves, A., and Shors, T.J. (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nature neuroscience 2, 260-265. Grandel, H., Kaslin, J., Ganz, J., Wenzel, I., and Brand, M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Developmental biology 295, 263-277. Hack, I., Bancila, M., Loulier, K., Carroll, P., and Cremer, H. (2002). Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nature neuroscience 5, 939-945. Hack, M.A., Saghatelyan, A., de Chevigny, A., Pfeifer, A., Ashery-Padan, R., Lledo, P.M., and Gotz, M. (2005). Neuronal fate determinants of adult olfactory bulb neurogenesis. Nature neuroscience 8, 865-872. Heisenberg, M. (1998). What do the mushroom bodies do for the insect brain? an introduction. Learning & memory (Cold Spring Harbor, NY 5, 1-10. Hevner, R.F., Hodge, R.D., Daza, R.A., and Englund, C. (2006). Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neuroscience research 55, 223-233. Hitchcock, P.F. (1997). Tracer coupling among regenerated amacrine cells in the retina of the goldfish. Visual neuroscience 14, 463-472. Hsu, Y.C., Pasolli, H.A., and Fuchs, E. (2012). Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92-105. lhrie, R.A., and Alvarez-Buylla, A. (2008). Cells in the astroglial lineage are neural stem cells. Cell and tissue research 331, 179-191. Jin, K., Sun, Y., Xie, L., Peel, A., Mao, X.O., Batteur, S., and Greenberg, D.A. (2003). Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Molecular and cellular neurosciences 24, 171-189. Joshi, P.M., Riddle, M.R., Djabrayan, N.J., and Rothman, J.H. (2011). Caenorhabditis elegans as a model for stem cell biology. Dev Dyn 239, 1539-1554. 43 Kani, S., Bae, Y.K., Shimizu, T., Tanabe, K., Satou, C., Parsons, M.J., Scott, E., Higashijima, S., and Hibi, M. (2010). Proneural gene-linked neurogenesis in zebrafish cerebellum. Developmental biology 343, 1-17. Kaslin, J., Ganz, J., and Brand, M. (2008). Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philosophical transactions of the Royal Society of London 363, 101-122. Kaslin, J., Ganz, J., Geffarth, M., Grandel, H., Hans, S., and Brand, M. (2009). Stem cells in the adult zebrafish cerebellum: initiation and maintenance of a novel stem cell niche. J Neurosci 29, 6142-6153. Kassen, S.C., Thummel, R., Burket, C.T., Campochiaro, L.A., Harding, M.J., and Hyde, D.R. (2008). The Tg(ccnbl:EGFP) transgenic zebrafish line labels proliferating cells during retinal development and regeneration. Molecular vision 14, 951-963. Kelsch, W., Mosley, C.P., Lin, C.W., and Lois, C. (2007). Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS biology 5, e300. Kempermann, G., Kuhn, H.G., and Gage, F.H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493-495. Kernie, S.G., and Parent, J.M. (2010). Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiology of disease 37, 267-274. King, R.S., and Newmark, P.A. (2012). The cell biology of regeneration. The Journal of cell biology 196, 553-562. Kirschenbaum, B., Doetsch, F., Lois, C., and Alvarez-Buylla, A. (1999). Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci 19, 2171-2180. Kizil, C., Kaslin, J., Kroehne, V., and Brand, M. (2011). Adult neurogenesis and brain regeneration in zebrafish. Developmental neurobiology 72, 429-461. Knight, J.K., and Raymond, P.A. (1990). Time course of opsin expression in developing rod photoreceptors. Development 110, 1115-1120. Kobayashi, C., Saito, Y., Ogawa, K., and Agata, K. (2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Developmental biology 306, 714-724. Kroehne, V., Freudenreich, D., Hans, S., Kaslin, J., and Brand, M. (2011). Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138, 4831-4841. 44 Kuhn, H.G., Dickinson-Anson, H., and Gage, F.H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16, 2027-2033. Lazarini, F., and Lledo, P.M. (2011). Is adult neurogenesis essential for olfaction? Trends in neurosciences 34, 20-30. Levine, J.M., Reynolds, R., and Fawcett, J.W. (2001). The oligodendrocyte precursor cell in health and disease. Trends in neurosciences 24, 39-47. Liu, H., and Guthrie, K.M. (2011). Neuronal replacement in the injured olfactory bulb. Experimental neurology 228, 270-282. Liu, W.L., and Shipley, M.T. (1994). Intrabulbar associational system in the rat olfactory bulb comprises cholecystokinin-containing tufted cells that synapse onto the dendrites of GABAergic granule cells. The Journal of comparative neurology 346, 541-558. Lois, C., and Alvarez-Buylla, A. (1993). Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. PNAS 90, 2074-2077. Losick, V.P., Morris, L.X., Fox, D.T., and Spradling, A. (2011). Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Developmental cell 21, 159-171. Mack, A.F., and Fernald, R.D. (1997). Cell movement and cell cycle dynamics in the retina of the adult teleost Haplochromis burtoni. The Journal of comparative neurology 388, 435-443. Magavi, S.S., Leavitt, B.R., and Macklis, J.D. (2000). Induction of neurogenesis in the neocortex of adult mice. Nature 405, 951-955. Malaterre, J., Strambi, C., Chiang, A.S., Aouane, A., Strambi, A., and Cayre, M. (2002). Development of cricket mushroom bodies. The Journal of comparative neurology 452, 215-227. Mannini, L., Rossi, L., Deri, P., Gremigni, V., Salvetti, A., Salo, E., and Batistoni, R. (2004). Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Developmental biology 269, 346-359. Marillat, V., Cases, 0., Nguyen-Ba-Charvet, K.T., Tessier-Lavigne, M., Sotelo, C., and Chedotal, A. (2002). Spatiotemporal expression patterns of slit and robo genes in the rat brain. The Journal of comparative neurology 442, 130-155. Marz, M., Chapouton, P., Diotel, N., Vaillant, C., Hesl, B., Takamiya, M., Lam, C.S., Kah, 0., Bally-Cuif, L., and Strahle, U. (2010). Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 58, 870-888. 45 Marz, M., Schmidt, R., Rastegar, S., and Strahle, U. (2011). Regenerative response following stab injury in the adult zebrafish telencephalon. Dev Dyn 240, 2221-2231. McHedlishvili, L., Epperlein, H.H., Telzerow, A., and Tanaka, E.M. (2007). A clonal analysis of neural progenitors during axolotl spinal cord regeneration reveals evidence for both spatially restricted and multipotent progenitors. Development 134, 2083-2093. Mensinger, A.F., and Powers, M.K. (1999). Visual function in regenerating teleost retina following cytotoxic lesioning. Visual neuroscience 16, 241-251. Merkle, F.T., and Alvarez-Buylla, A. (2006). Neural stem cells in mammalian development. Current opinion in cell biology 18, 704-709. Merkle, F.T., Mirzadeh, Z., and Alvarez-Buylla, A. (2007). Mosaic organization of neural stem cells in the adult brain. Science 317, 381-384. Ming, G.L., and Song, H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687-702. Mori, K., and Shepherd, G.M. (1994). Emerging principles of molecular signal processing by mitral/tufted cells in the olfactory bulb. Seminars in cell biology 5, 65-74. Morrison, S.J., and Kimble, J. (2006). Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441, 1068-1074. Mu, Y., Lee, S.W., and Gage, F.H. (2009). Signaling in adult neurogenesis. Current opinion in neurobiology 20, 416-423. Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, 0., Kawahara, N., Tamura, A., Kirino, T., and Nakafuku, M. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110, 429-441. Newmark, P.A., and Sanchez Alvarado, A. (2002). Not your father's planarian: a classic model enters the era of functional genomics. Nat Rev Genet 3, 210-219. Nguyen-Ba-Charvet, K.T., Picard-Riera, N., Tessier-Lavigne, M., Baron-Van Evercooren, A., Sotelo, C., and Chedotal, A. (2004). Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci 24, 1497-1506. Nishimura, K., Inoue, T., Yoshimoto, K., Taniguchi, T., Kitamura, Y., and Agata, K. (2011). Regeneration of dopaminergic neurons after 6-hydroxydopamine-induced lesion in planarian brain. Journal of neurochemistry 119, 1217-1231. Nottebohm, F. (2008). The discovery of replaceable neurons. In Neuroscience of Birdsong, H.P. Zeigler, ed. (Cambridge, Cambridge University Press), pp. 425-448. 46 Ohab, J.J., Fleming, S., Blesch, A., and Carmichael, S.T. (2006). A neurovascular niche for neurogenesis after stroke. J Neurosci 26, 13007-13016. Okamoto, K., Takeuchi, K., and Agata, K. (2005). Neural projections in planarian brain revealed by fluorescent dye tracing. Zoological science 22, 535-546. Orford, K.W., and Scadden, D.T. (2008). Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet 9, 115-128. Paratcha, G., Ibanez, C.F., and Ledda, F. (2006). GDNF is a chemoattractant factor for neuronal precursor cells in the rostral migratory stream. Molecular and cellular neurosciences 31, 505-514. Parent, J.M., Vexler, Z.S., Gong, C., Derugin, N., and Ferriero, D.M. (2002). Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Annals of neurology 52, 802-813. Parrish-Aungst, S., Shipley, M.T., Erdelyi, F., Szabo, G., and Puche, A.C. (2007). Quantitative analysis of neuronal diversity in the mouse olfactory bulb. The Journal of comparative neurology 501, 825-836. Pascual, A., and Preat, T. (2001). Localization of long-term memory within the Drosophila mushroom body. Science 294, 1115-1117. Pekny, M., and Nilsson, M. (2005). Astrocyte activation and reactive gliosis. Glia 50, 427-434. Pineda, D., Gonzalez, J., Callaerts, P., Ikeo, K., Gehring, W.J., and Salo, E. (2000). Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. PNAS 97, 4525-4529. Pineda, D., Rossi, L., Batistoni, R., Salvetti, A., Marsal, M., Gremigni, V., Falleni, A., Gonzalez-Linares, J., Deri, P., and Salo, E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. Ramaswamy, S., Goings, G.E., Soderstrom, K.E., Szele, F.G., and Kozlowski, D.A. (2005). Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain research 1053, 38-53. Raymond, P.A., Barthel, L.K., Bernardos, R.L., and Perkowski, J.J. (2006). Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC developmental biology 6, 36. Raymond, P.A., and Hitchcock, P.F. (2000). How the neural retina regenerates. Results and problems in cell differentiation 31, 197-218. 47 Raymond, P.A., Reifler, M.J., and Rivlin, P.K. (1988). Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. Journal of neurobiology 19, 431463. Reddien, P.W., and Sanchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annual review of cell and developmental biology 20, 725-757. Robel, S., Berninger, B., and Gotz, M. (2011). The stem cell potential of glia: lessons from reactive gliosis. Nature reviews 12, 88-104. Rothenaigner, I., Krecsmarik, M., Hayes, J.A., Bahn, B., Lepier, A., Fortin, G., Gotz, M., Jagasia, R., and Bally-Cuif, L. (2011). Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 138, 1459-1469. Sanchez Alvarado, A. (2000). Regeneration in the metazoans: why does it happen? Bioessays 22, 578-590. Sandeman, D.C., Bazin, F., and Beltz, B.S. Adult neurogenesis: examples from the decapod crustaceans and comparisons with mammals. Arthropod structure & development 40, 258-275. Scheffler, B., Walton, N.M., Lin, D.D., Goetz, A.K., Enikolopov, G., Roper, S.N., and Steindler, D.A. (2005). Phenotypic and functional characterization of adult brain neuropoiesis. PNAS 102, 9353-9358. Schmidt, M. (2007). The olfactory pathway of decapod crustaceans--an invertebrate model for life-long neurogenesis. Chemical senses 32, 365-384. Schnapp, E., Kragl, M., Rubin, L., and Tanaka, E.M. (2005). Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development 132, 3243-3253. Scotto-Lomassese, S., Strambi, C., Strambi, A., Aouane, A., Augier, R., Rougon, G., and Cayre, M. (2003). Suppression of adult neurogenesis impairs olfactory learning and memory in an adult insect. J Neurosci 23, 9289-9296. Seri, B., Garcia-Verdugo, J.M., Collado-Morente, L., McEwen, B.S., and Alvarez-Buylla, A. (2004). Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. The Journal of comparative neurology 478, 359-378. Silver, J., and Miller, J.H. (2004). Regeneration beyond the glial scar. Nature reviews 5, 146-156. Singer, M. (1952). The influence of the nerve in regeneration of the amphibian extremity. The Quarterly review of biology 27, 169-200. 48 Slotnick, B., Cockerham, R., and Pickett, E. (2004). Olfaction in olfactory bulbectomized rats. J Neurosci 24, 9195-9200. Squire, L.R. (1992). Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review 99, 195-231. Stenkamp, D.L. (2007). Neurogenesis in the fish retina. International review of cytology 259, 173-224. Stenkamp, D.L., Powers, M.K., Carney, L.H., and Cameron, D.A. (2001). Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. The Journal of comparative neurology 431, 363-381. Stone, L.S. (1950). Neural retina degeneration followed by regeneration from surviving retinal pigment cells in grafted adult salamander eyes. The Anatomical record 106, 89109. Sugimoto, K., Gordon, S.P., and Meyerowitz, E.M. (2011). Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends in cell biology 21, 212-218. Suhonen, J.O., Peterson, D.A., Ray, J., and Gage, F.H. (1996). Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383, 624-627. Takeda, H., Nishimura, K., and Agata, K. (2009). Planarians maintain a constant ratio of different cell types during changes in body size by using the stem cell system. Zoological science 26, 805-813. Tanaka, E.M., and Ferretti, P. (2009). Considering the evolution of regeneration in the central nervous system. Nature reviews 10, 713-723. Tanaka, E.M., and Reddien, P.W. (2011). The cellular basis for animal regeneration. Developmental cell 21, 172-185. Thored, P., Wood, J., Arvidsson, A., Cammenga, J., Kokaia, Z., and Lindvall, 0. (2007). Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke; a journal of cerebral circulation 38, 3032-3039. Umesono, Y., and Agata, K. (2009). Evolution and regeneration of the planarian central nervous system. Development, growth & differentiation 51, 185-195. Umesono, Y., Watanabe, K., and Agata, K. (1999). Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Development genes and evolution 209, 31-39. 49 Ventura, R.E., and Goldman, J.E. (2007). Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci 27, 4297-4302. Vihtelic, T.S., and Hyde, D.R. (2000). Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. Journal of neurobiology 44, 289-307. von Leithner, P.L., Ciurtin, C., and Jeffery, G. (2010). Microscopic mammalian retinal pigment epithelium lesions induce widespread proliferation with differences in magnitude between center and periphery. Molecular vision 16, 570-581. Waclaw, R.R., Allen, Z.J., 2nd, Bell, S.M., Erdelyi, F., Szabo, G., Potter, S.S., and Campbell, K. (2006). The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron 49, 503-516. Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816. Wehman, A.M., Staub, W., Meyers, J.R., Raymond, P.A., and Baier, H. (2005). Genetic dissection of the zebrafish retinal stem-cell compartment. Developmental biology 281, 53-65. Wetts, R., Serbedzija, G.N., and Fraser, S.E. (1989). Cell lineage analysis reveals multipotent precursors in the ciliary margin of the frog retina. Developmental biology 136, 254-263. Zupanc, G.K., and Horschke, I. (1995). Proliferation zones in the brain of adult gymnotiform fish: a quantitative mapping study. The Journal of comparative neurology 353, 213-233. 50 Chapter 2 dlx and sp6-9 control optic cup regeneration in a prototypic eye Sylvain W. Lapan1 2 1 and Peter W. Reddien ' 2, 3 'Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142 2 Department of Biology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02142 3 Howard Hughes Medical Institute Published as: Lapan, S.W., and Reddien, P.W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS genetics 7, e1002226. SUMMARY Optic cups are a structural feature of diverse eyes, from simple pit eyes to camera eyes of vertebrates and cephalopods. We used the planarian prototypic eye as a model to study the genetic control of optic cup formation and regeneration. We identified two genes encoding transcription factors, sp6-9 and dlx, that were expressed in the eye specifically in the optic cup, and not the photoreceptor neurons. RNAi of these genes prevented formation of visible optic cups during regeneration. Planarian regeneration requires an adult proliferative cell population with stem cell-like properties called the neoblasts. We found that optic cup formation occurred only after migration of progressively differentiating progenitor cells from the neoblast population. The eye regeneration defect caused by dlx and sp6-9 RNAi can be explained by a failure to generate these early optic cup progenitors. Dlx and Sp6-9 genes function as a module during the development of diverse animal appendages, including vertebrate and insect limbs. Our work reveals a novel function for this gene pair in the development of a fundamental eye component, and utilizes these genes to demonstrate a mechanism for total organ regeneration in which extensive cell movement separates new cell specification from organ morphogenesis. 52 INTRODUCTION Animal retinas are susceptible to damage and degeneration from injury and because of sensitivity to light. Multiple vertebrates have evolved the ability to regenerate ocular tissue following damage or degeneration. In zebrafish, proliferating marginal zone cells, specialized rod progenitors, and Muller glia reside within the retina and are sources of regenerative tissue (Stenkamp, 2007). In urodele amphibians, cells of the retinal pigment epithelium can act as a source of new retinal neurons in the adult (Okada, 1980). Some invertebrates, such as planarians, are also capable of eye regeneration. Unlike vertebrates, planarians can regenerate eyes completely de novo, using a population of cells that resides entirely outside of the eye. The eyes of planarians are substantially simpler than vertebrate camera eyes, but there are important similarities between the two structures nonetheless. Eye formation in planarians, vertebrates, and other animals involves common genes such as sine oculis and eyes absent (Mannini et al., 2004; Pineda et al., 2000). Furthermore, in both vertebrates and planarians, specialized pigment cells are organized such that they directly abut photoreceptive organelles in an optic cup. In vertebrates, cells of the retinal pigment epithelium (RPE) contact the outer segments of photoreceptor neurons from an adjacent layer of the optic cup. In planarians, the optic cup is entirely formed of pigment cells (it is commonly termed the "pigment cup") and photoreceptor neurons project rhabdomeres into the cup (Sato et al., 2005) (Figure 1A). A primary function of pigmented optic cups in simple eyes is to absorb incoming light prior to detection by photoreceptors (Nilsson, 2009), as this creates shade that allows the eye and brain to resolve the direction of incoming light. Light absorption is also an important function of the vertebrate RPE (Strauss, 2005), although vertebrate eyes use sophisticated imageforming mechanisms for vision with spatial resolution. 53 Planarian eyes can regenerate even after decapitation, from tissue that originally resided far from the head. Planarian regeneration is possible because of a population of proliferative cells, the neoblasts, that includes pluripotent stem cells (Wagner et al.) as well as all cycling somatic cells of the adult. The neoblasts are distributed throughout the body in the parenchyma of the adult (Reddien and Sanchez Alvarado, 2004), and new tissue is produced at wounds by localized increase in neoblast proliferation, followed by cell cycle exit and differentiation (Wenemoser and Reddien, 2010). Wounding in areas devoid of neoblasts ultimately results in localization of cycling cells at the wound site, indicating that cell migration may be important for repair of at least some injuries (Wenemoser and Reddien, 2010). Furthermore, immediate neoblast descendants are more peripherally located than the neoblasts, suggesting that cell movements occur during differentiation (Eisenhoffer et al., 2008; Newmark and Scnchez Alvarado, 2000). In prior studies, neoblasts and their descendants were examined in vivo as large populations of cells with unidentified lineage and fate. Therefore, very little is currently known about the cellular and genetic events that occur between the pluripotent state and the terminally differentiated state during regeneration of specific organs such as the eye. Here we identify the conserved transcription factors dlx and sp6-9 as novel regulators of planarian eye regeneration. These genes are expressed at early stages of pigment cup progenitor specification and are required for regeneration of the cup. We find that progenitors of pigment cup cells and photoreceptor neurons form distinct mesenchymal populations substantially before differentiation and morphogenesis. Our genetic characterization of the pigment cup allows us to identify lineage-specified pigment cup cells within the neoblast population, at surprisingly large distances from the final position of the regenerating eye, and we demonstrate that sp6-9*/dlx+ eye precursors differentiate in a spatially graded manner through the blastema prior to 54 reaching the eye. Therefore, in contrast to epithelium-based modes of eye development, planarian eye regeneration relies on a dramatic spatial decoupling of progenitor specification and morphogenesis. RESULTS The optic (pigment) cup is defined by expression of tyrosinaseand the transcription factors sp6-9 and dlx As in vertebrates, but unlike most protostomes, the planarian optic shading pigment is primarily composed of melanin (Hase et al., 2006). Consistent with this, we found that the Schmidtea mediterranea gene Smed-tyrosinase (Figure SI), homologs of which are required for melanin synthesis (Muller et al., 1988), was expressed exclusively in the pigment cup region of the planarian eye (Figures 1 B and 1 C and Figure S2). Whereas photosensing neurons in planarians express Smed-opsin (Senchez Alvarado and Newmark, 1999), pigment cells did not have detectable expression of Smed-opsin, nor the pan-neuronal markers Smed-synapsin and Smed-synaptotagmin (Figures 1 D and S3), indicating that pigment cup cells do not function directly in light detection and phototransduction. Eye development is controlled by similar transcription factors in diverse animals. We sought factors that might control formation of the pigmented optic cup in planarians during regeneration by broadly screening expression patterns of conserved transcription factor-encoding genes. We identified two genes, Smed-sp6-9 and Smed-dx, that were both expressed in the regenerating eye, specifically in the optic cup of the regenerating eye (Figures 1 E and 1 F). These genes were also expressed in cells outside of the eye (Figure S2 and S4), including in neurons of the brain and cells of the head rim and pharynx. 55 Smed-sp6-9 (sp6-9) clustered within the Sp6-9 zinc finger gene family (Figure SI), which diverged from the Spl-4 and Sp5 families prior to the evolution of the Bilateria (Schaeper et al.), suggesting Smed-sp6-9 is equally related to vertebrate Sp6, Sp7, Sp8, and Sp9 genes. Smed-dlx (dlx) is homologous to the Distal-less family of homeobox genes (Figure S1), which have broadly important roles in development (Panganiban and Rubenstein, 2002). otxA, sine oculis, and eyes absent genes, homologs of which encode transcriptional regulatory proteins required for the development of diverse animal eyes (Nilsson, 2004; Ranade et al., 2008), are expressed in the eyes of planarians (Mannini et al., 2004; Pineda et al., 2000; Umesono et al., 1999). We cloned S. mediterranea orthologs of these genes. In contrast to sp6-9 and dx,Smed-otxA (otxA) was expressed specifically in the photoreceptor neurons of the eye, but not the pigment cup (Figure 1G and S2) (Umesono et al., 1999). Smed-eyes absent (eya) and Smed-sine oculis1/2 (six1/2) were expressed in both photoreceptor neurons and pigment cups (Figures 1 H, 11 and S2) (Mannini et al., 2004; Pineda et al., 2000). We also identified an ortholog of dachshund (Figure S5A), a gene with important regulatory functions in Drosophila eye development (Silver and Rebay, 2005), but did not detect Smed-dachshund expression in the regenerating eye (Figure S5B). Expression of transcription factors was only weakly detected in the pigment cups of intact (non-regenerating) animals (Figure S2). As the pigment of the optic cup could obscure signal in intact eyes, we used RNAi of tyrosinase (see below) to reduce cup pigmentation prior to fixation of animals for FISH. Even with reduced pigmentation, however, expression of dlx, sp6-9, six-1/2 and eya was faintly detected in the intact optic cup (Figure S6). In summary, we have characterized eye-expressed transcription factors in planarians on the basis of expression in photoreceptor neurons and/or the optic cup during regeneration, and have identified two genes that are expressed specifically in the optic cup during regeneration. 56 dlx and sp6-9 are required for regeneration of pigment cups Dlx and Sp6-9 genes have essential roles during development of several animal tissues. We therefore examined whether dlx and sp6-9 loss of function could affect formation of optic cups during regeneration. RNAi of dlx or sp6-9 followed by decapitation resulted in planarians that did not regenerate visible optic cups, but did make photoreceptor neurons that contacted the brain (Figures 2A-C). Abnormal targeting of photoreceptor neuron processes in dlx(RNAi) and sp6-9(RNAi) animals was observed, possibly due to the physical absence of a cup, which typically encloses many processes (Figure 1C). tyrosinase(RNAi) animals had weakly pigmented optic cups, and displayed normal photoreceptor neuron morphology (Figure 2D). In contrast, RNAi of otxA resulted in animals that successfully made optic cups, but lacked photoreceptor neurons (Figure 2E). Consistent with six-1/2 expression in both pigment cups and photoreceptor neurons, RNAi of this gene strongly affected pigment cup regeneration and photoreceptor neuron formation (Figure 2F), as previously observed (Pineda et al., 2000). We were unable to observe an eye defect following RNAi of dachshund (n=8/8 animals regenerated pigment cups). dlx and sp6-9 were also required for eye regeneration following targeted removal of the eye without decapitation. Control animals regenerated a small pigment cup within 7 days of surgical excision of the eye. However, dlx(RNAi) and sp6-9(RNAi) did not regenerate pigment cups following this type of injury (Figure S7A). In order to examine whether dlx and sp6-9 are required for homeostatic maintenance of the eye in uninjured animals, dsRNA was applied by feeding over 3-7 weeks without injury (Figure S7B). dlx(RNAi) animals developed lesions in the region of the eye (n=5/9). Lesions also occurred in other areas of the body, including the tail and pre-pharyngeal region, and animals ultimately died from lysis. Homeostatic sp6-9(RNAi) animals exhibited gradual 57 loss in size and pigmentation of the optic cup (n=20/20), culminating in loss of visible optic cups in some animals (n=4/20) after 7 weeks of RNAi (Figure S7B). Our expression and functional analyses indicate that dlx and sp6-9 have essential roles in pigment cup maintenance as well as regeneration following diverse injuries to the eye. A population of cells directly posterior to the optic cup expresses markers of the optic cup In order to understand the mechanism by which dlx and sp6-9 act to promote eye regeneration, we sought to identify the source of pigment cup cells. smedwi-1 mRNA is a marker for neoblasts, whereas SMEDWI-1 protein is a marker for neoblasts and immediate neoblast descendants, because of the fact that SMEDWI-1 protein perdures beyond expression of smedwi-1 mRNA (Guo et al., 2006; Wenemoser and Reddien, 2010). In intact animals we were able to identify isolated cells in the dorsal anterior and in the brain that were positive for both SMEDWI-1 protein and sp6-9 mRNA or dlx mRNA (Figure S8), indicating that these genes are expressed in diverse neoblast-descendant progenitor populations during homeostasis. During regeneration following decapitation, we detected a dense trail of SMEDWI-1 /sp6-9+cells behind the eye primordium (Figure 3A). These cells were present in a band proximal and posterior to the eye, and at the same plane of the eye on the dorso-ventral axis. This is the region imaged and analyzed in all experiments referring to a cell "trail." The close proximity of trail cells to the optic cup, the expression of the optic cup gene sp6-9, and the presence of a marker for immediate neoblast progeny cells raised the possibility that these trail cells are progenitors of optic cup cells. To explore this possibility we first sought to determine the extent of molecular similarity between trail cells and optic cup cells. Using combinatorial FISH, we found that sp6-9' trail cells coexpressed other markers of the optic cup, including eya, six1/2, and dlx (Figures 3B-3D 58 and S9A-B). The identified trail cells therefore possess an expression profile that is uniquely similar to the optic cup; outside of the eye, no other differentiated cell types, including the neighboring sp6-9' head rim cells, were found to be positive for this combination of markers (Figure S10). To test whether sp6-9* trail cells shared molecular similarity with photoreceptor neurons as well as the optic cup cells, we looked for overlap between expression of sp6-9 and otxA, which is specifically expressed in the photoreceptor neurons in the eye. Cells double-positive for these markers or for dlxlotxA were observed only very rarely (Figure 3E and S9C), indicating that sp6-9+ trail cells share molecular identity with the pigment cup, but not with photoreceptor neurons. Numerous otxA+/eya+ cells were, however, detected in mesenchymal cells posterior to the eye (Figure 3F), consistent with the fact that eya is also expressed in photoreceptor neurons. In summary, we identified a population of sp6-9* cells posterior to the eye during head regeneration that shares a highly similar expression profile with optic cup cells (sp6-9+Id/x*Ieya*/six1-2*/otxA-), but not with any other cell type of the animal. Furthermore, we identified an adjacent population of mesenchymal blastema cells that share a similar expression profile with the photoreceptor neurons of the eye (sp6-9-/d1x/otxA+Ieya+). We hypothesized that the mesenchymal trails of sp6-9*/eya*/dlx*/six1-2*/otxAcells are progenitor cells that are the source of new pigment cup cells during eye regeneration after decapitation. Some sp6-9+/eya+ cells in the trail expressed tyrosinase (Figure 4A). tyrosinase expression serves as a marker for the differentiation of optic cup cells, and its expression in the trail indicates that these cells are indeed in the process of differentiation. sp6-9*/eya*/tyrosinase* cells of the trail were typically closer to the optic cup (Figure 4A and 4B) than were sp6-9*/eya+/tyrosinase- cells. Using single rather than triple probe in situ hybridization conditions, some tyrosinase* cells could be detected 150pm or more away from the pigment cup (Figure 4C). Expression of 59 tyrosinase in these distant cells was consistently much weaker, further indicating that proximity to the cup primordium correlates with extent of differentiation. tyrosinase expression in intact animals was not detected in any cells of the body outside of the optic cup, confirming that the expression profile of the sp6-9 trail cells identified here is uniquely similar to that of optic cup cells. We refer to these cells as "optic cup trail cells," and use detection of sp6-9 and eya co-expression to identify these cells in subsequent experiments. Optic cup trail cells originate in the neoblast population Differentiated cells in planarians are non-mitotic, and neoblasts are the only cycling somatic cells of the animal (Reddien and Senchez Alvarado, 2004). Surprisingly, at positions relatively far from the pigment cup, sp6-9*/eya' trail cells were observed that also expressed the proliferative marker (Hewitson et al., 2006) histone h2b (Figures 4D) and the neoblast marker (Reddien et al., 2005b) smedwi-1 mRNA (Figure S11). RTPCR analysis of FACS sorted "X1" neoblasts has indicated that the neoblast population contains heterogeneity of gene expression and possible commitment to specific lineages (Hayashi et al., 2010). smedwi-1 and h2b positive optic cup trail cells, described here, are the first lineage-committed neoblast subpopulation observed in vivo. It will be interesting to investigate whether these sp6-9+/eya+ cycling cells function as stem cells, or whether they are a transient, differentiating cell type. Neither h2b nor smedwi-1 expression was observed within the optic cup at any point during regeneration. Indeed, sp6-9*/eya*Ih2b+ cells were typically present in a region of the trail that was relatively distant from the regenerating eye primordium, in contrast to the sp6-9*/eya*/tyrosinase+ cells that were closer to the eye (Figure 4B and 4D). These data indicate that there exists a distal-to-proximal distribution of progenitor trail cells with respect to the eye that 60 divide in the neoblast population (distal), exit the cell cycle and begin differentiation (intermediate), and finally differentiate fully and aggregate (proximal). Optic cup trail cells are a source of new eye tissue during regeneration To further test the possibility that optic cup trail cells are the source of new optic cup tissue in regeneration, animals were irradiated with 6,000 Rads on the third day of regeneration following decapitation, a point at which aggregates of eye cells were first apparent. Irradiation permanently blocks all new cell division in planarians (Reddien and Senchez Alvarado, 2004). Nonetheless, optic cups underwent significant growth, measured in number of cells, following irradiation on day 3 of regeneration (Figure 5A and 5B). Simultaneously, the number of sp6-9*leya+ trail cells gradually decreased following irradiation. The extinction of trail cells 24-48 hours after irradiation correlated with the end of cup growth. Furthermore, the number of trail cells present shortly following irradiation approximately matched the number of cells gained by the optic cup with time following irradiation. When BrdU was injected at day three of regeneration, incorporation into sp6-9+Ieya+ cells was first detected distal to the cup in the trail, at 24 hours after injection (Figure 5C and 5D). Incorporation of BrdU first into distal cells of the trail provides further evidence these are the trail cells that have most recently undergone S-phase. or are actively in S-phase. At later time points following the BrdU pulse, however, a majority of sp6-9*/eya*/BrdU* cells were located in the cup aggregate (Figures 5C and 5D). Because most cycling sp6-9*/eya' cells are located at sites distal to the eye, and no cycling cells are found in any part of the eye primordium (Figure 4D), the presence of BrdU signal in the eye three days after the BrdU pulse indicates that a net movement of sp6-9*/eya+/BrdU+ cells from distal to proximal to the eye occurs during regeneration. Together, these data suggest a model in which optic cup progenitors arise after decapitation and migrate toward the eye while undergoing differentiation. 61 dlx and sp6-9 are required for eye progenitor formation after decapitation Because sp6-9 and dlx are expressed in the undifferentiated cells of the optic cup trail, we sought to determine whether they are required for generation of the optic cup progenitors or for later stages of cup formation. RNAi of dlx to eliminate gene function resulted in animals that completely lacked sp6-9+Ieya' optic cup trail cells during regeneration as well as sp6-9'/eya' double-positive signal in the optic cup primordium (Figures 6A, 6C and 6H; higher magnification images of RNAi phenotypes are in Figure S12). We obtained a similar result for sp6-9(RNAi) animals, which largely lacked dlx'/eya' progenitor cells (Figures 6B and 6D). eya' signal remaining in these animals reflects photoreceptor neuron expression of this gene. tyrosinase(RNAi) animals lacked visible eye pigment but displayed normal sp6-9*/eya' progenitor patterns (Figure 2D, 6E, and 6H), and eye markers were normally positioned, indicating that, unlike sp6-9(RNAi) and dlx(RNAi) animals, tyrosinase(RNAi) animals are able to regenerate optic cup tissue. otxA(RNAi) animals also had normal and abundant sp6-9+/eya+ progenitors, as expected given that these animals regenerate visible pigment cups (Figure 6F and 6H). RNAi of six1/2 led to severe reduction in numbers of both pigment cup progenitors and photoreceptor neurons following regeneration (Figure 6G and 6H). We verified the presence or absence of pigment cups in the above RNAi conditions using DIC light microscopy (Figure S13). Pigment cups (epithelial cells arranged in a crescent around a lumen) were observed in control, tyrosinase(RNAi), and otxA(RNAi) animals; no such cellular formations were observed in sp6-9(RNAi), dlx(RNAi), or six1/2(RNAi) animals. The RNAi experiments described above were performed by feeding dsRNA to animals for one week prior to cutting. In order to confirm that sp6-9 and dlx gene functions are required during regeneration, we applied dsRNA only after amputation by using an injection strategy. dsRNA was applied beginning at either day 2 or day 4 of 62 regeneration following decapitation. In both cases, pigment cups were typically present at day 7 of regeneration in dlx(RNAi) and sp6-9(RNAi) animals, as expected because some progenitors are formed before the onset of RNAi (Figures 61-L). However, RNAi animals lacked the tyrosinase* terminal trail cells posterior to the eye ( >97% reduction for dlx and sp6-9 RNAi at d2) and had pigment cups that were smaller than in control animal (Figures 61-L and S14). Therefore, inhibition of sp6-9 and dlx only during regeneration successfully impaired ongoing pigment cup cell specification. Despite a reduction in total numbers, the pigment cup cells that were generated successfully aggregated into concave cups properly positioned in the head. We conclude, based on these data, that dlx and sp6-9 are required for progenitor formation during regeneration of the optic cup (Figure 7). DISCUSSION A cellular model for regeneration of the eye All regions of the planarian body that can regenerate contain somatic dividing cells (neoblasts), which are typically identified by expression of cell cycle genes and smedwi1, a piwi homolog. Recent work has shown that some of the adult dividing cells are pluripotent stem cells, called cNeoblasts, which are capable of generating all essential cells of the body (Wagner et al., 2011). Here we show that some neoblasts have gene expression and distribution that is indicative of restriction to a specific cell type, the optic cup cells of the eye. Optic cup progenitors are induced in abundance within the parenchyma of the animal after decapitation, at a distance from the (now absent) site of the lost eye as well as the aggregated primordium of the newly regenerating eye. Differentiation and migration toward the eye primordium are temporally correlated, as expression of neoblast markers is downregulated and expression of the differentiation marker tyrosinase is upregulated in progenitor cells approaching the eye (Figure 7). 63 Therefore, we spatially and molecularly identify progenitors between the pluripotent state and the terminally differentiated state during regeneration of a specific tissue. The eye is an ideal system with which to address questions of how the identity of lost tissue impacts the generation of regenerative cells, as its pigmentation and restricted localization greatly simplify targeting for surgery. Therefore, many questions can now be asked about how specification, migration, and differentiation of a specific progenitor population are regulated following diverse injuries to a specific organ. For example, what is the minimal injury to the eye required for induction of eye progenitors? Previous work has suggested that optic cup cells and photoreceptor neurons in planarians derive from common progenitors cell that expresses terminal differentiation markers of both the optic cup cells (tryptophan hydroxylase) and photoreceptor neurons (arrestin) (Takeda et al., 2009). By contrast, our work indicates that progenitors for photoreceptor neurons and cup cells exist as distinct progenitor populations prior to terminal differentiation and aggregation in the eye primordium, based on distinct expression of dlx/sp6-9 and otxA in mesenchymal six*/eya* cells present posterior to the eye. With these results we cannot exclude that all eye cells derive from a common upstream, eye-specific progenitor. However, it is apparent that they exist as spatially distinct populations with distinct gene expression for a substantial period before differentiation and aggregation into an eye primordium. In most developing eyes, including in Drosophila and vertebrates, pigment cells and photoreceptor neurons are formed from a common epithelial eye field (Cagan, 2009). We find no epithelial eye field that contains proliferating or undifferentiated eye progenitors in the regenerating or intact planarian. Therefore, it will be interesting to determine whether the pigment cells and photoreceptor neurons likewise share a common eye-specific precursor population in this organism. 64 A significant consequence of development within an epithelial field is that position and numerical proportion of various cell types can be influenced by direct signaling from one cell type to another. For instance, in Drosophila eye development many cell fate decisions depend on direct cell-cell signaling via Notch/Delta, including the induction of primary pigment cells by adjacent cone cells (Nagaraj and Banerjee, 2007). Signaling between mesenchymal eye progenitors in planarians is less likely to depend on cell contact. Longer-range signaling molecules in planarians could be responsible for coordinating progenitor numbers and position. Perturbation with RNAi of Wnt (Adell et al., 2009; Gurley et al., 2008; Iglesias et al., 2008; Petersen and Reddien, 2008, 2009), Bmp (Molina et al., 2007; Orii and Watanabe, 2007; Reddien et al., 2007), or Fgf (Cebria et al., 2002) signaling in planarians can result in ectopic eyes. It is unknown, however, whether these pathways act directly in eye progenitor specification or whether ectopic photoreceptors are the indirect result of major regional tissue changes in these RNAi animals, such as expansion of the brain. Investigation of the signaling molecules that directly act on eye progenitors might promote understanding of how progenitor numbers are coordinated during de novo organ regeneration. Transcription regulatory genes and eye regeneration The planarian eye has important similarities to many animal eyes, including expression of regulatory transcription factors such sine oculis (Pineda et al., 2000) and eyes absent (Mannini et al., 2004), downstream genes such as opsin, arrestin, and tyrosinase, as well as the overall morphology of a pigmented cup that encapsulates photoreceptive organelles. We identify planarian dlx and sp6-9 as novel regulators of optic cup progenitor formation during planarian eye regeneration. Dlx and Sp6-9 transcription factors are well conserved throughout the Eumetazoa and can function as an evolutionarily conserved module. Together, DIx and Sp6-9 genes regulate limb 65 outgrowth in both insects and vertebrates (Estella and Mann, 2010; Hertveldt et al., 2008; Kawakami et al., 2004; Schaeper et al., 2009; Talamillo et al.) and are important for development of the vertebrate tooth (Hertveldt et al., 2008; Nakamura et al., 2004; Thomas et al., 1997), another appendage-like structure. Dlx and Sp6-9 family genes are also both expressed in the anterior of the body axis in Drosophila embryos and the basal deuterostome Saccoglossus (Lemons et al.), although a coordinated role for these two genes in the anterior of the body axis has not yet been supported by functional data. Multiple Dlx genes are expressed in the vertebrate eye from early stages of retina formation (de Melo et al., 2005; Dhawan et al., 1997), and a Dlx gene is expressed in the adult Platynereis cup-shaped eye (Arendt et al., 2009). Planarian eye regeneration differs from eye development in a number of other animals in that it does not appear to rely on orthologs of the pax6(eyeless) (Pineda et al., 2002) or dachshund genes, two important components of the Drosophila retinal determination gene network (RDGN). Pax6 genes are important regulators of eye development across many animal lineages (Kozmik, 2008). dachshund is required for Drosophila eye development (Mardon et al., 1994), and dachshund orthologs are expressed, but not functionally required, during mouse eye development (Davis et al., 2006). Therefore, potentially ancestral eye specification genes (Silver and Rebay, 2005) might not be involved in planarian eye regeneration. One possible explanation for this is that differences in specification mechanisms may exist between embryonic development and adult regeneration. The roles for eye regeneration genes are currently unknown in planarian embryonic eye development. Another possibility, if these genes indeed had roles in eye specification in the ancestor of Bilateria, is that planarians have diverged over evolutionary time with respect to their reliance on pax6 and dachshund. Planarians also differ from vertebrates, but are similar to Drosophila, in the role of Orthodenticle homologs in pigment cell specification in the eye. Vertebrate RPE 66 development depends on otx2 (Martinez-Morales et al., 2003), and regeneration of the newt RPE is associated with expression of otx (Sakami et al., 2005). By contrast, in planarians the only eye-expressed orthodenticle homolog, otxA, is expressed specifically in the photoreceptor neurons, and not the pigmented cells (Figure 1H) (Umesono et al., 1999) and is primarily required for photoreceptor neuron specification (Fig 1N). Similarly, in Drosophila development, orthodenticle is required for photoreceptor neuron development, and a role in pigment cells of the eye has not been described (Vandendries et al., 1996). Therefore, the function of Orthodenticle homologs in maintenance and specification of pigmented eye cells may differ between protostomes and deuterostomes. Despite these differences, the demonstrated Eumetazoa-wide conservation of numerous other eye genes, as well as the demonstrated capacity of Dlx and Sp6-9 to execute deeply conserved developmental programs, indicates that investigation of this gene pair in diverse animals will be an important direction for the study of optic cup formation and regeneration. Furthermore, the identification of a highly localized eye progenitor population based on expression of dlx and sp6-9 will facilitate the exploration of progenitor dynamics during regeneration. 67 MATERIALS AND METHODS Gene cloning Genes were cloned from cDNA using gene-specific primers designed from EST databases and gene predictions (Genscan and Maker) (Cantarel et al., 2008). For cDNA library generation, RNA from mixed stage regenerating animals was isolated in Trizol, and used as template for first-strand reverse transcription with Superscript Ill (Invitrogen). PCR using cDNA template and gene-specific primers was typically followed by a secondary, interval PCR to improve specificity and yield. The following primer sequences were used with gateway adapters or addition of T7 promoter sequence (see below) for cloning genes. dachshund 5' primer: GTGGGCTTCACACGGTTTAT nested 5' primer: TTGAAGAGACTAGAAATCGTTCCA 3' primer: TTGCACAAACTTTGCAGGAG dlx 5' primer: AATGAACCTCCCACTGCA nested 5' primer: CAGGATCAGAAACCCAATCC 3' primer: CGGTTATTCGAAAAATTAACTGG eyes absent 5' primer: GGCCTTTCAAAAGACGACTC 3' primer: AAGACTCAATGCGTGGTGAA opsin 5' primer: TGGTTTCATCGGTGGTCTTT nested 5' primer: TGGGTTTATATCCATCAAQACAAT 3' primer: TTTTTGCACCCGTTTTCAT otxA 5' primer: CCACAAATCCCTCTCTACGG nested 5' primer: ACGTAGCTGGGATCAACACC 3' primer: TGGACCTGACAAATTGTTCC six 5' primer: ATCGATATCCACGAGCCAAG 68 3' primer: CCAGATTCGCATTCGTTACTT nested 3' primer: ACAGGACTCCGAACAAATCA sp6-9 5' primer: TTCAATAAATAACGTTGAGAGCAA nested 5' primer: ATCAATCTTGGCTATTGGAACG 3' primer: TTCACAATTGTTTGTTAACGACTC tyrosinase 5' primer: TGCTCGTAATCACAATAGGCATAG nested 5' primer: TTTGCATCTTTCTTACCTTTGAGT 3' primer: TTTCTTAATAGCCAAATTTCAAAGA For gateway cloning, the following adapters were appended to the above primers 5' primer adapter: AAGCTGGAGCTCCACCGCGG 3' primer adapter: GGGCGAATTGGGTACCGGG cDNA amplified by PCR was cloned into pGEM (pGEM T-Easy, Promega) for use in riboprobe and dsRNA reactions (see below). For RNAi experiments by feeding, gateway recombination was used to clone genes in pPR244, a dsRNA-expression vector, as described (Reddien et al., 2005b). For determination of complete gene sequences, 5' and 3' RACE libaries were generated from mixed stage planarian RNA (FirstChoice RLM-RACE, Ambion). Nested primers were designed to either the 5' or 3' prime end of the known gene sequence, and candidate bands following the second (nested) PCR were excised, purified, cloned into pGEM, and sequenced. RNA synthesis To generate template for riboprobe synthesis, amplified cDNAs were first cloned into pGEM (as above). Template was generated with same primers as were used for cloning, except that gateway adapters were not present on primers, and T7 promoter sequence was appended to the 3' primer. Transcription reactions were performed with T7 (Promega) and either DIG-, FITC- (Roche), or DNP- (Perkin Elmer) labeled ribonucleotides. RNA was purified using ethanol precipitation with 7.5M ammonium 69 actetae (1:2). Pellets were resuspended in formamide (50pl per 2 5pl synthesis reaction), and stored at -20'. To generate template for dsRNA synthesis in vitro, generic primers recognizing gateway adapter sequences were used with T7 appended to either the forward or reverse primer, and PCR was performed using amplified cDNA (with gateway adapters) cloned into pGEM. T7 transcription reactions for sense and antisense RNA were performed separately, and pooled prior to phenol-chloroform extraction followed by ethanol precipitation with sodium acetate (1:10). RNA was annealed using the following program in a thermocycler: 70'C for 10', 37'C for 30', 4"C for 10' RNAi RNAi was performed by feeding intact animals with E. coli expressing dsRNA under an inducible promoter. HT1 15 competent cells were transformed with pPR244 vector (Reddien et al., 2005a) containing the gene of interest. Negative control experiments used a 1 kb region of unc-22, a C. elegans gene with no significant nucleotide sequence similarity to a planarian gene. Cultures were grown in 2xYT media to an OD600 of 0.350.45, and then induced for two hours with 1 mM IPTG. Cultures were pelleted and resuspended in a volume of 70% liver/30% water equal to 1 /3 0 0 th of the original volume of the culture, and stored at -80' C. Worms were fed three times prior to amputation. Amputation was performed on the day following the final feeding, and worms were fixed following one round of regeneration. For targeted eye surgery, a microsurgical blade (MSP, 15', 3mm depth) was inserted into the eye and the optic cup was excised upon removal of the blade. Surgery was performed one day after the third RNAi feeding, and one additional feeding was administered 4 days after surgery. For homeostasis RNAi experiments, animals were fed every four days. 70 For RNAi by injection, dsRNA was diluted to 4pg/pl in water. For standard RNAi application (dachshund RNAi experiment), animals were amputated and injected after 30 min, and again after 24 hours. After 3 days of regeneration, animals were amputated again and injected once more after 30 min. For RNAi injection in figures 61-6K, animals were injected once, either on day 2 or day 4 of regeneration after decapitation. Injection was performed with a Drummond Nanoject 11 and 3.5" Drummond capillaries (3-000-203G/X). Animals were immobilized for injection with use of a peltier cooling block. Several injections of 32 nI were applied as needed to observe swelling of animals with liquid. Histology and imaging Whole-mount fluorescent in situ hybridization (FISH) and antibody staining was performed as described (Pearson et al., 2009) except that FITC-tyramide was used at 1:500, and HRP enzyme was inactivated using 4% formaldehyde. Tyramide was generated by conjugation of succinimidyl esters of rhodamine, FITC, Cy5 and AMCA with tyramide-HCL (Roche) (Hopman et al., 1998). Riboprobes (see below) were used at 1:800 dilution in hyb solution, except for opsin and tyrosinase, which were used at 1:1200. Anti-ARRESTIN (VC-1) antibody (1:5000 dilution) was kindly provided by Kiyo Agata, and SMEDWI-1 antibody (1:1000 dilution) was obtained as described (Guo et al., 2006; Wenemoser and Reddien, 2010). For BrdU experiments, animals were injected with a solution in 5 mg/mI of BrdU (Fluka) in planarian water. Similar injection methods were used as for dsRNA injection (see above), and animals were only injected once. Animals were then fixed and labeled according to the regular FISH protocol, and following FISH development animals were treated with 2N HCI for 45 minutes at RT. BrdU was detected with rat-anti-BrdU (1:100) antibody (Oxford Biotech) followed by incubation with anti-rat-HRP (1:100) (Abcam) and development with commercial tyramide (Invitrogen). 71 Optical sectioning was performed using an Apotome, Axiocam digital camera, a Zeiss Axiolmager, and Axiovision software. Images in figures 5A, S4 and S8 were generated with a Zeiss confocal microscope (LSM 700). Brightness, contrast, and gamma were adjusted as needed to improve visibility. In most images, optical sections are overlayed to show co-expression in cells across a depth of tissue. Stacks of sections were manually examined at the level of individual optical sections to determine true instances of co-expression, and instances of artifact overlapping signal created by digital overlaying were excluded from images and analyses. Phylogenetic analyses S. mediterranea amino acid sequence predictions were aligned (ClustalW) with sequences of putatively homologous proteins from other metazoans. Sequences were trimmed by gblocks under the lowest stringency settings. Phylogenetic trees for tyrosinase, sp6-9, dlx, and otxA were constructed using Bayesian inference (MrBayes) with >2,000,000 generations, and >1,500 burn-in trees discarded. Branch labels display posterior probabilities. ACKNOWLEDGMENTS We thank Irving Wang for illustrations and members of the Reddien lab for comments on the manuscript. 72 73 A Vertebrate Planarian 74 Figure 1. The planarian optic pigment cup expresses dlx and sp6-9. Anterior is up in all images, and all eyes are shown at day 6 of regeneration except in (B). Fluorescent images are fluorescent in situ hybridizations (FISH) unless otherwise noted. (A) Schematic highlighting similarities between the vertebrate (left) and planarian (right) optic cup. Light-sensing organelles (orange), neuronal cell bodies and processes (blue), and pigmented cells of the optic cup (brown) are depicted. Neural circuitry of the vertebrate retina is highly simplified. (B) Smed-tyrosinase (tyro) is expressed in the optic (pigment) cup of an intact planarian. (C) Planarian photoreceptor neurons, labeled by an anti-ARRESTIN antibody (Sakai et al., 2000) are adjacent to the pigment cup and extend rhabdomeres (Rh) into the cup lumen. (D) The optic cup does not express opsin or synaptotagmin (synt). (E-1) Expression of transcription factors in the planarian eye during head regeneration. sp6-9 (E) and dlx (F) are expressed in the optic cup. (G) otxA is expressed in the photoreceptor neurons, similar to the case for D. japonica (Umesono et al., 1999). six1/2 (H) and eya (1)are expressed in both the optic cup and photoreceptor neurons, similar to the case for other planarian species (Mannini et al., 2004; Pineda et al., 2000). Scale bars, 100pm (B, D), 50 pm (C, E-1), 75 Control RNAi sD6-9 RNAi d/x RNAi tyrosinase RNAI otxA RNAi six 1/2 RNAi 76 Figure 2. dlx and sp6-9 are required for optic cup regeneration. Pigment cups are visualized in live animals on day 7 of regeneration after decapitation. Photoreceptor neurons are visualized with anti-ARRESTIN (ARR) antibody. Insets in ARR panels show a photoreceptor from the dorsal side. Penetrance in (A) is n=10/10 (presence of pigment cups) and n=6/6 (photoreceptor neurons that contact brain); in (B) n=0/10 and n=9/9; (C) n=0/10 and 9/9; (D) n=0/7 and n=7/7; (E) n=10/10 and n=0/8; (F) n=0/1 0 and n=3/1 1. Scale bars, 1 OOpm for fluorescent images, 200pm for live worm images. 77 --------i 78 Figure 3. A population of mesenchymal cells posterior to the eye during regeneration expresses optic cup transcription factors. Anterior is up in all images, and all eyes and trails are in blastemas at day 6 of regeneration following decapitation. In all high magnification images of trails only one of two eyes is shown, and images are rotated slightly so that the trail fits into a vertical frame. Fluorescent images are FISH. (A) During regeneration sp6-9 is expressed in the pigment cups (Pcup) and in trails of cells directly behind the pigment cups on the dorsal side of the animal. Inset: sp6-9+ trail cells, but not the eye primordium, are positive for SMEDWI-1 protein. Asterisk indicates eye primordium. (B-D) Double FISH for transcription factors showing that trail cells express combinations of genes also expressed in the cup primordium. sp6-9-expressing (B) and d/x-expressing (C) cells in the Pcup and trail also express eya. (D) sp6-9 and dlx are co-expressed in the Pcup and in many trail cells. (E) sp6-9-expressing cells did not express the photoreceptor neuron marker otxA. (F) Numerous eya-expressing cells did express otxA. Arrows show double positive cells. Pigment cup is outlined in (B-E). Scale bars, 100 pm (A), 50 pm in (B-F). 79 C0 00 191, *0 7119% 16. 0 0 - CD 8 8$ CD 0Co Avg distance from Pcup (pm) UFE ) ( * * * ou Figure 4. Optic cup trail cells exhibit a gradient of differentiation that correlates with distance from the cup primordium. Anterior is up in all images, and all eyes and trails are in blastemas at day 6 of regeneration following decapitation. (A) sp6-9*/eya+ trail cells strongly expressing tyrosinase are close to the optic cup. (B) Quantification of distance of trail cells from the Pcup (mean ± s.e.m; n=4 eyes for each category; significance by two-tailed t-test is shown relative to second bar (sp6-94 /eya+) ***P<.001 , *P<.05). tyro++ indicates strong, non-granular signal. (C) weak tyrosinase expression can be detected in cells far from the optic cup. (D) Some sp6-9*/eya+ cells express the proliferation marker histone h2b. Solid arrows show triple-positive cells and the open arrow shows double-positive cells. Scale bars, 50 pm. 81 A ~d3.5 A d3 d3 4d5d d4 dS d7 'V (D m .. B O IE~ a~~te amett 40. . 353025. - Trd~ffod Paup.Wr opra ~20 Jis 20 40 U so 100 82 120 0 Trot puft C,P94W Puss Tfatpul" 4 mw 11 '-- Figure 5. Optic cup trail cells are a source of new optic cup tissue. All fluorescent images are FISH, except for fluorescent detection of BrdU by antibody. (A) Time course of eye regeneration in unirradiated animals and animals irradiated at day 3 of regeneration following decapitation. In irradiated panels arrows indicate double positive cells. (B) Experimental design diagram and quantification of cell numbers in pigment cups (Pcups) and trails of irradiated and unirradiated animals over time (mean s.d; n29 eyes/trails; significance by two-tailed t-test is shown for the Pcup cell numbers in irradiated vs. un-irradiated ***P<.05 ). (C) BrdU injection at day three of regeneration after decapitation only labels trail cells after one day, but labels Pcup cells after a longer time delay. Inset: magnification of sp6-9 signal in BrdU/sp6-9-positive cell. Arrowheads indicate sp6-9+/eya /BrdU* Pcup cells. (D) Quantification of BrdU+/sp6-9+/eya* cell numbers in the trail and Pcup 24h and 72h after BrdU injection. (mean ± s.d; n=5 eyes; significance by two-tailed t-test ***P<.001 , **P<.005). Scale bars, 50 pm. 83 H 10 SIIf L7- 4 w 2- ~' 84 Figure 6. dlx and sp6-9 are required for regenerative optic cup cells. All fluorescence is FISH. Day 7 regeneration blastemas are shown, anterior is up. (AG) Photoreceptor defects following RNAi of eye-expressed genes. Control RNAi animals have co-localization of (A) sp6-9 with eya , or (B) sp6-9 with eya in the eyes and progenitor trail, n=10/10 animals for each. (C), dlx(RNAi) animals do not regenerate sp69'Ieya' cells, n=10/10. (D) sp6-9(RNAi) animals do not regenerate dlx*Ieya+ cells, n=10/10. (E-F) tyrosinase(RNAi) and otxA(RNAi) animals are able to regenerate sp69+Ieya+ cells. (G) six-1/2(RNAi) regenerate greatly reduced numbers of sp6-9'Ieya+ cells. (H) Quantification of the number of optic cup trail cells for RNAi conditions in (AG). For control(2) and sp6-9 RNAi, dlx and eya coexpression is used to count trail cells. For all other conditions sp6-9 and eya coexpression is used. (I-K) RNAi of sp6-9 and dlx beginning on either day 2 or day 4 of regeneration results in smaller pigment cups and fewer terminal trail cells, assessed by tyrosinase expression. Boxes with dashed outline in (1)enclose terminal trail cells. (L) Quantification of number of tyrosinase+ trail cells in the indicated RNAi conditions. Arrows indicate double positive cells. Scale bars, 50 pm. 85 otxA+/ eya+/ AV of tyrosinase+ six1-2+ I I I 004=~ie 0 I 0 0 0 0 86 LQ h2b+1 0 smedwi-1+ 0 Figure 7. A model for cell state changes in pigment cup regeneration. Lines indicate domain of expression of the adjacent gene labels in the optic cup trail during regeneration. Regenerative photoreceptor neuron cells are shown as spatially separated for clarity. This model proposes that optic cup progenitors are specified at distance from the aggregated cells of the optic cup primordium, within the neoblast population (h2b', smedwi-1*). Progenitors undergo changes in gene expression, including loss of neoblast markers and activation of differentiation markers, as migration towards the eye primordium proceeds. Ultimately, terminally differentiating progenitors incorporate into the eye and undergo a mesenchymal-to-epithelial transition. 87 SPSPQXP-7932M SMEDTYROSINASE SMEDSP69 -- mSp1_NP_727350 Drop6_NP_991195 DTyrOaseNP571008 MpP126 SPOAAU04515 -Spe-NP_9jqm ..D._...p._ P. rp7 NP4 998 -- Mmjyrosdre.eAAA40016 DrSpNPrP9P125 Mm_49-NP_001005343 - GQ-SpQ-AAUt4S16 DrSp3 NP 001082M?7 MmSP3ZNP_001-016=5 r pNP seM836375 Gg_Tro -aGS3AALM4516 001230 Dr_Sp4_NP_%UI41 i NP Dsp 5P2gum G MmLdopudwOmsTauWmEDLOOG0D ClrSpC0NP3_9927 ......... g-SP5_NP_001030149 H,_LdopacrwwmsTatomsraseNP_001913 Mm _................ 2 Sp5_NPT_0718X Drp,_NPi19372 0.66SpS5.XP769110 Dr LdopachroreTakmbrxs._NP 001035064 amtp1_NP_064791 ff POMiCAJ3810.1 7 N&_MwBAG15S.1 ,*!HsMalxlNP_002439.2 ;W,,*Nk4_XP00222332.1 W*-Nk2Q-1NP_004378.1 1 Dr-Dbxla-NP -a 571380.1 Mm. oty1NP_035163 rNP 571325OTX1_0 C(_OtKAF306499_1 Dm_nfrNP_524433.1 Mnt-Ot.SACOS3862 HsDli14P 30221.2 GgDIx1_NP_001039307.2 Hs_2D6x_NP_0052132 GD S ---------- 14NP_001074359.1 SMEDOTXA DrDbdaNP571398.1 DrIxA._NP 071375.1 099 HoDIz4aNP_612138.1 CLDICNP_001027821.1 k Cx_ AAP79300.1 rMm_pax6_AA3815 SMED DLX LU DpaNP_671379 B7 PDlx1_CAJ3S79I.1 DmDxANP_523857.1 Dm_"y"us_NP_524628 Cec 043NP_497904.1 Nv-Db(tABGS7787.1 |0. Cl DB, Dm_Arx_NP_722629 NP 001027672.1 B0 Dx XP 002212363.1 Dm~orthopedaP_00109738S CDoxAjP0010270.1 0md Dil? MmAm4CAM22100 88 Figure SI. Orthology of S. mediterranea genes. Posterior probabilities are shown on branches. See methods for details. Dm, Drosophila melanogaster; Dr, Danio rerio; Hs, Homo sapiens; Gg, Gailus gallus; Gt, Girardia tigrina; Dj, Dugesia japonica; Nv, Nematostella vectensis; Sp, Strongylocentrotus purpuratus; Pd, Platynereis dumerilii; Ci, Ciona intestinalis; Sk, Saccoglossus kowalevskii; Bf, Branchiostoma floridae; Ce, Caenorhabditis elegans; Cb, Caenorhabditis briggsae. 89 90 Figure S2. Expression of eye genes in intact animals. FISH showing expression of indicated gene in whole mount intact animals. Expression of some pigment cup-expressed genes in intact animals is partly obscured by unbleachable eye pigment. Dorsal is shown in left panel, ventral on right for each gene. Scale bars, 200 pm. 91 M-- 92 Figure S3. synapsinand synaptotagmin are pan-neuronally expressed and synapsin does not label the pigment cup. All fluorescence is FISH. Ventral view of 6 day regenerating animals showing panneuronal expression of (A) synapsin (B) synaptotagmin orthologs in the brain and elsewhere. As for synaptotagmin (Figure 1 D), synapsin signal (C) is not detected in the optic cup. Scale bars, 100 pm. 93 synaptotagmin 94 overlap Figure S4. Expression domains of dlx and sp6-9 in relation to neurons of the head rim and brain. All fluorescence is FISH, animals are intact (non-regenerating), and anterior pole is facing up. (A) and (B) are dorsal views of the anterior left side of the animal, (C) and (D) are ventral views of the anterior of the animal. (A) dlx is expressed in diffuse cells throughout the dorsal anterior, some of which express neuronal markers. (B) sp6-9 is prominently expressed in the head rim epidermis, as well as sparse underlying cells. These cells abut neurons but largely do not express synaptotagmin. (C) dlx is expressed in many neurons within the primary lobes of the brain as well as more lateral regions. (D) sp6-9 is expressed in a small subset of cells located in the ventral brain, most of which express synaptotagmin. (E) dlx is also expressed in cells of the pharynx and pharynx cavity at the midbody of the animal. Pr, photoreceptors; Phx: pharynx. Scale bars, 50 pm. 95 mmdach1_AAH78644 - A 0.59 mmdach2_CAM22742 0.76 - drdach2_NP_694487 SMEDDACHSHUND 0.87 dmdachshundAAC46506 mmskiNP_035515 0.77 - gg-ski_NP_001034407 0.92 1_- drskiNP_571010 dmsnoABV53645 B 96 Figure S5. dachshund is not detectably expressed in the regenerating eye and does not have an obvious eye phenotype following dsRNA injection. (A) Orthology of smed-dachshund. Posterior probabilities are shown on branches, see methods for details. dm, Drosophila melanogaster; dr, Danio rerio; gg, Gallus gallus; mm, mus musculus. (B) Expression analysis with FISH does not indicate expression of smed-dachshund in the photoreceptor neurons or the optic cup in 6 day regenerating heads. opsin (green) and tyrosinase (blue) expression are used to label photoreceptor neurons and optic cup, respectively. Scale bar, 50 pm. 97 Vl 00 MERGE FISH DIC Figure S6. Expression of transcription factors in de-pigmented optic cups of intact animals. RNAi of tyrosinase was used to reduce melanin pigment in the optic cup. (A) DIC image of an optic cup in an animal not treated with tyrosinase RNAi. (B) Expression of selected transcription factors in intact eyes of tyrosinase RNAi animals (FISH). The crescent-shaped structure in the DIC image is the optic cup. Arrows indicate regions of the optic cup with expression of the gene labeled in the panel. Scale bar, 20 pm. 99 A tO after surgery dlx(RNAi) sp6-9(RNAi) 7 days after surgery B control sp6-9(RNAi) dix(RNAi) 100 control Figure S7. sp6-9 and dlx are required for regeneration of the optic cup after excision and for homeostatic maintenance of the optic cup. (A) The optic cup was surgically removed after 3 RNAi feedings, at which point animals in all RNAi conditions appeared similar. 7 days after surgery, only control animals showed signs of optic cup regeneration. Regeneration of the optic cup was apparent in n=10/10 control RNAi animals, n=0/10 dlx(RNAi) animals and n=0/10 sp6-9(RNAi) animals. (B) RNAi of dlx in uninjured animals resulted in worm lysis within 3 weeks. n=5/9 worms surviving at three weeks of homeostasis had lesions in the area of the eye. RNAi of sp6-9 in uninjured animals resulted in pigment cups that were reduced in size and pigmentation in n = 20/20 animals after 7 weeks. 4/20 of these animals lost pigment cups completely. Scale bars, 500 pm (A), 200 pm (B). 101 SMEDWI-1 102 overlap Figure S8. Expression of dlx and sp6-9 in neoblasts or immediate neoblast descendants in intact animals. All fluorescence is FISH, animals are intact (non-regenerating). (A) and (B) are dorsal views, (C) and (D) are ventral views. (A) d/x-expressing cells on the dorsal side of the animal that also express SMEDWI-1 protein can be found both posterior and anterior to the photoreceptors. (B) A small population of sp6-9-expressing cells in the prepharyngeal region posterior to the photoreceptors also expresses SMEDWI-1 protein. These may represent optic cup progenitors that function during homeostasis. (C) Some d/x-expressing cells at the periphery of the brain lobes are positive for SMEDWI-1. (D) Most sp6-9 positive neurons (located in a ventral region of the brain) are fully differentiated, but some cells in the anterior of this domain express SMEDWI-1. Scale bars, 50 pm. 103 104 Figure S9. Additional image data for figure 2. Anterior is up in all images, and all eyes and trails are in anterior blastemas at day 6 of regeneration following decapitation. Fluorescent images are FISH. (A) six1/2 expression and eya expression overlap fully in the eye aggregate and in the trail. (B) six1/2 expression and sp6-9 expression overlap partly in the eye aggregate and partly in the trail. (C) d/x-expressing cells do not detectably express otxA. Arrowheads indicate double-positive cells. Scale bars, 100 pm. 105 A sp6-9 eya dlx sp6 cc 0 C: B 106 eya otxA Figure S10. Overlapping expression of transcription factor combinations is not found outside of the eye region. (A) FISH showing expression of indicated genes in whole mount intact animals. Both rows show overlayed optical sections imaged from the dorsal surface. The second row is imaged at a more ventral level than the first. Arrows indicate regions with double positive cells. In intact animals, sp6-9, dlx, and eya expression is difficult to detect in intact pigment cups, but is apparent in new cells that are incorporating as part of homeostatic maintenance. (B) Overlayed optical sections taken from ventral surface of the animal showing that sp6-9* nerve cord cells do not detectably express eya, unlike optic cup cells. Scale bar, 50 pm. 107 108 Figure S11. Some sp6-9'/eya' trail cells also express smedwi-1. Anterior is up, and the eye and trail are in an anterior blastema at day 6 of regeneration following decapitation. Fluorescent images are FISH. Note that SMEDWI-1 protein is a marker for neoblasts and their immediate descendants, whereas smedwi-1 mRNA labels only neoblasts. Scale bars, 50 pm. 109 tyrosinase RNAi otxA RNA six-1/2 RNAi Control RNAi sp6-9 RNAi I Control RNAi dx RNA Figure S12. Higher magnification images of RNAi phenotypes. All fluorescence is FISH. Anterior is up, fixed animals are on day 7 of regeneration. Arrowheads indicate double-positive cells. Scale bars, 50 pm. 111 Control RNAi tyrosinase RNAi sp6-9 RNAi dlx RNAi 112 otxA RNAi six1/2 RNAi Figure S13. Detection of presence of pigment cups by DIC. Specimens are the same as in figures 4 and S12. Anterior is up, and arrows indicate presence of pigment cup. Scale bar, 100 pm. 113 * I 0 0 0 1* * * * -1* I 0 0 OD * I 0) 0 0 inj. d4 I I . 0D 0 dix inj.d4 sp6-9 Control inj. d4 dix inj. d2 sp6-9 inj. d2 inj. d2 Control I I l 0D 0 o I 0D 0 Mean Area of Pigment Cup -I I 0 0) -i I 0 0 0) Figure S14. RNAi of dlx and sp6-9 during regeneration leads to smaller pigment cups. Animals were injected with dsRNA on the indicated day of regeneration and pigment cups and terminal trail cells were visualized by tyrosinase expression at day 7 of regeneration. Graph shows average area of tyrosinase+ pigment cup primordium in pm 2 . Error bars are s.e.m; n>12 eyes for each category; significance by two-tailed t-test is shown relative to second bar control, ***P<.001. 115 REFERENCES Adell, T., Salo, E., Boutros, M., and Bartscherer, K. (2009). Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development (Cambridge, England) 136, 905-910. Arendt, D., Hausen, H., and Purschke, G. (2009). The 'division of labour' model of eye evolution. Philosophical transactions of the Royal Society of London 364, 2809-2817. Cagan, R. (2009). Principles of Drosophila eye differentiation. Current topics in developmental biology 89, 115-135. Cantarel, B.L., Korf, I., Robb, S.M., Parra, G., Ross, E., Moore, B., Holt, C., Sanchez Alvarado, A., and Yandell, M. (2008). MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome research 18, 188-196. Cebria, F., Kobayashi, C., Umesono, Y., Nakazawa, M., Mineta, K., Ikeo, K., Gojobori, T., Itoh, M., Taira, M., Sanchez Alvarado, A., et al. (2002). FGFR-related gene noudarake restricts brain tissues to the head region of planarians. Nature 419, 620-624. Davis, R.J., Pesah, Y.I., Harding, M., Paylor, R., and Mardon, G. (2006). Mouse Dach2 mutants do not exhibit gross defects in eye development or brain function. Genesis 44, 84-92. de Melo, J., Du, G., Fonseca, M., Gillespie, L.A., Turk, W.J., Rubenstein, J.L., and Eisenstat, D.D. (2005). DIx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development (Cambridge, England) 132, 311-322. Dhawan, R.R., Schoen, T.J., and Beebe, D.C. (1997). Isolation and expression of homeobox genes from the embryonic chicken eye. Molecular vision 3, 7. Eisenhoffer, G.T., Kang, H., and Senchez Alvarado, A. (2008). Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell stem cell 3, 327-339. Estella, C., and Mann, R.S. (2010). Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS genetics 6, e1001001. Guo, T., Peters, A.H., and Newmark, P.A. (2006). A Bruno-like gene is required for stem cell maintenance in planarians. Developmental cell 11, 159-169. 116 Gurley, K.A., Rink, J.C., and Sanchez Alvarado, A. (2008). Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science (New York, NY 319, 323-327. Hase, S., Wakamatsu, K., Fujimoto, K., Inaba, A., Kobayashi, K., Matsumoto, M., Hoshi, M., and Negishi, S. (2006). Characterization of the pigment produced by the planarian, Dugesia ryukyuensis. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society 19, 248-249. Hayashi, T., Shibata, N., Okumura, R., Kudome, T., Nishimura, 0., Tarui, H., and Agata, K. (2010). Single-cell gene profiling of planarian stem cells using fluorescent activated cell sorting and its "index sorting" function for stem cell research. Development, growth & differentiation 52, 131-144. Hertveldt, V., Louryan, S., van Reeth, T., Dreze, P., van Vooren, P., Szpirer, J., and Szpirer, C. (2008). The development of several organs and appendages is impaired in mice lacking Sp6. Dev Dyn 237, 883-892. Hewitson, T.D., Kelynack, K.J., and Darby, l.A. (2006). Histochemical localization of cell proliferation using in situ hybridization for histone mRNA. Methods in molecular biology (Clifton, NJ 326, 219-226. Hopman, A.H., Ramaekers, F.C., and Speel, E.J. (1998). Rapid synthesis of biotin-, digoxigenin-, trinitrophenyl-, and fluorochrome-labeled tyramides and their application for In situ hybridization using CARD amplification. J Histochem Cytochem 46, 771-777. Iglesias, M., Gomez-Skarmeta, J.L., Salo, E., and Adell, T. (2008). Silencing of Smedbeta-catenin-1 generates radial-like hypercephalized planarians. Development (Cambridge, England) 135, 1215-1221. Kawakami, Y., Esteban, C.R., Matsui, T., Rodriguez-Leon, J., Kato, S., and lzpisua Belmonte, J.C. (2004). Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development (Cambridge, England) 131, 4763-4774. Kozmik, Z. (2008). The role of Pax genes in eye evolution. Brain research bulletin 75, 335-339. Lemons, D., Fritzenwanker, J.H., Gerhart, J., Lowe, C.J., and McGinnis, W. (2010). Cooption of an anteroposterior head axis patterning system for proximodistal patterning of appendages in early bilaterian evolution. Developmental biology 344, 358-362. 117 Mannini, L., Rossi, L., Deri, P., Gremigni, V., Salvetti, A., Salo, E., and Batistoni, R. (2004). Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Developmental biology 269, 346-359. Mardon, G., Solomon, N.M., and Rubin, G.M. (1994). dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development (Cambridge, England) 120, 3473-3486. Martinez-Morales, J.R., Dolez, V., Rodrigo, I., Zaccarini, R., Leconte, L., Bovolenta, P., and Saule, S. (2003). OTX2 activates the molecular network underlying retina pigment epithelium differentiation. The Journal of biological chemistry 278, 21721-21731. Molina, M.D., Salo, E., and Cebria, F. (2007). The BMP pathway is essential for respecification and maintenance of the dorsoventral axis in regenerating and intact planarians. Developmental biology 311, 79-94. Muller, G., Ruppert, S., Schmid, E., and Schutz, G. (1988). Functional analysis of alternatively spliced tyrosinase gene transcripts. The EMBO journal 7, 2723-2730. Nagaraj, R., and Banerjee, U. (2007). Combinatorial signaling in the specification of primary pigment cells in the Drosophila eye. Development (Cambridge, England) 134, 825-831. Nakamura, T., Unda, F., de-Vega, S., Vilaxa, A., Fukumoto, S., Yamada, K.M., and Yamada, Y. (2004). The Kruppel-like factor epiprofin is expressed by epithelium of developing teeth, hair follicles, and limb buds and promotes cell proliferation. The Journal of biological chemistry 279, 626-634. Newmark, P.A., and Sinchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental biology 220, 142-153. Nilsson, D.E. (2004). Eye evolution: a question of genetic promiscuity. Current opinion in neurobiology 14, 407-414. Nilsson, D.E. (2009). The evolution of eyes and visually guided behaviour. Philosophical transactions of the Royal Society of London 364, 2833-2847. Okada, T.S. (1980). Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Current topics in developmental biology 16, 349-380. Orii, H., and Watanabe, K. (2007). Bone morphogenetic protein is required for dorsoventral patterning in the planarian Dugesiajaponica. Development, growth & differentiation 49, 345-349. Panganiban, G., and Rubenstein, J.L. (2002). Developmental functions of the Distalless/DIx homeobox genes. Development (Cambridge, England) 129, 4371-4386. 118 Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and Sanchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238, 443-450. Petersen, C.P., and Reddien, P.W. (2008). Smed-beta-catenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science (New York, NY 319, 327-330. Petersen, C.P., and Reddien, P.W. (2009). A wound-induced Wnt expression program controls planarian regeneration polarity. Proceedings of the National Academy of Sciences of the United States of America 106, 17061-17066. Pineda, D., Gonzalez, J., Callaerts, P., Ikeo, K., Gehring, W.J., and Salo, E. (2000). Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. Proceedings of the National Academy of Sciences of the United States of America 97, 4525-4529. Pineda, D., Rossi, L., Batistoni, R., Salvetti, A., Marsal, M., Gremigni, V., Falleni, A., Gonzalez-Linares, J., Deri, P., and Salo, E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development (Cambridge, England) 129, 1423-1434. Ranade, S.S., Yang-Zhou, D., Kong, S.W., McDonald, E.C., Cook, T.A., and Pignoni, F. (2008). Analysis of the Otd-dependent transcriptome supports the evolutionary conservation of CRX/OTX/OTD functions in flies and vertebrates. Developmental biology 315, 521-534. Reddien, P.W., Bermange, A.L., Kicza, A.M., and Senchez Alvarado, A. (2007). BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development (Cambridge, England) 134, 4043-4051. Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R., and Sanchez Alvarado, A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Developmental cell 8, 635649. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and Senchez Alvarado, A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science (New York, NY 310, 1327-1330. Reddien, P.W., and Sinchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annual review of cell and developmental biology 20, 725-757. 119 Sakai, F., Agata, K., Orii, H., and Watanabe, K. (2000). Organization and regeneration ability of spontaneous supernumerary eyes in planarians -eye regeneration field and pathway selection by optic nerves. Zoological science 17, 375-381. Sakami, S., Hisatomi, 0., Sakakibara, S., Liu, J., Reh, T.A., and Tokunaga, F. (2005). Downregulation of Otx2 in the dedifferentiated RPE cells of regenerating newt retina. Brain research 155, 49-59. Senchez Alvarado, A., and Newmark, P.A. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proceedings of the National Academy of Sciences of the United States of America 96, 5049-5054. Sato, Y., Kobayashi, K., Matsumoto, M., Hoshi, M., and Negishi, S. (2005). Comparative study of eye defective worm 'menashi' and regenerating wild-type in planarian, Dugesia ryukyuensis. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society 18, 86-91. Schaeper, N.D., Prpic, N.M., and Wimmer, E.A. (2009). A conserved function of the zinc finger transcription factor Sp8/9 in allometric appendage growth in the milkweed bug Oncopeltus fasciatus. Development genes and evolution 219, 427-435. Schaeper, N.D., Prpic, N.M., and Wimmer, E.A. (2010). A clustered set of three Spfamily genes is ancestral in the Metazoa: evidence from sequence analysis, protein domain structure, developmental expression patterns and chromosomal location. BMC evolutionary biology 10, 88. Silver, S.J., and Rebay, I. (2005). Signaling circuitries in development: insights from the retinal determination gene network. Development (Cambridge, England) 132, 3-13. Stenkamp, D.L. (2007). Neurogenesis in the fish retina. International review of cytology 259, 173-224. Strauss, 0. (2005). The retinal pigment epithelium in visual function. Physiological reviews 85, 845-881. Takeda, H., Nishimura, K., and Agata, K. (2009). Planarians maintain a constant ratio of different cell types during changes in body size by using the stem cell system. Zoological science 26, 805-813. Talamillo, A., Delgado, I., Nakamura, T., de-Vega, S., Yoshitomi, Y., Unda, F., Birchmeier, W., Yamada, Y., and Ros, M.A. (2010). Role of Epiprofin, a zinc-finger transcription factor, in limb development. Developmental biology 337, 363-374. 120 Thomas, B.L., Tucker, A.S., Qui, M., Ferguson, C.A., Hardcastle, Z., Rubenstein, J.L., and Sharpe, P.T. (1997). Role of DIx-1 and DIx-2 genes in patterning of the murine dentition. Development (Cambridge, England) 124, 4811-4818. Umesono, Y., Watanabe, K., and Agata, K. (1999). Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Development genes and evolution 209, 31-39. Vandendries, E.R., Johnson, D., and Reinke, R. (1996). orthodenticle is required for photoreceptor cell development in the Drosophila eye. Developmental biology 173, 243255. Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science (New York, NY 332, 811-816. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental biology 344, 979991. 121 122 Chapter 3 Transcriptome analysis of the planarian eye identifies ovo as a specific regulator of eye regeneration Sylvain W. Lapan 1' 2 and Peter W. Reddien 1,2,3 'Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142 2 Department of Biology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02142 3 Howard Hughes Medical Institute Published as: Lapan, S.W., and Reddien, P.W. (2012). Transcriptome Analysis of the Planarian Eye Identifies ovo as a Specific Regulator of Eye Regeneration. Cell rep, Aug 2, 2012 (epub ahead of print) . 123 124 SUMMARY Among the millions of invertebrate species with visual systems, the genetic basis of eye development and function is well understood only in Drosophila melanogaster. We describe an eye transcriptome for the planarian Schmidtea mediterranea. Planarian photoreceptors expressed orthologs of genes required for phototransduction and microvillus structure in Drosophila and vertebrates, and optic pigment cells expressed solute transporters and melanin synthesis enzymes similar to those active in the vertebrate retinal pigment epithelium. Orthologs of several planarian eye genes, such as bestrophin-1 and Usher syndrome genes, cause eye defects in mammals when perturbed and were not previously described to have roles in invertebrate eyes. Five previously undescribed planarian eye transcription factors were required for normal eye formation during head regeneration. In particular, a conserved, transcription factorencoding ovo gene was expressed from the earliest stages of eye regeneration and was required for regeneration of all cell types of the eye. 125 INTRODUCTION The vertebrate eye is a structure of striking complexity in form and function. Consequently, understanding the evolution and development of eyes has been a classic challenge. The human eye is also subject to numerous pathologies that are poorly understood, such as heritable retinopathies and age-related degeneration. Genetic studies in model invertebrates have the potential to advance the understanding of eye evolution and development, and of the functions of conserved genes associated with eye disorders. The relevance of invertebrate models to vertebrate systems depends in part on whether homology exists between most bilaterian eyes, a difficult point to establish based on morphological studies alone. Establishing some degree of common ancestry between vertebrate and invertebrate (primarily Drosophila) visual systems has been a success of comparative molecular genetics. Two findings figure prominently in understanding the relationship between diverse animal eyes. First, two photoreceptor neuron categories, rhabdomeric (microvillar) and ciliary, exist throughout the Bilateria and rely on conserved R-opsin and C-opsin signal transduction pathways, respectively (Arendt, 2003). Second, embryonic development of multiple eye types involves Pax-6, Sine oculis, Eyes absent, and Otx gene family members. These observations have led to the suggestion that photoreceptor cell types were already present prior to the existence of the Bilateria, and that the common ancestor of the Bilateria utilized transcription factors for eye development still commonly used in extant eyes (Nilsson, 2009). To explore many conserved features of eye biology, additional invertebrate model eyes must be established. The morphology, cell type composition, and set of gene activities present in ancestral bilaterian eyes remains largely unknown, and can only be inferred using data from multiple extant eyes belonging to diverse animal phyla. Furthermore, invertebrate models are not yet established for extensive genetic study of 126 several common aspects of eye biology not characteristic of Drosophila eyes, such as the formation of optic cups. Finally, many genes associated with eye disease have not yet been identified and studied in invertebrate eyes, and no invertebrate system exists for studying regenerative repair of eye damage, an increasingly important therapeutic approach. The planarian Schmidtea mediterranea is an emerging invertebrate model system that is highly amenable to gene function studies with RNAi. Planarians are ideal for the study of eye evolution because they are members of the Lophotrochozoa, the sister grouping of phyla to the Ecdysozoa (which includes Drosophila and C. elegans), and therefore exist at an important and understudied position in the animal phylogeny (Tessmar-Raible and Arendt, 2003). Planarian eyes are true cerebral eyes, as they connect via axon tracts to the brain (Agata et al., 1998), and express orthologs of Otx (Umesono et al., 1999), Sine oculis (Pineda et al., 2000), and Eyes-absent (Mannini et al., 2004). Planarians have rhabdomeric photoreceptor neurons (PRNs), pigment cells (PCs), and a pigmented optic cup structure - features that are common among cerebral eyes. Photoreceptive organelles in the planarian eye face the optic cup in an inverse orientation, similar to the orientation of ciliary vertebrate photoreceptors with respect to the retinal pigment epithelium (RPE). Finally, planarians possess unique abilities to regenerate entire eyes, and replenish eye tissue throughout adulthood. These abilities require a population of adult regenerative cells (neoblasts) that includes pluripotent stem cells (Wagner et al., 2011). Here we use eye purification and RNA-seq to identify most genes active in planarian eyes. These data demonstrate conserved features of the R-opsin signaling cascade, candidate rhabdomeric microvillus-regulating genes, and unexpected similarities between rhabdomeric and ciliary cell types. In addition, a high degree of similarity exists between the types of solute transporters expressed in planarian optic 127 pigment cells and the vertebrate RPE, suggesting that optic pigment cells have an ancient role as an accessory eye cell type distinct from photoreceptors. We describe several new, conserved transcription factors expressed in the planarian eye and that are functionally required for eye regeneration, including a central role for the conserved gene ovo in formation of all eye progenitors. Results Planarian eye purification and the eye transcriptome To obtain pure eye tissue for gene expression analysis, we developed a dissociation protocol for isolating -200 morphologically intact planarian eyes in a 1-2 hour period (Figure 1A). Greater than 96% of cells in the purified eye preparation were indeed eye cells, as determined using an opsin RNA probe for photoreceptor neurons (PRNs) and melanin as a marker for pigmented optic cup cells (PCs) (Figure 1A). RNA from purified eyes was used to generate a cDNA library for quantitative RNA sequencing (RNA-seq). Animals were fed prior to eye harvesting to stimulate growth and incorporation of new cells in eyes. Genes previously described to be expressed in planarian eyes all displayed at least 4-fold enrichment in our eye reads, compared to reads from control tissue (ventral, anterior tissue enriched for neurons), in terms of fragments per kilobase per million fragments mapped (FPKM) values (Figure 1C). pax6A, pax6B, rax, six3, and dachshund are orthologs of genes with roles in eye development in other organisms, but have been described to lack detectable expression and function in the planarian eye (Lapan and Reddien, 2011; Mannini et al., 2008; Pineda et al., 2002; Pineda and Salo, 2002); all of these genes displayed FPKM values of zero (Figure 1 C), demonstrating specificity of the data and further suggesting that these genes have no role in planarian eye regeneration. We conclude that transcript levels in the RNA-seq dataset can be highly predictive of gene expression in vivo. 128 For an in situ expression screen we selected unpublished planarian genes with similarity to genes in Drosophila and vertebrates and >4-fold expression enrichment in the eye. Of these approximately 600 genes (Table S1), nearly 200 were tested by in situ hybridization (Table S2) in regenerating heads 7 days after amputation. PRNs and PCs can be identified by position and whether the eye expression domain curves toward (PRNs) or away from (PCs) the midline. 93% of genes screened by in situ had detectable expression in eyes. 60% were expressed in PRNs, 21% were expressed in the pigmented optic cup cells, and 12% were detected in both cell types (Table S2). Planarian phototransduction genes Rhodopsin signaling components displayed eye-enriched expression in the RNA-seq dataset (Figure 1 B-D) and in situ hybridizations revealed expression in photoreceptor neurons for two R-opsin orthologs (Senchez Alvarado and Newmark, 1999), two 0arrestin orthologs (Agata et al., 1998), Ga-q (Smed-gna-q), PLC-3 (Smed-plcb), INAD (Smed-mpdz), PKC, and two Trp channel-encoding genes (Smed-trpc-1, Smed-trpc-2) (Fain et al.) (Figures1 B,E and 2B). Planarian PRNs also had abundant expression of genes encoding enzymes of the phosphoinositide cycle (Figures 1 D and 2B), which replenishes PIP2 after its hydrolysis by PLC (Wang and Montell, 2007). These include retinal degeneration a (Smed-dagk), retinal degeneration b (Smed-pitp), dpis (Smedpis), and cds (Smed-cds). Genes with predicted roles in cGMP signaling and intracellular calcium regulation, which are involved in phototransduction in multiple organisms, were also expressed in planarian eyes. Specifically, PRNs expressed orthologs of IP3-receptors (Smed-ip3r) and STIM (Smed-stim) (Figures 2B and SI), which regulate intracellular calcium levels (Smyth et al., 2010). PRNs also expressed genes encoding the cGMP pathway components guanylate cyclase (Smed-gucy-1, Smed-gucy-2), cGMP129 dependent phosphodiesterase (Smed-cgs-pde), RGS7/9 (Smed-rgs6/7/9), a cGMPgated ion channel (Smed-cng), and a hyperpolarization-activated cyclic nucleotide-gated channel (Smed-hcng) (Figures 1 D, 2B and S1). Many of these components mediate signaling downstream of C-opsin in vertebrates (Arendt, 2003), but we were unable to identify an ortholog of C-opsin in planarians. GO analysis of the RNA-seq data indicated that Rhodopsin signaling, cGMP signaling, and intracellular calcium regulation were all enriched categories in the dataset of eye-expressed genes (Table S3). Expression of microvillus-related genes in photoreceptor neurons Unexpectedly, orthologs of genes involved in the function and development of auditory hair cells displayed enriched expression in planarian eyes and were prominently represented in GO analysis (Figure 2B, C and Table S3). Like planarian photoreceptors, hair cells are sensory neurons with abundant apical microvilli. Microvilli are actin-based structures, and we identified enriched PRN expression of genes encoding actin (Smedactin-1), the actin-regulating proteins Ezrin/Radixin/Moesin (Smed-erm), Clic (Smedclic), Maguk (Smed-maguk), Gelsolin (Smed-gs), and the actin-based motor Myosin VI (Smed-myoVI). These genes have all been shown to have roles in morphogenesis and function of vertebrate hair cells (Gagnon et al., 2006; Kitajiri et al., 2004; Mburu et al., 2006; Mburu et al., 2010; Self et al., 1999). Auditory hair cells of human and mouse also express Usher syndrome genes, which function in morphogenesis of microvillus bundles (Frolenkov et al., 2004). Planarian PRNs expressed orthologs of three Usher syndrome genes, Myosin VIIA/Ush1B (Smed-myoV/IA), Sans/UshiG (Smed-sans), and Cadherin 23 (Smed-cdh23) (Figure 2B-C). These genes are also expressed in vertebrate photoreceptors, despite the fact that the latter are ciliary. The function of Usher syndrome proteins in vertebrate photoreceptors is not understood (Williams, 2008), but many localize to the periciliary region and Usher syndrome can result in aberrant 130 photoreceptor cilia morphology (Hunter et al., 1986). Many of the hair cell-related genes described here, including Usher syndrome genes, have not been described to be expressed in Drosophila photoreceptors. Therefore, our analysis identifies unexpected similarity between a rhabdomeric photoreceptor and auditory hair cells, identifying genes that are candidates to regulate microvillus-like apical membrane specialization. Genes of the pigmented optic cup The vertebrate retinal pigment epithelium (RPE) supports photoreceptor function by providing organic molecules, maintaining extracellular ion concentrations, phagocytosing aging photoreceptor components, and absorbing light energy (Strauss, 2005). Many genes expressed in planarian pigment cells were orthologs of genes important for the support function of RPE cells. The metabolic rate of the vertebrate retina necessitates high levels of available glucose. Planarian PCs expressed orthologs of Glut3 (Smedglut3) (Figure 2B-C) and MCT transporters (Smed-mct-1 and Smed-mct-2) (Figure 2BC), which transport glucose to the retina (Ban and Rizzolo, 2000) and remove lactic acid waste (Bergersen et al., 1999), respectively. Lactic acid uptake is coupled to proton uptake (Lin et al., 1994; Lin and Miller, 1991) and two genes encoding pH-regulatory proteins, a Na+/H+ exchanger regulatory cofactor (Smed-nhe-rf) and a sodium bicarbonate cotransporter (Smed-nabct), were expressed specifically in the planarian PCs (Figure 2B-C). High metabolic activity in the retina results in excess water production, which is eliminated using chloride gradients across the RPE (Strauss, 2005). Orthologs of the RPE-expressed chloride channels Bestrophin-1 (Smed-best-a) and Cftr (Smed-cftr) were strongly expressed in the pigment cells of planarian eyes (Figure 2BC). Planarians are similar to vertebrates, but differ from most other invertebrates, in the use of melanin as the primary eye shading pigment (Hase et al., 2006; Strauss, 131 2005). Among the most abundantly expressed genes in planarian PCs were those encoding orthologs of enzymes required for melanin synthesis in vertebrates, including Tyrosinase (Lapan and Reddien, 2011) (Figure 1 B), Aromatic amino acid hydroxylase (Nishimura et al., 2007) (see "tph", Figure 1B); and Dopa decarboxylase (Smed-ddc) (Figures 2B-C). PCs also expressed orthologs of Quinoid dihydropteridine reductase (Smed-qdr), which produces an essential cofactor of tyrosine/phenylalanine hydroxylase (Schallreuter et al., 2008); and Glutathione-S-transferase (Smed-gst), which catalyzes addition of glutathione during pheomelanin polymerization (del Marmol et al., 1996) (Figures 2B-C). Peroxide is generated by melanin-synthesizing enzymes and is required for melanin polymerization, but it can also cause cytotoxicity in melanocytic cells (Mastore et al., 2005). The GO category for "Response to hydrogen peroxide" was significantly enriched in the eye data (Table S3). Two orthologs of catalase, Smed-cat-1 and Smedcat-2, were expressed in the planarian PCs. Similar to tyrosinase, Smed-cat-1 was one of the most strongly and specifically expressed genes in the planarian pigment cells (Figure 2B-C and Table S2). Glutathione-S-transferase might also function as an antioxidant enzyme in the eye (Giblin, 2000; Singhal et al., 1999). Therefore, planarian and vertebrate optic pigment cells express similar melanin-synthesizing enzymes and enzymes that protect from oxidative damage. Heterogeneity of the PRN and PC populations Recently, planarian photoreceptor neuron heterogeneity has been described, with prohormones and smad6/7-2 expressed in distinct domains of the PRN population (Collins et al., 2011; Gonzalez-Sastre et al., 2012). Smed-soxB, Smed-best-b, Smed-pcdhP-1, and Smed-pthr were expressed only in anterior photoreceptors (Figure S2A). SmedfzdP-1 was expressed primarily in dorsal and anterior photoreceptors, as well as 132 progenitors near the eye (Figure S2A). Smed-zfp-2, Smed-pctaire, and Smed_19866 were expressed only in posterior photoreceptors (Figure S2B). We also identified a gene, Smed-actin-2, expressed in subsets of PCs, indicating that heterogeneity also exists among pigment cup cells (Figure S2C). Identification of candidate regulators of eye formation and an eye-specific transcription factor-encoding gene, Smed-ovo Molecular regulators remain to be identified for key steps in planarian eye formation, including progenitor migration, homotypic cell aggregation, mesenchymal-to-epithelial transition, and cupping morphogenesis. in situ screening identified enriched expression in the planarian eye for many genes that are good candidates to encode proteins regulating these processes, including 14 kinases, 5 protocadherins, and 18 signaling receptors (Figures 2B-C and SI). Transcription factors that control eye development in both Drosophila and vertebrates have received close attention for their capacity to elucidate the evolution of eye development. Here we identified 10 new conserved genes predicted to encode transcription factors with enriched expression in planarian eyes (Figure 2B-C). The most prominently enriched of these was a member of the Ovo family of zinc finger transcription factor-encoding genes (Figure S3), which includes the Drosophila gene ovo (shavenbaby) and mouse ovoll-3 genes. Expression of Smed-ovo (ovo) was detected in both PRNs and PCs, as well as in mesenchymal cells posterior to the eye in day 7 head blastemas (Figures 2B and 3A). After head amputation, ovo* cells first became apparent throughout the dorsal anterior region, near the wound, by 2 days post-amputation (Figure 3A). At later regeneration time points ovo* eye aggregates formed in the anterior blastema, while dispersed cells posterior to the eye remained visible. This pattern was similar to a recently defined population of regenerative eye progenitors in planarians 133 (Lapan and Reddien, 2011). Consistent with this, nearly all ovo+ cells at day 2 and day 7 of regeneration also displayed expression of six-1/2 and eya, which marks eye progenitors (Figure 3B-C). In regenerating or intact animals, no ovo expression was detected outside of eyes or eye progenitors (see below). Furthermore, in the control (ventral anterior tissue) sequencing data, the FPKM value for ovo was zero (Table S2 and Figure S4A). Other previously known eye-expressed transcription factors, eya, six-1/2, sp6-9, and otxA also have expression in the brain and/or elsewhere in the body (Lapan and Reddien, 2011; Pineda et al., 2000; Umesono et al., 1999). Therefore, ovo is the only known planarian transcription factor with expression exclusively in the eye and eye progenitors. It is one of only three transcription factor-encoding genes, together with six-1/2 and eya, that is expressed in all cells of the eye. ovo expression is required for eye regeneration RNAi of ovo followed by decapitation resulted in animals that failed to regenerate eyes (Figure 3D). These animals also lacked detectable tyrosinase mRNA and Arrestin protein (Figure 3E), and no eye structure could be detected by differential interference contrast microscopy (DIC) (Figure S4B). We studied ovo(RNAi) animals using six1/2/eya co-expression to label all eye progenitors, and otxAleya and sp6-9/eya coexpression to label PRN and PC progenitors, respectively (Lapan and Reddien, 2011). ovo RNAi resulted in failure to generate any eye aggregate (primordium), and loss of most eye progenitors (Figures 4A-B). We observed no defects that were unrelated to the eyes, consistent with the highly specific expression of this gene. For example, other cell types of the head, such as sp6-9*/eya- cells of the head rim appeared unaffected (Figure 4A). Therefore, eyes and eye progenitors are strongly and specifically affected in ovo(RNAi) animals. 134 Formation of ovo' progenitors requires expression of other eye transcription factors In gene regulatory networks involved in Drosophila and vertebrate eye development, several transcription factors that regulate eye formation are required for the expression of each other (Silver and Rebay, 2005; Zuber et al., 2003). We tested whether ovo+ progenitor cells are sensitive to expression of other planarian eye transcription factors. RNAi of six-1/2 and eya resulted in animals with greatly reduced or absent ovo signal in the eyes and eye progenitor region (Figures 4C-D). RNAi of the cell type-specific transcription factors otxA and sp6-9, required for PRN and PC regeneration respectively, also impacted the presence of ovo* progenitors (Figures 4E-F). RNAi of otxA led to a 34% reduction in ovo' cells; this reduction was primarily a result of loss of ovo* PRN progenitors (Figure 4E-F), because most remaining ovo+ cells were sp6-9*. Similarly, RNAi of sp6-9 led to a 60% reduction in ovo* cells, primarily a result of loss of ovo+ PC progenitors, because most remaining ovo+ cells were otxA* (Figure 4E-F). These large decreases indicate that ovo-expressing cells, even in early stages of eye progenitor formation, are sensitive to the expression of six-1/2, eya, otxA, and sp6-9 transcription factors. ovo is expressed in a population of eye progenitors during homeostasis and is required for maintenance of intact eyes The specificity of ovo expression for eyes and eye progenitors allowed us to ask for the first time whether subsets of neoblasts in uninjured animals are specialized for particular differentiation paths. In intact animals undergoing normal adult homeostasis, ovo was expressed not only in the eye but also in sparse cells posterior to the eye and anterior to the pharynx (Figure 5A). Adult planarians undergo perpetual tissue turnover (Newmark 135 and Sanchez Alvarado, 2000), but the identity and origin of progenitors that replenish eyes in uninjured animals is unknown. ovo* cells posterior to the eye in intact animals expressed eya and six-1/2 (Figure 5B), and those nearer to the eye also expressed markers for differentiated photoreceptor neurons (trpc-1) or pigment cells (tyrosinase) (Figure S5A). Therefore, ovo' cells that exist posterior to the eye in intact animals have a gene expression profile seen elsewhere only in cells of the eye. The localization of the ovo' cells posterior to the eye in intact animals is consistent with the location of eye progenitors during regeneration following head amputation (Lapan and Reddien, 2011). Strikingly, these cells were eliminated within one day of irradiation (Figure 5A), a treatment known to specifically eliminate the dividing cells of the adult animal (the neoblasts) (Reddien and Sanchez Alvarado, 2004). Furthermore, many ovo' cells in this prepharyngeal population expressed the neoblastspecific markers histone h2b (Figure 5C) and smedwi-1 (Figure S5B) (Reddien et al., 2005.). It is unknown whether these ovo* neoblasts are self-renewing or constantly produced from a more naive cell. These results suggest that cycling eye progenitors constitutively exist in the neoblast population. ovo RNAi in intact animals resulted in gradual loss of eyes, with pigment cups undetectable following two months of RNAi (Figure 5D). These un-amputated ovo(RNAi) animals also lacked six-1/2*Ieya+ cells in the region of putative homeostatic eye progenitors (Figure 5E-F). Reciprocally, homeostatic RNAi of six-1/2 and eya, in addition to causing loss of eye tissue, caused a loss of ovo* cells in the prepharyngeal region (Figure 5G-H). Overall, these data suggest that ovo, six-1/2, and eya are expressed in a population of eye progenitors during homeostasis as well as regeneration and are required for maintenance of this population. 136 Smed-soxB, Smed-foxQ2, Smed-kf, and Smed-meis regulate eye differentiation and morphogenesis. Photoreceptor neuron differentiation RNAi of genes encoding four additional conserved transcription factors, Smed-soxB, Smed-foxQ2, Smed-kf, and Smed-meis (Figure S3) resulted in PRN aggregates that were smaller in cross section than in the control (Figure 6A-C). All of these genes were expressed in PRNs and eye progenitors during regeneration (Figure S6A). Photoreceptor aggregates in klf(RNAi) animals were also displaced posteriorly relative to the pigment cup and descended unusually far toward the interior of the animal along the D-V axis, embedding within the brain (Figures S7A-B). Small eye phenotypes were not accompanied by an evident decrease in the number of ovo' progenitors in these RNAi animals (Figure S6B-C and 6F), suggesting that these genes function primarily following progenitor specification. Because soxB expression was restricted to anterior PRNs, we assessed the presence of photoreceptor neuron subsets in soxB(RNAi) animals. Anterior PRNs (eye53-1* (Collins et al., 2011) and best-bk) were strongly reduced or eliminated in soxB(RNAi) animals (Figure 6D). By contrast, posterior PRNs (pctaire+) were not affected (Figure 6D). soxB RNAi did not significantly impact numbers of PC (sp6-9+/ovo*) or PRN (sp6-9-/ovo*) progenitors (Figure 6E-F), and soxB is not abundantly expressed in the ovo* progenitor population (Figure S6A). However, soxB eye signal is eliminated by ovo RNAi (Figure S6D). These data indicate that whereas ovo (along with six1/2, eya, and otxA) acts in general PRN progenitor specification (Figures 4A and 4E), soxB acts subsequently to promote differentiation of an anterior subset of PRNs. optic cup morphology 137 Several RNAi phenotypes affected the pigment cup shape. Loss of photoreceptor cells by RNAi of PRN-specific genes, such as otxA, results in pigment cups with diminished apertures (Lapan and Reddien, 2011). Accordingly, in k/f and foxQ2 RNAi animals, optic cups were more closed than in the control, or even completely circularized (Figure 6AB), possibly explained if rhabdomeres provide support for optic cup opening. In meis(RNAi) animals, however, optic cups were also elongated and often more than one cupped structure was visible in each eye - a defect not characteristic of photoreceptor cell loss (Figures 6B and S7C). Although meis expression was not detected in the pigment cup, meis transcripts are detectable in tyrosinase' PC progenitors (Figure S7D). Meisi loss in mouse also disrupts optic cup morphogenesis, with optic cup duplication observed (Hisa et al., 2004). Developing eyes of planarian embryos express transcription factors involved in eye regeneration Most eye formation studies in other organisms are performed in developing embryos. Therefore, we investigated whether the transcription factors that govern planarian eye regeneration might also be expressed during planarian embryonic development (Figure 7A). Recent work has shown that beginning at stage 6, Schmidtea polychroa embryos have expression of eya, six-1/2, otxA in the eyes, and lack expression of pax6A, rax, and dachshund in the eye domain (Martin-Duran et al., 2012), similar to the case in regeneration. We found that from early stages of S. mediterranea embryonic eye formation, ovo was expressed in the eyes and in mesenchymal cells posterior to the eye that were reminiscent of regenerative progenitor eye trails (Figure 7B). Double FISH with ovo enabled us to determine whether regeneration-related eye genes were expressed in the embryonic eye primordium and/or presumptive earlier progenitors. Embryonic eyes expressed all known transcription factors that function during eye regeneration: eya, six138 1/2, otxA, sp6-9, kif,meis, foxQ2, and soxB (Figure 7C). Regionalization of expression in the embryonic eye mirrored the case in regeneration (Lapan and Reddien, 2011); for instance, otxA was expressed in the lateral eye primordium whereas sp6-9 was expressed in the medial eye primordium, and both were expressed in only a subset of ovo* trail cells (Figure 7C). Furthermore, neither the eye primordium (Martin-Duran et al., 2012) nor the ovo* trail cells (Figure 7D) expressed pax6A, rax, dachshund, or another Pax6 ortholog, pax6B. Therefore, findings regarding genetic regulation of eye regeneration are relevant to planarian embryonic development. DISCUSSION Planarian eye formation in regeneration, homeostasis, and embryogenesis The cellular events bridging the pluripotent state and cellular differentiation during planarian regeneration and homeostasis are poorly understood, and the eye is emerging as a system to study adult lineage specification in this animal (Lapan and Reddien, 2011). ovo is the first identified gene that is expressed in all eye progenitors and is specifically expressed in eye cells, and therefore is a marker that facilitates study of progenitor dynamics during various modes of planarian eye formation. Our investigations of ovo expression lead to three new conclusions concerning planarian eye formation. 1) Isolated eye progenitors are present early in regeneration (by two days after decapitation), prior to the appearance of an aggregated eye primordium. Therefore, specification at a distance from the aggregated eye occurs from the onset of regeneration rather than only at later stages of eye growth (Lapan and Reddien, 2011). 2) Eye progenitors exist in uninjured intact animals in a dorsal pre-pharyngeal domain, similar to regenerative progenitors but at lower density. These data indicate that some neoblasts are specialized for specific lineages during normal tissue turnover conditions 139 of adult life. 3) During embryogenesis, ovo* cells are present in the eye and in a trail-like formation posterior to the eye. Further experiments are required to formally determine whether, like regenerative progenitors (Lapan and Reddien, 2011), homeostatic and embryonic eye progenitors move toward the eye primordium. However, our ovo expression analyses indicates that migration of differentiating mesenchymal cells, rather than patterning of an epithelium, might be a feature of all modes of eye formation in planarians. Our findings advance the use of the planarian eye as a system for exploring the extent of similarity between embryonic, regenerative, and homeostatic modes of organ formation within a species and between species. Eye purification and transcriptome analysis Canonical phototransduction cascades have been described for ciliary and rhabdomeric phototransduction based on functional investigations in vertebrates and Drosophila, respectively (Arendt, 2003). However, a diversity of phototransduction strategies likely exists among animals. For instance, horseshoe crab (Limulus) and Drosophila light sensation both begin with an R-opsin/PLC cascade, but Drosophila phototransduction culminates in Trp channel opening (Katz and Minke, 2009). By contrast, most evidence from Limulus indicates that opening of cyclic nucleotide-gated (CNG) ion channels (preceded by IP3-dependent intracellular calcium release and changes in cGMP levels) is responsible for photoreceptor depolarization (Garger et al., 2004). We observed robust expression for orthologs of nearly all central Drosophila phototransduction components in planarian photoreceptors, including two Trp channels. However, we also detected enrichment for intracellular calcium regulation and cGMP pathway-like components, including CNG channels. Based on the high levels of Trp channel gene expression, we hypothesize that these are the primary mediators of membrane 140 depolarization following light detection. The roles of CNG channels in planarian eyes are unknown, but electrophysiological recordings combined with RNAi could be used to test possible functions, such as in the regulation of light response or light/dark adaptation. The planarian pigmented optic cup has numerous similarities to the RPE of vertebrates. Melanin is not commonly found in protostome eyes; however, it is the primary pigment of both planarian PCs and the RPE. We expand upon this similarity by demonstrating that the enzymes used in melanin production and for protection from oxidative damage (a consequence of melanin production) are similar between these cell types. Because of the wide divergence in identity of ocular shading pigment among invertebrates (Vopalensky and Kozmik, 2009), the common synthesis of melanin in planarians and vertebrates need not indicate common ancestry of optic pigment cells. However, we identified additional similarities between planarian PCs and the vertebrate RPE with respect to expression of numerous metabolite and solute transporters, consistent with a common function of these cell types as components of a transport epithelium. This similarity supports a model in which pigmented cells already existed as an accessory cell type separate from photoreceptor neurons in the ancestral bilaterian eye. The RPE is commonly affected in eye diseases, including age-related macular degeneration (AMD), edema, and inherited retinal degeneration syndromes. A molecular explanation for pathogenesis is lacking for most of these diseases. Planarian PCs express orthologs of two RPE chloride channels underlying eye diseases. Best-I is mutated in Best vitelliform macular dystrophy, and Cftr mutations underlie vision defects in cystic fibrosis patients (Marmorstein et al., 2009; Xiao et al., 2010). Furthermore, RPE degeneration in AMD correlates with peroxide accumulation and declining Catalase function, which is also responsible for degeneration of other melanocytic cell types (Venza et al., 2011; Wood et al., 2009). Molecular similarities between Drosophila ocular 141 pigment cells and the vertebrate RPE have been difficult to identify (Charlton-Perkins and Cook, 2010). The planarian eye should therefore prove useful as an invertebrate model system for investigating orthologs of mammalian RPE genes involved in pathogenesis, as well as for inferring ancestral functions of ocular pigment cells. Transcription factor control of planarian eye formation Comprehensive sequencing of RNA from intact eyes identified three genes that encode transcription factors and that are expressed in all cells of the eye and their progenitors six-1/2, eya, and ovo. The requirement for six-1/2 and eya in planarian eyes and eye progenitors is previously described (Lapan and Reddien, 2011; Mannini et al., 2004; Pineda et al., 2000) and both are homologs of well-known eye development genes (Figure 7E). We propose a model for eye regeneration in which six-1/2, eya, and ovo expression specifies all eye progenitor formation, with additional expression of otxA specifying PRNs and additional expression of sp6-9 and dlx specifying PCs (Figure 7F). How do available planarian eye data impact models about transcriptional regulators governing eye formation in the common ancester of the Bilateria? Planarian data support the inclusion of Otx and Eya as ancestral bilaterian eye genes, and of Six1/2 as an ancestral eye gene for the protostomes (Figure 7E). Our data also support the possibility that Meis and SoxB family genes encode transcription factors with ancestral roles in eye biology. Meis genes regulate eye progenitor proliferation in Drosophila and vertebrates as well as optic cup morphology in mouse (Figure 7E) (Bessa et al., 2002; Heine et al., 2008; Hisa et al., 2004). The SoxB-family sox-neuro and fish-hook genes are expressed in the Drosophila eye disc (Mukherjee et al., 2000) and Sox2 required for neural progenitor maintenance and differentiation of retinal ganglion cells in vertebrates (Matsushima et al., 2011). 142 In contrast to Otx, Eya, Six-1/2, Meis, and SoxB, several transcription factors that have prominent roles in Drosophila and/or vertebrate eye formation - Pax6, Dachshund, Rax, and Six3 - have no apparent role in planarian eye formation (Figure 7E) (Lapan and Reddien, 2011; Mannini et al., 2008; Martin-Duran et al., 2012; Pineda et al., 2002), a conclusion extended by our eye transcriptome and embryonic eye expression data. Therefore, planarian data does not support the argument that these genes are ancestral regulators of eyes. Loss of a role for any of these genes in eye biology during planarian evolution is clearly a reasonable possibility, especially in cases such as Pax6 where data from multiple other phyla support an eye role for the gene. In addition to the case of the planarian eye, however, the development of Drosophila larval ocellus, branchiostome eye cups, Limulus eyes, and Platynereis adult eyes also does not depend on Pax6 (Vopalensky and Kozmik, 2009). Furthermore, orthologs of the Drosophila eye regulator Dachshund do not have major functions in mouse eye development (Davis et al., 2008), and an ortholog of the vertebrate eye regulator Rax is not found in Drosophila (Arendt et al., 2004). Six3 family transcription factors predominate in vertebrate eye development, whereas most invertebrates depend on Six-1/2 (Vopalensky and Kozmik, 2009). Analysis of additional animal eyes will therefore be an important direction for further testing evolutionary hypotheses related to these genes and eye evolution. We describe a number of transcription factors conserved among the Bilateria that regulate planarian eye formation, but for which the functions in other animal eyes has not been extensively investigated (Figure 7E). These include ovo, k/f, and foxQ2. Our prior work also indicated prominent roles for dlx and sp6-9 in optic cup formation (Lapan and Reddien, 2011). One gene encoding an Ovo-like transcription factor, shavenbaby, is known to exist in Drosophila. This gene is well-studied in epidermal morphogenesis, but roles for this gene in the eye have not been reported. The chromosomal locus responsible for the blind-sterile phenotype in mouse contains the ovol2 gene (Li et al., 143 2002). These mice exhibit small eye size as well as early onset cataracts; however, other genes are contained in this locus and no causal role for ovo/2 in this phenotype is known. Mouse ovol2 is expressed in the surface ectoderm of the ventral forebrain at E8.75-9.5 (Mackay et al., 2006), the site of optical eminence formation. Interestingly, ovol2 knockout mice die at E10.5 without displaying any sign of optic vesicle formation typical of this stage, despite the fact that D-V expression domains examined in the neural tube are maintained and overall embryonic development is not delayed (Mackay et al., 2006; Unezaki et al., 2007). We demonstrate an unbiased technical approach to systematically survey the molecular basis of eye formation and function. This approach identified novel and conserved genetic features of eyes in planarians, further indicating the power of planarians as a system for study of animal eyes. Few transcription factors in animals display eye-specific expression and are required for formation of all eye cells, and ovo is a new one. 144 MATERIALS AND METHODS Tissue isolation Large asexual Schmidtea mediterranea were used for eye preparation. Animals were fed twice prior to eye harvest. Head tips were amputated posterior to the eyes and collected into CMFB + 1 mg/mL collagenase (Sigma) + 0.05% NAC and agitated. Freed eyes were rinsed and transferred to Trizol for RNA collection. For control (ventral anterior) tissue, heads were amputated above the pharynx and then coronal amputation was made to remove dorsal tissue, including eyes. cDNA sequencing and analysis -1 ug of RNA was used to construct Illumina libraries for 36bp single-end sequencing on the GAll platform. Libraries were constructed using Illumina reagents and according to commercial protocol. FPKM were determined using the Cufflinks program Cuffdiff. Gene cloning For cDNA library generation, RNA from heads was isolated using Trizol purification, and used as template for first-strand reverse transcription with Superscript Ill (Invitrogen). Cloning was otherwise permformed as described (Lapan and Reddien, 2011). RNA synthesis and RNAi For probes used in RNAi experiments, riboprobe template was produced with the same primers as were used for cloning, except that gateway adapters were not present on primers, and T7 promoter sequence was added to the 3' primer. Probe synthesis and RNAi were otherwise performed as described (Lapan and Reddien, 2011). In homeostasis RNAi experiments, animals were fed dsRNA food every four days for six weeks. 145 Histology and imaging Whole-mount fluorescent in situ hybridization (FISH) and antibody staining was performed as described (Lapan and Reddien, 2011; Pearson et al., 2009). Embryos were killed in 2.7% HCI following manual decapsulation and were otherwise treated the same as adult worms for histological analysis. Images were acquired with a Zeiss confocal microscope (LSM 700). ACKNOWLEDGEMENTS Thanks to Jessica Witchley for generating the Illumina library. Thanks to Josien van Wolfswinkel, Mansi Srivastava and Jared Owen for bioinformatic assistance. P.W.R. is an early career scientist of the Howard Hughes Medical Institute and an associate member of the Broad Institute of Harvard and MIT. We acknowledge support from the NIH (R01GM080639) and the Keck Foundation. 146 Ca+ A Rhabdomeric Y R C44 44 lDissodate ca+ "+ Cilary CGMP-VNW dow GOORTh" , i:: cow Camp GNP B Fmp CGMP D a. U- rnauuomenc 12- oth I _10o- ma I IS. a. . 1 1 1 e aI. U- 4-I 4 20 I it: 0~ K ib c 11 eq A NN Ml A 1111110 a A[_ f f_6_l aA__i d A, 147 1I II Is I Is fiI _11__II r 1 -mIEI fI 1EI i 7&,Tt:_ F t;:Ar I - Figure 1. cDNA sequencing of purified planarian eyes. (A) Planarian eye purification. opsin RNA probe and pigment (melanin) were used to assess purity of purified eyes. (B) Schematic of signaling downstream of R-opsin and C-opsin. (C) Plot of relative FPKM values for planarian genes previously described in the literature with expression in the eye (black), or lacking expression in the eye (red). (D) Enriched expression was detected for genes typical of the R-opsin cascade (left set), the C-opsin cascade (right set), or either form of phototransduction (middle set). Table shows FPKM values for genes in the eye sample and summarizes expression data from mouse (Mm), Drosophila (Dm) and planarian (Sm; see also Figure 2). PR, photoreceptor neuron; PC, optic pigment cell; RG, retinal ganglion cell. Retinal ganglion cells are vertebrate retinal neurons with some photoreceptive ability, but which rely an R-opsin cascade-like phototransduction mechanism (Sexton et al., 2012). Scale bars, 200 pm (B, left and middle); 50 pm (B, right). 148 rol 12 Ii flUWOld iI0 -04W mII Figure 2. An in situ hybridization screen of genes with enriched expression in planarian eyes. (A) Schematic of tissue types in the head that appear in expression patterns. PRN expression domains curve toward the midline, and PC expression domains curve away from the midline (B) in situ hybridization of eye-enriched transcripts, grouped by predicted function. Full names and additional orthology information are given in Table S1. (C) Plot of the log 2 of fold enrichment (FPKM eye sample/FPKM control sample) for genes screened (see also Table S2). Values for rhabdomeric phototransduction and cGMP pathway genes are shown in Figure 1. Table shows FPKM values for genes in the eye sample and summarizes expression data from vertebrate (V), Drosophila (Dm), and planarian (Sm). PR, photoreceptor neuron; PC, optic pigment cell; HC, inner ear hair cell; asterisk indicates that planarian gene orthology could only be assigned to a broad gene family for which functions have been identified in the eyes of indicated species. Scale bar, 200 pm. 150 A dO d3 d2 d5 dG 0 0 , B D ovo/six-1/2 control R NAi six-112 ovo nvn RNAi ,~ C E 151 control RNAI ovo RNAi Figure 3. Smed-ovo is expressed specifically in the eye and eye progenitors, and is required for eye regeneration. (A) Regeneration time course showing ovo expression after amputation of head and tail. Bottom panel is a magnified view of the anterior wound/blastema. (B) ovo' cells express the eye progenitor marker six-1/2 at early and late time points of regeneration (d2: 96% +/- 5% overlap, d7: 100% overlap, a = 0.05). (C) ovo' cells also express the eye progenitor marker eya (d2: 90% +/- 6% overlap, d7: 100% overlap, a =0.05). (D) RNAi of ovo leads to failed eye regeneration (n=10/10). Dashed lines indicate approximate site of head amputation 12 days prior. (E) ovo(RNAi) animals lack expression of ovo, tyrosinase, and Arrestin after head regeneration (n=10/10). Scale bars, 200 pm (A), 50 pm (B,C,E), and 1 mm (D). 152 A sp6-9 eya (PCs) otxA eya (PRNs) B six eya (PRNs + PCs) control RNAI Dovo RNAi . Iy r3 -. 0 - e* 0 E30 & 20. I -- - 1 -- E 5PRN+PC PC PRN slx+/9ym+ sp&-9+/1y"+ otxA+/oyv+ Progenitor type C D six-1/2 RNAI control RNAI 40 eya RNAI o 0 38 Econtrol RNAI +0 m *six-1/2 RNAI Eieya RNAI 25 E20 1i - E c 10 s~7 6 ovo sp -9 ovo otxA 0 F E S l 40 W 35 ';fl|flfl 153 30?' 1511 Econtrol RNAI motxA RNAi sp6-9 RNAi L.. Figure 4. ovo is required for formation of regenerative eye progenitors, and ovo* progenitor presence requires other eye transcription factors. (A)-(B) ovo RNAi eliminates regenerative eye progenitors as assessed by markers for PRN (otxA*/eya+), PC (sp6-9/eya'), and general eye progenitors (six-1/2+/eya+). Arrows indicate double-positive cells. (C)-(D) Most regenerative ovo* progenitor cells are eliminated by six-1/2 and eya RNAi. (E)-(F) sp6-9 RNAi eliminates most ovo* pigment cell progenitors (ovo*/otxA-), and otxA RNAi eliminates most ovo* photoreceptor neuron progenitors (ovo*/sp6-9-). n = 8 eyes + trails for all quantifications. Error bars represent SD. *** p < .005. Scale bars, 50 pm (A,C,E), and 20 pm (A, insets). 154 A unirradiated 12 1 Irradiated + 24h m unirradiated A B a irradiated 0w 0 04- E C 20- - C D C 0 0 E Econtrol RNAi F m ovo RNAI R z lo. 0C 0. H 1816 - S2- 14 - G a 12- 010- 80 4.2- 0 155 Econtrol RNAi * six-1/2 RNAi Oeya RNAi Figure 5. ovo is expressed in a population of homeostatic eye progenitors. (A) In starved intact animals, ovo is expressed in isolated dorsal cells between the eyes and pharynx. These cells are eliminated within 24 hours of irradiation. (B) ovo' cells posterior to the eyes in intact animals also express six-1/2 and eya. (C) ovo' cells posterior to the eyes in intact animals also express the neoblast marker histone h2b (60% +/- 15%, a =0.05). (D) ovo(RNAi) animals that are not amputated fail to maintain eyes (n=10/10). (E)-(F) ovo(RNAi) animals that are not amputated fail to maintain six-1/2*/eya+ cells posterior to the eyes (n=4 animals). For image clarity only six-1/2 signal is shown. (G)-(H) six-1/2(RNAi) and eya(RNAi) animals fail to maintain ovo+ cells posterior to the eyes (n=4 animals). Arrows indicated double-positive cells in (B) and (C). Error bars represent SD. *** p < .005. Scale bars, 100 pm (A), 50 pm (B,C,F,H), and 200 pm (D). 156 B tyro ARR A D- soxB RNAi control RNAi z .0 ".9 U) a) .0 0 I0 a) 10- a) C - 25.. I 100 E F *ovo+ 35. - cnaq-04+Isono+ m-I-p 30 IT 2520- Mcontrol RNAi 2500 - ~2000 - 1s * foxQ2 RNAi *kf RNAi m soxB RNAi El meis RNAi 10. s. 0 *** *** CIO 21000 - z 0: 500 M. 0- 157 Figure 6. Smed-soxB, Smed-meis, Smed-kif, and Smed-foxq2 are required for eye regeneration. (A) Eye regeneration defects at 7 days of regeneration in live RNAi animals. (B) Detection of tyrosinase transcript and Arrestin protein reveals defects in optic cup formation and photoreceptor neuron regeneration. Arrow indicates duplicated optic cup in meis(RNAi) animal. (C) Quantification of cross-section area (pm 2) of photoreceptor neuron aggregate (mean + SD, n=8 eyes). (D) soxB RNAi results in loss of anterior subtypes of photoreceptor neurons, but not posterior subtypes (n=8 eyes for each condition). (E)-(F) soxB RNAi does not affect numbers of ovo+ progenitors, nor the ratio of PC progenitors (sp6-9*/ovo') to PRN progenitors (sp6-9-/ovo*). Error bars represent SD. *** p < .005. Scale bars, 200 pm (A), 100 pm (D), 20 pm (B, E). 158 A F Anterior Posterior C +ovo D +ovo +meis +kIf +ovo 4 +foxQ2 +soxB\ +sp6-9 f +dlx +otxA \ ovo six-1/2 eya E SIx1/2 SIx3 I Mm Fe Eye Pax6 Otx Dechs Rax x x Dmxx Sm Required for: x * Neural Retina and Cmorphology Pigment Celia Ovo Sp6-9 ? ? ? ? Dix ? Moes SoxB FoxQ21 Klf x ? ? x NR or PC []Subset of NR or PC [jexpressed, function unknown 159 [x] not expressed [7 unknown Figure 7. Expression of transcription factors in planarian embryos. (A) Freshly decapsulated planarian embryos of the stage used for expression analyses. The dark structure is the embryo capsule. (B) ovo is expressed during embryonic development in eyes and trails posterior to eyes (arrows) (C) Double-fluorescent in situ hybridization with ovo is used to show presence of gene expression in eye primordium and eye progenitors during embryonic development. Arrows indicate the location of ovo+eye primordia. (D) Absence of gene expression for indicated genes in the eye primordium and eye progenitors during embryonic development. (E) Table summarizing roles of transcription factors involved in eye formation in either mouse (Mm), Drosophila (Dm), or planarian (Sm). NR, neural retina; PC, optic pigment cells. (F) Model of steps in planarian eye regeneration that are regulated by transcription factors. Scale bars, 200 pm (A), 50 pm (B), 20 pm (C,D). 160 Contig name gnlAdamidiisotrg24812 gntlAdamidiisotg25522 Comp2dcO seqi Eye gnhIAdanrd"I1sotg22517 comp4-c0_seql comp328 c0seq com>471_cOSeql comp34_cOseq1 FPKM Control FPKM 21716.5 16079 2 4437.45 13757 9 5151.79 480.508 1 32104 6 5209 2.9824 1 0365 630.016 1.15426 198581 4 7793 1686.79 101.313 1.623293 gnlAdamidi soSg25558 118 349 989 51 1 908914 16 4005 1 1 447579 comp3295_oDO "qi comp1201 cO seql 26.6038 COMPU07 0O-veci 60.3208 cO seql :omp2649 cO_aeqi 86.6588 135."92 comp768cO seql 210 466 368864 gn][Adamid*4o~17297 2327.69 172 373 83.5876 41.1133 308856 1.5096i4 134.979 2.44M3 7598.3 277.01 145.898 comp3056 complOO cOseqi :omp7449_c0_wq1 comp5a10-ooeqi gnlAdamid;isotig24868 grillAdanididso0 343 gnillAdamidillsot182 comp107_cO seq1 12529 7 487.409 228.223 gnllAdamidilisolig13603 mp 6_q_seq1 81.M27 nomp1952 _r_seq1 113011 65.167 omp$721_ *_weqi omp457_cOmeqi comp977_cOseq1 romp4196 cO seq 323 | 932 113.84 878806 2.33682 535763 243.338 9.50619 4.49436 .1419M4 2 29714 1.332899 0.81004 2 39735 1 860108 S; B 3502 gntlAdamidiisotig11945 e planarlan gone name Beat Human hlat hit value BeStDrosophua Smed-opsi melanopsin isoform 1 [Homo 4.39E-54 ninaE-PA 'Smed-arrestin beta-arresin-1 isoform [Hom 497E-66krz-PB *Smed-tyrosinase L-dopachrome tautomerase iso 4.33E-59]drpr-PB Smed-tph __tryptophan 5-hydroxytase 1 Hc 1.1E-162 Trh PA 4 3586 Smed-cat-1 homeobox protein SIX I (Homo WAD domain phosphncino repeat putative 5-977922231 5954151598 5.914902583 5.914$83M7 5.90363227 2.7E-82 1.02M98Atg18-PF 1.582881 Corticotroprn-releasing factor 5.823149948 0 01997365 0 5.85W00M2 5834354387 Smeed-crf-r 1 1E-35 0 0 rE 8 00E-21 0 5.791066439 5.789514907 ftizzled-2 precroir [Homo sop Glutathione-requinng prostagla, 5702644663 Smed-gst 0 CG8422-PA MA be 5,686246431 w tumohir s Smed_05608 5.680121843 Srned 12493 5 666184692 Smed-ddryk :uncharacterized pro0.n Clorf2 iTyrosine-protein kinase-like 7 p 5.624W51S4 prematurely terminated m RNA 562048061 Smed-nohl NOL lNOP2Sun domain tal-2 flHlomno saples) 5.4116022111 5.5718m11 faml! ploxin domain-contairing protei Forkhead box protein J3 Usher syndrome type-1G prote 5 569423207 Smed-foxq2 5 562086426 Smed-sans 161 0 0 Cyp9b2-PA 4.7114 tPA 6.9 CG14194-PA 2.58163 00E-17 Discoidin domain receptor Vps2PA 7.00E-22 1.00E-21 C( 2 OOE-35 6.04018 CG13035-PB soform B 0002 0 PNUTS-PC CG 11152-PA CG13320-PA, 0 7.00E-20 0.000157494 4.08M9 0 221015 0 1.38651 0,5244 0.4 0.00051 CG17097-PB 9 0 1.55377 3.00E-19 Glutathione S-transferase S1 6 00E-31 0 7.00E-19 0 0.8219M 00111861 O.6188 1 08E-12 8.34734 ATPO-birmfing casset sub-famil O.89442 protocadherin Fat 3 precursor [ 0.000876 Rpl-PA 5802455645 6 protein soform 2.64E-60 00149898 1.28E-141 2 48E-84 golgin subfamily A mer 0 000192 CG43154-PE bW-2-11ke 1i I [H CG31869-PC glutamate receptor lonotropic 5 87E-30 CG9935-PB enteropelpdase precursor [Hor 1 85E-05 CG31221-PC sia-aIpa-2.Ga-be-1.44&cf 3.1462 CGI7751-PB zinc 5nger MYM-type protein 1 0 000249 5986504233 a value 5.53E-48 O0Catatase so-PA Catalase 'Smed-skx-/2 Blast hit 0 0.578072 2.00E-39 isoform A 5.00E-15 Table S1. Information for genes with enriched expression in planarian eyes The table shows the most enriched contigs (eye versus control). Contigs with no clear ortholog (E value > 0.1) are in gray. Genes that are mentioned in the text or that appear in a main figure are named based on orthology, if possible. Genes that were screened but that are described only in supplemental material are given a numerical identifier. "Adamidi" sequences are from a reference planarian transcriptome (Adamidi et al., 2011), and sequences beginning in "comp" are derived from de novo read assemblies. An asterisk indicates that a gene was previously described as eye-expressed in the literature. 162 Name Smed-cat- 1 smed-trpc-2 Smed_02759 Smed-glut3 Smed-ovo Smed-trpc-1 Smed_20991 Smed_53sley Smed-ptcb Smed-ddc Smed_12493 Smed-nabct Smed-ninag Smed-cdh23 Smed-pitp Smed-cpo Smed_34307 Smed-kif Smed-crf-r Smed-gst Smed 05608 Smed-ddryk Smed-noll Smed-foxq2 Smed-sans Smed-pkc Smed 19866 Smed-ip3r Smed-5ht-r2 Smed_59sley Smed-dagk Smed-actin-2 Smed-gucy-2 Smed 31312 Smed_04497 Smed-gls Smed-pthr Smed-myoVilA Smed-best-a Smed-pcdhP-4 Smed-arrb-2 Smed 09268 Smed_12504 Smed_16656 Smed-5ht-ri Smed-zfp-2 Smed-pkd2 Smed-fzdP-1 Smed-gna-q Smed-gpcrP- 1 Smed-best-b Smed 17151 Smed-cftr Smed 13049 Smed-eag Smed-mct- I Smed-pcdhP-2 Smed-pcdhP-3 Smed-tfr Smed-gpcrP-2 Smed 12518 Smed 22239 Smed-unc5-1 Smed 08711 Smed-mpdz Smed 35828 Smed-eds Smed-nhe-rf Smed 08951 Smed 32587 Smed 03464 Smed 00858 Smed-ip3k Smed-syk Smed_25626 Smed 02478 Smed-cgs-pde Smed-smad6/7-2 Smed 34343 log2 (Eye FPKMIBraIn FPKM) Signal Strength Score Brain FPKM Eye FPKM 5 10.75435045 2.98247 5151.79 5 8.698691419 4.77937 1985.81 5 8.43189502 7.53843 2603.36 5 8.406331991 1.116786 378.903 5 8.376870887 1.208495 401.73 5 8.169576265 1.214679 349.743 4 7.79414652 1.971778 437.653 5 7.606286757 1 194.859 5 7.53559815 2.89304 536.779 5 7.225093689 4.49461 672.453 4 7.019188344 3.51598 584.785 3 6.974265594 1.142957 143.712 5 6.958422524 4.74126 589.641 4 6.457710321 8.46878 744.364 5 6.39844112 20.3814 1719.32 5 6.259386225 4.63376 354.974 4 6.195681874 2.14384 157.137 4 6.084843847 1.49256 101.31 4 5.834354387 3.68864 210.466 5 5.702644663 145.898 7598.3 5 5.686246431 243.338 12529.7 4 5.666184692 4.49436 228.223 3 5.62048061 2.29714 113.011 4 5.569423207 2.39735 113.84 4 5.562086426 1.860108 87.8806 4 5.509942253 29.4074 1340.03 4 5.433374225 7.84411 338.963 3 5.429473052 10.15379 437 585 4 5.413494459 2.16985 92.4813 4 5.395199591 3.59733 151.39 4 5.384762184 14.7802 617.526 3 5.197625308 1.751542 64.2779 3 5.172801076 6.0023 216.514 2 5.120442623 1.969894 68.5251 4 5.087274624 11.6787 397024 3 5.028363929 5.30725 173204 4 5.02122168 5.61335 182.289 4 5.01695011 20.5287 664.682 4 5.006829463 4.46413 143.53 4 4.983527334 7.45615 235.888 3 4.952478911 8.36127 258.891 4 4.861741781 14.4963 421.49 0 4.859875154 1 29.0381 2 4.846079622 4.74307 136.419 4 4.769853678 5.36872 146.467 4 4.764528253 2.57172 69.902 5 4.739715671 12.3557 330.114 3 4.692171096 5.97391 154.434 4 4.546526536 52.7266 1232.17 4 4.476667786 5.20629 115.915 3 4.466153546 1.950509 431116 3 4.383881018 5.44671 113.714 4 4.359631141 13.7207 281.68 4 4.276076994 24.933 483.061 4 4.275558864 3.71202 71.8922 4 4.273401384 20.5779 397.945 3 4.167035732 366319 65.8055 4 4.110508292 6.42183 110.929 3 4.064340545 3.75508 62.8214 2 4.048800774 5.67026 93.8455 4 3.996012401 4.06858 64.9176 4 3.920359115 7.11632 107.746 3.916195274 12.4156 187.439 3 3.904607662 6.78406 101.6 4 3.894411225 21.7159 322.933 C 3.853310079 15.3869 235.842 2 3.792285059 69.6299 964.689 4 3.783383135 17.5404 241.519 3.753003892 131.654 1775.01 4 3.704920241 7.74402 100.9854 4 3.701176191 34.0766 443.222 4 3.697736251 33.6389 436.487 4 3.669868388 49.131 625.311 3.661704072 11.958 151342 4 3.645155163 25.6887 321.398 C 3.632500515 14.2139 196.725 3.597287183 18.727 226.652 3.570781005 16.4627 195.62 2 3.490931131 7.3291 82.3997 163 Table S2. Summary of in situ hybridization results for screened genes In situ hybridization results are categorized by expression in photoreceptor neurons (PRN), pigment cells (PC), or both. "none" indicates that no signal was detected by in situ hybridization. "not clear" indicates that signal was present in the animal but was not clearly enriched in the eye. In situ hybridization signal strength above background was approximated visually with a number on a scale of 5 (highest) to 1 (lowest). 164 Human GO term GO:0006182-cGMP biosynthetic process Drosophila GO term Pvat Fold Enrichment Pval 7.695841393 0.049608106 0:0046671-negative regulation of retinal cell prograt 7.6696258 0. 049882337 7.695841393 0.049608106 GO:0007521-muscle cell fate determination 7.669683258 0. 049882337 6.840747905 0.000771981 G0:0045488-regulation of R8 cell spacing in compout 7889683258 0. 049882337 6.158873114 0.077429012 GO:0016330-second mitotic wave during compounds 7.669683258 0. 049882337 6.156673114 0.077429012,GO:0042676compound eye cone cell fate commitme 6.817496229 0. 014736816 6 158673114 0 077429012 GO 0016199-axon midline choice point recognition 6.507610037 0 000251487 0-02376037G00006182-cGMP biosynthetic process 5,863498204 6.135746606 0. 077843927 5 130580928 0.035315281 GO:0007451-dorsalventral lineage restriction imagin 6.135746606 0. 077843927 5.130580928 8.04033E-10 GO0046088-cGMP metabolic process 5,843568197 0.023942849 4664146299 0.016747721 G0:0042693-muscle cell fate commitment 5.843568197 0.023942849 4225167823 0003994858 G0:007432-salivary gland boundary specification 5 57795148 0.002426409 4.225167823 0 003994858 G0:0016339-calcium-dependent cell-cell adhesion 5.453996983 C.00025761 4.104448743 0.065386845 GO0006874-allular calcium ion homeostasis 5.113122172 0. 035580433 4 104448743 0.065388845 G0016056-rhodopsin mediated signaing pathway 5.113122172 0.01164053 4,015221596 0.000992979 G0016198-axon choice point recognition 5.113122172 0.001265757 3 847920696 0.015049167 GO:0016203-muscle attachment 4,844010479 0.000220857 3 847920696 0.015049167 GO:0030005-cllular di-, tr-valent inorganic cation ho 4719805082 0. 005731069 3.731317039 0.083626517 GO:0008875-cellular metal ion homeostasis 4.544997486 0.049590929 3.731317039 0.083626517 GO:0055074-calcoum ion homeostasis 4.544997486 0. 049590929 3.705405115 8.889E-05G:0019991-septate junction assembly 44739819 0. 002848656 3.600393634 7.99062E-07 :GO0030003-cellular cation homeostasis 4.382676147 0. 08211798 3.59139265 0.009722891 G0:0009187-cycc nucleotide metabolic process 4.305787092 0' 01426024 3.420373952 0.025096267 GO0048675-axon extension 4.090497738 0. 011348769 3.420373952 0.025098267 GO0007043-cel-cell junction assembly 4.090497738 0' 000362649 3.240354271 0.031415 I2IGO:0034329-call junction assembly 4.090497738 0. 000362649 3.240354271 0031415862 GO:0007156-homophlic cell adhesion 3.978728 0, 005577253 3.184486094 2.13936E-05 G0:0016337-cell-cell adhesion 3.933170901 8.80849E-06 3 12295013 0.019713173 GO:0055068-di-, tri-valent inorganic cation homeosta 3.834841629 0, 015212456 3,078336557 0 038646852 GO:0043297-apical junction assembly 3.834841629 0.00138517 3078338557 0 038W4852 0:0016318-ommatidial rotation 3 767563706 0. 007493205 0 0 74 7 8 3 007570199 8.43851E-05 GO:O -leg disc morphogeness 3767563706 0]007493205 3007570199 8.43851E-05 G00055065-metal ion homeostasis 3.718634307 0. 084211502 2.992827208 0.024189219 G0:0060538-skeletal muscle organ development 3.698854337 4. 45629E-06 2.931749102 0.000201131 G0:0006916-anti-apoptosis 3.652230123 0. 240557775 2 894162575 0 003282184 GO0045197establishment or maintenance of epithel 3.60926271 0. 019865352 2.821808511 1 56308E-05 GO:0007419-ventral cord development 3.57918552 0 009842127 2 813533412 0.000199568 GO 009190-cyclc nucleotide biosynthetic process 3.408748115 0. 051179232 2 687436877 0.005823962 G0:0035127-post-embryonic limb morphogenesis 3.408748115 0. 025360775 2.687438677 0.005823962 GO0035109imagina disc-derived limb morphogenei 3.408748115 0. 025360775 2.676814397 0.0859802468G0:0007480-imaginal disc-derived leg morphogenesi 3.408748115 0. 025360775 2.V67814397 0 065980246 GO0007291-sperm individuaization 3.408748115 0. 025360775 2 676814397 0.065980246 GO:0030855-epithehal cat differentiation 3.408748115 0. 012871272 2.676814397 0.065980246 GO.0048588-developmental cell growth 3.408748115 0. 006369991 2.676814397 0.065980248 Go:0008105-asymmetric protein localization 3.408748115 0. 006369991 2.648031447'0 026341839 G.0007602-phototransduction 3.408748115 0. 303217102 2848031447 00283418390G:0009582-detection of abiotic stimulus 3 316619787 0C000548783 2 638574192 0 016817791 GO:0045216-oeli-cell junction organization 3.298788498 0. 002103297 2 63105688 0.010788689 G0:0034330-cat junction organization 3.298788498 0. 002103297 2.631056886 0.010788689 000048859formation of anatomical boundary 3.287007111 0.0104128135 2,583735719 2 35002E-07 G000009954-proximadistal pattem formation 3229340319 0.0131742049 2.565280464 0 030991957 GO:0009581-detection of external stimulus 3.229340319 0.0100705888 2.565280464 0.008230525 G3 0030010-estabashment of cell polarity 3.195701357 0.163168158 2.565280464 0 008230525 GO 0009583-detection of tight stimulus 3.195701357 0.0 102679212 2565280484 0.005323851 G00042067-establishment of ommatidial polarity 3.173662038 0.0105225754 2.487544893 0.036162719G0:0048645-organ formation 3.146536721 0.0110203394 2.460575139 8.5889E-09 G0:0080173-8mb development 3.146536721 0. 110203394 2.438599947 4.11375E-14'G430035110-leg morphogenesis 3.146536721 0. 110203394 2.430265703 0.027016726 GO:0035108-8mb morphogenesis 3.146536721 0.0110203394 2.430265703 0.027016726 GO:0006873-ellular ion homeostasis 3.148536721 0.0110203394 2.430265703 0.027016726 G00635088-establishment or maintenance of apical/ 3,112335235 0.0119946467 2.401539158 0 01318819 GO:0007519-sskeletal muscle tissue development 307873303 0. 106533117 2.401539158 0.01318819 GO:0030001--metal ion transport 3.033208068 2. 62711E-09 2 373841624 8.59413E-06 GO:0035315-hair cell differentiation 3.029998324 0.0112648596 2.387951198 0 031204612 GOM0035316-non-sensory hair organization 3.029998324 0.0 11248596 2.367951198 0.001073298 GO:0042052-rhabdomere development 3.007718925 0.0 76492098 2.367951198 0.001073298 G 0010001-glial cell differentiation 3.007718925 00 76492098 2.352868706 1 6784E-06 GO:0007449-proximal/distal pattem formation, imagir 3.007718925 0.0 76492098 2.308752418 0.035816227 GO0007616-long-term memory 3.007718925 0 76492098 2.308752418 0.035816227 G0:0007164-establishment of tissue polarity 2.993047125 0. 00141547 2280925155 001000088 GO0042462-eye photoreceptor ce deveiopment 2.960228626 0. 02710384 2.256372345 616455E-07 GO0055080-cation homeostasis 2.921784098 0,C47282884 2.252441383 0.040888721 G0:0007474-imaginal disc-derved wing vein specific; 2.921784098 0.C47282884 2.244620406 0.007570957 GO:0009913-epiderma cell diferentiation 2.921784098 0.01548875 2.238790223 0.015143645 GO:0055082-celkilar chemical homeostasis 2.921784098 0.01548875 2238022463 1.93988E-08 IGO0007398-ectoderm development 2,890025575 0.0 01136363 2.218620942 0 06236909 00010160-formation of organ boundary 2.863348416 0.C29835293 2,210087785 0.008650312 G.o0001738-morphogenesis of a polarized epitheliun 2.82853587 0.0 01394258 2.198811826 0 046369281 GO:0001736-establishment of planar polarity 2.812217195 0.0 04069165 2.198811828 0.017250992 00:0016319-mushroom body dlevalonmn 2.78897573 0.0 11951834 0.017250992 GO:0016319-mushtoom body development 2.198811826, Fold Enrichment GO:0042428-serotonin metabolic process GO:0007213-muscarinic acetylcholine receptor signa GO:0050910-detection of mechanical stimulus involvi GO0050957-equibroception GO:0046068-cGMP metabolic process GO 0042177-negative regulation of protein catabolic GO0050982-detecion of mechanical stimulus GO00007156-homophilic cell adhesion GO:0050909-sensory perception of taste 0:0009582-detection of abiotic stimulus GO:0007215-glutamate signaling pathway GO:0015804-neutral amino acid transport GO:0009187-cyclic nucleotide metabolic process GO 0006821-chloride transport GO:0007200-activation of phospholipase C activity by GO:0008277-regulation of G-protein coupled receptor 00.0060079-regulation of eacitaxory postsynaptic me GO 0043271-negative regulation of ion transport 30:0015698-inorganic anion transport GO 0006814-sodium ion transport 00009581-detection of external stimulus GO0007202-activation of phospholipase C activity G00010863-positive regulation of phospholipase C a GO0010517-regulation of phospholipase activity GO 0010518-positive regulation of phosphoipase act GO0006820-anion transport GO0042472-inner ear morphogenese GO:080193-positive regulation of lipase activity GO0009612-response to mechanical stimulus GO0031644-regulation of neurological system proce 00051969-regulation of transmission of nerve impu GO0042471-ear morphogenesis 000050804-regulation of synaptic transmission G00007188-G-protein signaling, coupled to cAMP nt' 0:0016337-cell-cell adhesion 00006813-potassium ion transport 00030802-regulation of cyclic nucleotide biosynthe 00030808-regulation of nucleotide biosynthetic pro GO0060191-regulation of lipase activity 00050778-positive regulation of immune response, GO 0042542-response to hydrogen peroxide 00 0051783-regulation of nuclear division 0:0007088-regulation of mitosis GO:0048839-inner ear development 0:0000302-response to reactive oxygen species GO:0006836-neurotransmitter transport 00007411-axon guidance GO:0051606-detection of stimulus G00015672-monovalent inorganic cation transport GO:0048015-phosphoinosid-mediated signaling 0:0019933-cAMP-mediated signaling 0.0030799-regulation of cyclic nucleotide metabolic 00:0007187-G-protein signaling, coupled to cyclic nu 30:0043583-ear development 0:0030001-metal ion transport 30:000681 1-ion transport 300006865-amino acid transport 30 0045761-regulation of adenylate cyclase activity 00 0031279-regulation of cyclase activity 300006140-regulation of nucleotide metabolic proce 0:0048584-positive regulation of response to stimul 300007268-synaptic transmission 00:0030817-regulation of cAMP biosynthetc process 300050953-sensory perception of light stimulus 30:0007601-visual perception 30 0019226-transmission of nerve impulse 30.0051339-regulation of lyase activity 300048511-rhythmic process GO 0006816-calcium ion transport GO:0007267-cell-cell signaling 0.0030814-regulation of cAMP metabolic process 3O0007018-microtubule-based movement 30:0019935-cycc-nuceotide-mediated signaling 30:00006812-cation transport 30:0040017-positive regulation of locomotion 30.0048942-carboxylic acid transport 30:0006576-biogenic amine metabolic process 30:0015837-amine transport 165 Table S3. GO categories enriched in eye transcriptome versus whole planarian Worksheets display enriched or depleted GO biological process (BP) categories among orthologs of planarian eye-enriched genes (relative to the whole planarian transcriptome). 166 C 0 .CO0 negative control 29, a -- C 0 ether-a-go-go sk3 Z 0 0 M. v 0 C ## Inpp5 ninag Ip3 kinase trpc-2 pde c g protein gamma g protein aipna, o g protein beta arrrestin beta-2 gucy-1 07503 sIc25 bestrophin-b 32281 75954 SIC7 V 0 C- 0 sIc12m7 U3404 SICZO-Z 0 11992 gaba-r ii 0 U) 'U 0( 23489 Dak4 $ 0 08951 0 00868 20991 26028 ran-gap 0 actin-2 DAG lipase I couch potato nol1 23802 aurora kinase 04497 cathepsin 167 1% 19866 09268 08711 traf4 acun-i Figure SI. Additional genes from an in situ hybridization screen of eye-enriched genes, related to Figure 1. Colorimetric in situ hybridization probing for genes with enriched eye expression in cDNA sequencing data. Genes are grouped by gene ontology categories. See Tables S1 and S2 for additional enrichment, orthology, and naming information. For genes with numerical identifiers, the best blast hit is listed. "p-tases", phosphatases. 168 fzdP-1 actin-2 best-b ptcdP-1 snys 19866 nthr nctaire w Figure S2. Identification of heterogeneity within the PRN and PC populations, related to Figure 2. (A) Fluorescent in situ hybridization showing expression of indicated genes in anterior photoreceptor neurons. Right side of each column shows co-expression with tyrosinase or opsin (both in green). Right eyes are shown. Dashed outline indicates pigment cup in top image. (B) Expression of genes in posterior photoreceptor neurons. (C) actin-2 is expressed in a subset of optic pigment cells. The pigment cup is on the left and photoreceptor neurons are on the right in all panels. Expression is shown in 7 day blastemas. Scale bars, 10 pm. 170 KLF2 mm 038720 NP OVOI__drNP_001108216 RTP02 OVO NP_726972 dm NP mm NP 497759 cc KLFI 0Q SMED-OVO NP dm 572185 075673 cc __NP_001022204 MUA ce LIN4O KLF15 NP 497632 SMED-KLF NP KLFT__ 1VO1l NP_014552 _h OVOLI _mm KLF8_ 00 0.91 dr 776141 001079472 NP LUNA_dm_NP_995811 _NP_064319 KLF5 OVOL2_mmNP_694455 mm-_NP_0I3909 KLFPImmNP_034765 K OVOL2__hs_NP_067043 L-4_dr _NP_001106955 KLF4__ __NP_034767 KLF2__mm__NP__032478 ZHTrB16_mm_-NP_0W10294% -- KLF2 MYONEtLON33m d, NP 571932 ---NP_085013 SPTF2 UTT1ONHUAD PRMD14_drNP__001157303.1 SPI HAMLETdm ccNP_495933 m NP d,_ NI'_ 511110 038700 NP_724130 I'IOXQIYn1_m_NI'_032265 PKNOXI11.NP SLOPPYPAIRED 057879.2 0.62 --3338 PNOX2_drNP_705940.1 FOXLI MEISI 034919.1 NP mm 000 UNCI32ce FOXB2 MEI.S3_m._NP_032653.2 0.0 FOXB - SMED-MEIS I -063 =mNP mm NP 0616Ca-dm__NP_524495.1 F04E4 MFIS2 dr NP 001073150.2 FOXQ2 c_ -UNC62 1024173.1 NP HOMOTHORAX 0.77 ED102C NP01 163581.1 dm PKH10 M1152 032049.1 071773.2 I-mm_%T_899121 571968.1 NP NP.496411.1 ISIOI__dr __NP__57104(.1 MEIS33__drNP__571853.1 MEIS .dr_ dm_ NP 1 _mmNP 032050.2 dr _NP 957278.1 FOXI I dm NP dr NP 001090411.1 651951.1 SMED-FOXQ2 cc_ NP492676 NP__01153041.1 mm FOXN4_drNP_571174 3TGIF mm ACHYNTIA SOX15 mm NP 001157547.1 _NP NP dm 033261.1 SOX9 , rOXN4 725102.1 dr NP S3X9ggNP 093q SOXIOdr 13.9 571718 _999612 NP 571950.1 SOX15_dmNP_523739.2 [SOX mm NP 011264.2 SOXIO__1mmNP_035567.1 NP SOX3_dr SOX3_mm_ 001001811.2 -NP__033263 SMED-SOXB SxNU11 dm NP 5247M.1 SOX2 -- SOX21 dm NP 648694.1 SOXIA-dr-NP3998283.1 SOXI m.__NP__M1259.2 -SOX2_drNP998283.1 SOX14_mmNP SOX14 dr NP 035570 001032769AI 171 mm NP 683737 476730.1 Figure S3. Assessment of gene orthology by Bayesian analysis, related to Figure 3. Branch nodes display posterior probabilities determined by Bayesian inference. SOX1/2/3/14/21, Fish-hook, and SoxNeuro belong to the SoxB family (Bowles et al., 2000). FoxQ2 orthologs are missing in placental mammals, but are present in other vertebrates, Drosophila, and C. elegans (Shimeld et al., 2010; Tu et al., 2006). Trees are rooted by outgroups. 172 A 21 kb L~frJ~f 034 kb 3515IF 16 33.s i h L 3. 09.0 IJ 5 L M1S6 Sb W4.551 51 L. MAS 30 ~534SNS..S443qI 351 U545 SS555(Fi'lM)~ Imax ns"F-mri W1 twain rmid hlgmFZOFPW): 557 a"t coverage a" feI B ovo RNAi Control RNAi 173 34.414 kbSS.Se 55 34.01,,5 L A L - m S..p4554..s&5.41 kb L 34, Figure S4. ovo is specifically expressed in the eye and ovo RNAi results in loss of eye cells, related to Figure 4. (A) No reads (above background) align to the ovo locus (comp452 contig) in the control (brain-enriched) sample. Shown on the right is a gene that is represented equally in both eye reads and control reads. (B) DIC images of 7 day head blastemas. Optic cups have a characteristic hollow crescent shape (arrow) at this stage, and these structures are not observable in the ovo RNAi condition (n=10/10). Scale bar, 50 pm. 174 A B ovo ovo smedwi-1 175 Figure S5. ovo* cells posterior to the eye in intact animals can also express markers of differentiated eye cells or neoblasts, related to Figure 5. (A) Double fluorescent in situ hybridization in starved intact animals is used to detect coexpression of ovo with markers for differentiated photoreceptor neurons (trpc-1) and pigment cells (tyrosinase). (B) Overlapping expression of ovo with the neoblast marker smedwi-1 is shown. Arrows indicate double-positive cells. Scale bars, 50 pm. 176 Control RNAi ovo RNAi w 0 meis RNAi kifRNAi progenitors z - Control RNA number of ovo+ fox RNAi ;0 X 0 0 Figure S6. Smed-meis, Smed-kif, Smed-foxQ2, and Smed-soxB expression and relationship to ovo expression, related to Figure 6. (A) Smed-meis, Smed-klf, Smed-foxQ2, and Smed-soxB are expressed in the photoreceptor neuron primordium during regeneration. Arrows indicate double-positive eye progenitors. (B) - (C) RNAi of Smed-meis, Smed-kf, and Smed-foxQ2 does not cause significant decrease in the numbers of ovo' progenitors. sp6-9 expression is used to label the pigment cup. n=8 eyes for each RNAi condition, error bars show SD. (D) soxB expression in the eye (arrow) is eliminated in ovo(RNAi) animals. All images are from day 7 regenerating head blastemas analyzed by FISH. Scale bars, 20 pm (A,D), 50 pm (B). 178 D P A Control RNAi kf RNAI V C A 'Z3 B 179 Control RNAi meis RNAi Figure S7. Defects in eye morphology resulting from Smed-kif and Smed-meis RNAi (A) 3-D z-stack reconstruction showing a side view of a regenerating eye. The photoreceptor neuron aggregate (green) is displaced ventrally and posteriorly in Smedklf(RNAi) animals, but the pigment cup (yellow) remains in the normal position (ventral displacement: n=6/6 animals in k/f RNAi; n=0/6 in control). Arrows indicate a second (non-eye related) sp6-9 expression domain along the D-V boundary. (B) 3-D z-stack reconstruction viewed from dorsal surface showing that photoreceptor neuron aggregates (opsin') are buried within the cerebral ganglion in Smed-kf(RNAi) animals, whereas photoreceptor neuron aggregates in control animals are above the cerebral ganglion. (C) meis RNAi followed by head amputation results in regeneration of eyes with morphologically aberrant pigment cups. Many defective cups have a duplicated cup structure (aberrant cup morphology: meis RNAi, 10/16; control RNAi, 2/24. Duplicated cup: meis RNAi, 8/16; control RNAi, 0/24). (D) Late pigment cell progenitors (tyrosinase') express low but detectable levels of meis transcript (arrows). All images are from day 7 head blastemas. Scale bars, 20 pm (C), 10 pm (D). 180 REFERENCES Agata, K., Soejima, Y., Kato, K., Kobayashi, C., Umesono, Y., and Watanabe, K. (1998). Structure of the planarian central nervous system (CNS) revealed by neuronal cell markers. Zoological Science 15, 433-440. Arendt, D. (2003). Evolution of eyes and photoreceptor cell types. The International Journal of Developmental Biology 47, 563-571. Arendt, D., Tessmar-Raible, K., Snyman, H., Dorresteijn, A.W., and Wittbrodt, J. (2004). Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science 306, 869-871. Ban, Y., and Rizzolo, L.J. (2000). Regulation of glucose transporters during development of the retinal pigment epithelium. Brain research 121, 89-95. Bergersen, L., Johannsson, E., Veruki, M.L., Nagelhus, E.A., Halestrap, A., Sejersted, O.M., and Ottersen, O.P. (1999). Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience 90, 319-331. Bessa, J., Gebelein, B., Pichaud, F., Casares, F., and Mann, R.S. (2002). Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes & Development 16, 2415-2427. Charlton-Perkins, M., and Cook, T.A. (2010). Building a fly eye: terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Current topics in developmental biology 93, 129-173. Collins, J.J., 3rd, Hou, X., Romanova, E.V., Lambrus, B.G., Miller, C.M., Saberi, A., Sweedler, J.V., and Newmark, P.A. (2011). Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biology 8, e1000509. Davis, R.J., Harding, M., Moayedi, Y., and Mardon, G. (2008). Mouse Dach1 and Dach2 are redundantly required for Mullerian duct development. Genesis 46, 205-213. del Marmol, V., Ito, S., Bouchard, B., Libert, A., Wakamatsu, K., Ghanem, G., and Solano, F. (1996). Cysteine deprivation promotes eumelanogenesis in human melanoma cells. The Journal of investigative dermatology 107, 698-702. Fain, G.L., Hardie, R., and Laughlin, S.B. (2010). Phototransduction and the evolution of photoreceptors. Curr Biol 20, R1 14-124. Frolenkov, G.I., Belyantseva, I.A., Friedman, T.B., and Griffith, A.J. (2004). Genetic insights into the morphogenesis of inner ear hair cells. Nature Reviews Genetics 5, 489498. 181 Gagnon, L.H., Longo-Guess, C.M., Berryman, M., Shin, J.B., Saylor, K.W., Yu, H., Gillespie, P.G., and Johnson, K.R. (2006). The chloride intracellular channel protein CLIC5 is expressed at high levels in hair cell stereocilia and is essential for normal inner ear function. J Neurosci 26, 10188-10198. Garger, A.V., Richard, E.A., and Lisman, J.E. (2004). The excitation cascade of Limulus ventral photoreceptors: guanylate cyclase as the link between InsP3-mediated Ca2+ release and the opening of cGMP-gated channels. BMC neuroscience 5, 7. Giblin, F.J. (2000). Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther 16, 121135. Gonzalez-Sastre, A., Molina, M.D., and Salo, E. (2012). Inhibitory Smads and bone morphogenetic protein (BMP) modulate anterior photoreceptor cell number during planarian eye regeneration. The International journal of developmental biology 56, 155163. Hase, S., Wakamatsu, K., Fujimoto, K., Inaba, A., Kobayashi, K., Matsumoto, M., Hoshi, M., and Negishi, S. (2006). Characterization of the pigment produced by the planarian, Dugesia ryukyuensis. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society 19, 248-249. Heine, P., Dohle, E., Bumsted-O'Brien, K., Engelkamp, D., and Schulte, D. (2008). Evidence for an evolutionary conserved role of homothorax/Meisl/2 during vertebrate retina development. Development 135, 805-811. Hisa, T., Spence, S.E., Rachel, R.A., Fujita, M., Nakamura, T., Ward, J.M., DevorHenneman, D.E., Saiki, Y., Kutsuna, H., Tessarollo, L., et al. (2004). Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. EMBO 23, 450-459. Hunter, D.G., Fishman, G.A., Mehta, R.S., and Kretzer, F.L. (1986). Abnormal sperm and photoreceptor axonemes in Usher's syndrome. Archives of ophthalmology 104, 385389. Katz, B., and Minke, B. (2009). Drosophila photoreceptors and signaling mechanisms. Frontiers in cellular neuroscience 3, 2. Kitajiri, S., Fukumoto, K., Hata, M., Sasaki, H., Katsuno, T., Nakagawa, T., Ito, J., Tsukita, S., and Tsukita, S. (2004). Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. The Journal of Cell Biology 166, 559570. Lapan, S.W., and Reddien, P.W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genetics 7, e1002226. 182 Li, B., Dai, Q., Li, L., Nair, M., Mackay, D.R., and Dai, X. (2002). Ovol2, a mammalian homolog of Drosophila ovo: gene structure, chromosomal mapping, and aberrant expression in blind-sterile mice. Genomics 80, 319-325. Lin, H., la Cour, M., Andersen, M.V., and Miller, S.S. (1994). Proton-lactate cotransport in the apical membrane of frog retinal pigment epithelium. Experimental eye research 59, 679-688. Lin, H., and Miller, S.S. (1991). pHi regulation in frog retinal pigment epithelium: two apical membrane mechanisms. The American Journal of Physiology 261, C1 32-142. Mackay, D.R., Hu, M., Li, B., Rheaume, C., and Dai, X. (2006). The mouse Ovol2 gene is required for cranial neural tube development. Developmental biology 291, 38-52. Mannini, L., Deri, P., Picchi, J., and Batistoni, R. (2008). Expression of a retinal homeobox (Rx) gene during planarian regeneration. The International journal of developmental biology 52, 1113-1117. Mannini, L., Rossi, L., Deri, P., Gremigni, V., Salvetti, A., Salo, E., and Batistoni, R. (2004). Djeyes absent (Djeya) controls prototypic planarian eye regeneration by cooperating with the transcription factor Djsix-1. Developmental biology 269, 346-359. Marmorstein, A.D., Cross, H.E., and Peachey, N.S. (2009). Functional roles of bestrophins in ocular epithelia. Progress in retinal and eye research 28, 206-226. Martin-Duran, J.M., Monjo, F., and Romero, R. (2012). Morphological and molecular development of the eyes during embryogenesis of the freshwater planarian Schmidtea polychroa. Development Genes and Evolution. Mastore, M., Kohler, L., and Nappi, A.J. (2005). Production and utilization of hydrogen peroxide associated with melanogenesis and tyrosinase-mediated oxidations of DOPA and dopamine. FEBS journal 272, 2407-2415. Matsushima, D., Heavner, W., and Pevny, L.H. (2011). Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development 138, 443-454. Mburu, P., Kikkawa, Y., Townsend, S., Romero, R., Yonekawa, H., and Brown, S.D. (2006). Whirlin complexes with p55 at the stereocilia tip during hair cell development. PNAS 103, 10973-10978. Mburu, P., Romero, M.R., Hilton, H., Parker, A., Townsend, S., Kikkawa, Y., and Brown, S.D. (2010). Gelsolin plays a role in the actin polymerization complex of hair cell stereocilia. PloS one 5, el 1627. 183 Mukherjee, A., Shan, X., Mutsuddi, M., Ma, Y., and Nambu, J.R. (2000). The Drosophila sox gene, fish-hook, is required for postembryonic development. Developmental biology 217, 91-106. Newmark, P.A., and Senchez Alvarado, A. (2000). Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Developmental biology 220, 142-153. Nilsson, D.E. (2009). The evolution of eyes and visually guided behaviour. Philosophical transactions of the Royal Society of London 364, 2833-2847. Nishimura, K., Kitamura, Y., Inoue, T., Umesono, Y., Yoshimoto, K., Takeuchi, K., Taniguchi, T., and Agata, K. (2007). Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesiajaponica. Neuroscience Research 59, 101-106. Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and S nchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238, 443-450. Pineda, D., Gonzalez, J., Callaerts, P., Ikeo, K., Gehring, W.J., and Salo, E. (2000). Searching for the prototypic eye genetic network: Sine oculis is essential for eye regeneration in planarians. PNAS 97, 4525-4529. Pineda, D., Rossi, L., Batistoni, R., Salvetti, A., Marsal, M., Gremigni, V., Falleni, A., Gonzalez-Linares, J., Deri, P., and Salo, E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. Pineda, D., and Salo, E. (2002). Planarian Gtsix3, a member of the Six/so gene family, is expressed in brain branches but not in eye cells. Mechanisms of development 119 Suppl 1, S167-171. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and Ssnchez Alvarado, A. (2005). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Reddien, P.W., and Sanchez Alvarado, A. (2004). Fundamentals of planarian regeneration. Annual Review of Cell and Developmental Biology 20, 725-757. Senchez Alvarado, A., and Newmark, P.A. (1999). Double-stranded RNA specifically disrupts gene expression during planarian regeneration. PNAS 96, 5049-5054. Schallreuter, K.U., Kothari, S., Chavan, B., and Spencer, J.D. (2008). Regulation of melanogenesis--controversies and new concepts. Experimental dermatology 17, 395404. 184 Self, T., Sobe, T., Copeland, N.G., Jenkins, N.A., Avraham, K.B., and Steel, K.P. (1999). Role of myosin VI in the differentiation of cochlear hair cells. Developmental biology 214, 331-341. Sexton, T., Buhr, E., and Van Gelder, R.N. (2012). Melanopsin and mechanisms of nonvisual ocular photoreception. The Journal of Biological Chemistry 287, 1649-1656. Silver, S.J., and Rebay, I. (2005). Signaling circuitries in development: insights from the retinal determination gene network. Development 132, 3-13. Singhal, S.S., Godley, B.F., Chandra, A., Pandya, U., Jin, G.F., Saini, M.K., Awasthi, S., and Awasthi, Y.C. (1999). Induction of glutathione S-transferase hGST 5.8 is an early response to oxidative stress in RPE cells. Investigative ophthalmology & visual science 40, 2652-2659. Smyth, J.T., Hwang, S.Y., Tomita, T., DeHaven, W.I., Mercer, J.C., and Putney, J.W. (2010). Activation and regulation of store-operated calcium entry. Journal of cellular and molecular medicine 14, 2337-2349. Strauss, 0. (2005). The retinal pigment epithelium in visual function. Physiological reviews 85, 845-881. Tessmar-Raible, K., and Arendt, D. (2003). Emerging systems: between vertebrates and arthropods, the Lophotrochozoa. Current opinion in genetics & development 13, 331340. Umesono, Y., Watanabe, K., and Agata, K. (1999). Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Development Genes and Evolution 209, 31-39. Unezaki, S., Horai, R., Sudo, K., Iwakura, Y., and Ito, S. (2007). OvoI2/Movo, a homologue of Drosophila ovo, is required for angiogenesis, heart formation and placental development in mice. Genes Cells 12, 773-785. Venza, I., Visalli, M., Cucinotta, M., Teti, D., and Venza, M. (2011). Association between oxidative stress and macromolecular damage in elderly patients with age-related macular degeneration. Aging clinical and experimental research 24, 21-27. Vopalensky, P., and Kozmik, Z. (2009). Eye evolution: common use and independent recruitment of genetic components. Philosophical transactions of the Royal Society of London 364, 2819-2832. Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816. 185 Wang, T., and Montell, C. (2007). Phototransduction and retinal degeneration in Drosophila. Pflugers Arch 454, 821-847. Williams, D.S. (2008). Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision research 48, 433-441. Wood, J.M., Decker, H., Hartmann, H., Chavan, B., Rokos, H., Spencer, J.D., Hasse, S., Thornton, M.J., Shalbaf, M., Paus, R., et al. (2009). Senile hair graying: H202-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. Faseb J 23, 2065-2075. Xiao, Q., Hartzell, H.C., and Yu, K. (2010). Bestrophins and retinopathies. Pflugers Arch 460, 559-569. Zuber, M.E., Gestri, G., Viczian, A.S., Barsacchi, G., and Harris, W.A. (2003). Specification of the vertebrate eye by a network of eye field transcription factors. Development 130, 5155-5167. 186 Chapter 4 Medially-expressed Hedgehog gene in the planarian CNS regulates formation of a glia-like cell type Sylvain Lapan, 2 , Irving Wang 1' 2 , Steven Sando 2, Joshua Meisel 1 and PeterW. Reddien ' 2, 2 3 'Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142 2 Department of Biology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02142 3Howard Hughes Medical Institute Sylvain Lapan performed all experiments, except for those represented in Figures 6C-G and 7AC, which were performed in collaboration with Irving Wang. Steven Sando was a one month rotation student who assisted with screening genes affected by Hh RNAi. Joshua Meisel was a research technician who performed race on several transcription factors described in the work. 187 SUMMARY Comparative analyses of development in bilaterally symmetric animals suggest common evolutionary origins of the central nervous system (CNS). We identified similarities between the planarian and vertebrate CNS with respect to the relative expression domains of orthologous transcription factors and the function of a Bmp-4 ortholog. We propose that the medial-lateral (M-L) axis of the planarian brain is comparable to the dorso-ventral (D-V) axis of the vertebrate neural tube, with medial corresponding to ventral. Sonic Hedgehog is expressed ventrally in the neural tube of vertebrates and has a prominent role in regulating expression of transcription factors along the D-V axis of the spinal cord. An ortholog of Hedgehog in planarians, Smed-hedgehog, is expressed specifically in the medial CNS. Perturbation of planarian Hedgehog signaling affected maintenance of a glia-like population in the CNS, but did not alter the M-L expression domains of orthologs Nkx6, Nkx2, Pax6, or Single minded. These data raise the possibility that a medial/ventral position of Hedgehog expression in the CNS is a conserved feature of bilaterian nervous systems, but that the common function of Hedgehog is specification of glia-like cells rather than regional control of transcription factor expression. 188 INTRODUCTION Most bilaterally symmetric animals have a centralized nervous system with complex morphology and cell type composition. Whether the common ancestor of this group had a nervous system that was centralized has been the subject of much attention. There is increasing evidence from molecular and genetic studies that fundamental patterning mechanisms at work during nervous system development are conserved across distant animal groups. The relative position of the expression domains of nkx2/vnd, gsxlind, and msx/msh in neurogenic regions with respect to the site of expression of the signaling ligand Bmp4/Dpp is similar between vertebrate and Drosophila embryos (Arendt and Nubler-Jung, 1999; Mizutani and Bier, 2008). Furthermore, transcription factor expression domains along the antero-posterior axis of the enteropneust nerve net closely parallel the distribution of these domains within the centralized nervous systems of vertebrates (Lowe et al., 2003). In vertebrates, infolding during neurulation causes a topological change in the neuroectoderm that transforms an M-L axis into a D-V axis. In protostomes, which do not undergo neurulation, most patterning in the neuroectoderm occurs along the M-L axis. Many parallels have been observed between the M-L axis of the developing neuroepithelium in the annelid Platynereis dumerilii and the D-V axis of the embryonic vertebrate neuroepithelium. These include the distribution of differentiated cell types, transcription factor expression domains, and site of action of Bmp-4 (Denes et al., 2007). Therefore, the nervous system of early bilaterians was likely patterned by mechanisms that remain relevant in nervous systems of extant organisms. Characterization of nervous systems in more diverse phyla is required to refine existing theories of the evolution of metazoan CNS patterning. Numerous features of the planarian Schmidtea mediterranea make it an ideal organism for the study of CNS 189 pattern formation. Planarians, members of the lophotrochozoan superphylum (Dunn et al., 2008), have a ventrally located, bi-lobed cephalic ganglion connected to two longitudinal nerve cords (Umesono and Agata, 2009). The brain develops during embryogenesis in planarians, but can also be completely regenerated in adult animals following decapitation (Hyman, 1951). The planarian nervous system can undergo size changes of several orders of magnitude during growth or during shrinking due to starvation, and cells lost during normal tissue turnover are actively replaced in adults. Therefore, patterning mechanisms persist in the adult planarian in order to orchestrate brain formation during regeneration, homeostasis, and scaling. A prominent feature of vertebrate CNS development is the expression of diffusible Hedgehog ligand from ventral positions to pattern the D-V axis (Dessaud et al., 2008). However, a comparable role for Hedgehog signaling outside of vertebrates has not been identified. We characterized the function of a planarian ortholog of Hedgehog, which is expressed specifically in the medial CNS. The distribution of conserved transcription factor expression domains and differentiated cell types, as well as the site of BMP action, indicate that the medial planarian CNS position has notable similarity to the ventral region of the developing neural tube. Hedgehog signaling was required for maintenance of a putative glial cell type in the medial planarian brain, but did not impact expression of conserved brain-expressed transcription factors that are Hedgehogsensitive in vertebrate patterning. Therefore, our findings support a role for Hh in formation of a specific cell type in the nervous system, but not in broad control of D-V/ML regionalization in an invertebrate brain. 190 RESULTS Hedgehog and Patched are expressed in the medial CNS of planarians One homolog of Sonic hedgehog is present in the planarian genome (Smedhedgehog) (Figure S1), and is expressed in the medial region of the cephalic ganglion and in the ventral nerve cords (Figure 1A), as recently described (Rink et al., 2009; Yazawa et al., 2009). hedgehog' cells expressed choline acetyl-transferase (Smedchat), which is expressed in many neurons of the planarian CNS (Figure 1 B). hedgehog was expressed during brain regeneration by day three after amputation (Figure 1 C). From days 2-4 of regeneration, hh expression was also present at the tip of anterior blastemas (Figure 1D). In pathways described in other protostomes and deuterostomes, Hedgehog ligand signals through the Patched receptor (Chen and Struhl, 1996; Stone et al., 1996). One ortholog of Patched, Smed-patched (ptc), is present in the planarian genome and is broadly expressed, including strong expression in the cephalic ganglion and longitudinal nerve cords (Figure 1E) (Rink et al., 2009; Yazawa et al., 2009). A medial-to-lateral distribution of midline, serotonergic, hb9' and sensory neurons in the planarian brain To assess whether the medial expression of hedgehog in the planarian brain may be homologous to the ventral expression of Sonic hedgehog in the vertebrate neural tube, we looked for similarities between the M-L axis of the planarian brain and the D-V axis of the vertebrate neural tube, beginning with the distribution of neural cell types. The distribution of chat expression was used as an indicator of position along the M-L and DV axes of the brain. At the most ventral position, the cephalic ganglia are similar in width to the nerve cords. More dorsally, the ganglia gradually widen into two lobe-like structures at the end of each nerve cord, with elongated aggregates of neurons (the 191 brain branches) extending from the lateral (outer) lobe. Within the lobe is a region that has few cell bodies but is neurite rich (Okamoto et al., 2005), termed the spongy region. Sensory, motor (hb9*), serotonergic, and midline neurons are distributed along the M-L axis of the Platynereis dumerilii neuroepithelium in a similar pattern as in the developing vertebrate spinal cord/hindbrain along the D-V axis (Denes et al., 2007). In both cases, midline neurons are present in the most ventral/medial region, followed laterally/dorsally by serotonergic neurons, then hb9' motoneurons, and finally sensory neurons at the most dorsal/lateral position. Smed-g-alpha (Nakazawa et al., 2003), encoding a g-protein alpha subunit, was used to label cells of the brain branches (Figure 2B). Cells expressing the mechanosensory channel-encoding gene, cintillo (Figure 2C) (Oviedo et al., 2003), were localized within the branch region. The g-a/pha-expressing cells of the brain branches were also localized near a region of high ift88 expression (Figure 2D), which encodes an intraflagellar transport (IFT) protein. IFT88 is present specifically in cilia (Rosenbaum and Witman, 2002), which are required for sensory transduction in neurons of many animals (McEwen et al., 2008; Tsujikawa and Malicki, 2004). Therefore, the brain branches may represent a lateral region of the CNS with sensory functions. Ion channels belonging to the TRP family have diverse roles in response to sensory stimuli (Venkatachalam and Montell, 2007). Three homologs of TRP channels, Smed-trp-m, Smed-trp-a, and Smed-pkd2 (Nakazawa et al., 2003) were expressed in neurons of the lateral-most regions of the cephalic nervous system (Figures 2E-G). A subset of cells expressed both trp-m and trp-a, suggesting that this region contains a heterogeneous population of sensory neurons. Because neurons concentrated in the lateral-most region of the brain are also sparsely distributed in the sub-epidermal space throughout the animals (Figure 2H), we refer to these cells as peripheral nervous system 192 (PNS) cells. Thus, sensory CNS and sensory PNS neurons were identified, and both of these occupied lateral positions in the nervous system of the head. We next assessed whether neurons with molecular similarity to midline neurons of the vertebrate ventral spinal cord were present in the medial planarian brain. Netrin and Single-minded genes are expressed in midline neurons of Drosophila (Harris et al., 1996; Mitchell et al., 1996; Thomas et al., 1988), and homologs are expressed in midline neurons of Platynereis and vertebrates (Denes et al., 2007; Fan et al., 1996; Serafini et al., 1996). The medial region of the planarian brain expresses two orthologs of Netrin, Smed-netrin-1 and Smed-netrin-2 (Cebri6 and Newmark, 2005) (Figures 21). (netrin-1 is also broadly expressed near the ventral surface of the worm; this expression appears as lateral signal in optical sections, but was not concentrated in the head, in contrast to PNS markers examined.) Neurons in the medial planarian CNS express an ortholog of single-minded (sim) (Figure 2J). The similarity between the medial planarian brain and the ventral spinal cord is further supported by the medial expression in planaria of a homolog of Slit (Cebria et al., 2007). Slit proteins act as midline repellents in axon pathfinding (Kidd et al., 1999). We next examined the distribution of cell types between the midline and lateral sensory regions. Serotonergic neurons in Schmidtea mediterranea are distributed in a highly similar pattern to that reported in the related species Dugesiajaponica (Nishimura et al., 2007). Expression of a Smed-tryptophan hydroxylase (tph) gene, which encodes an enzyme required for serotonin synthesis, was detected in the medial brain as well as in the outer lobe and slightly lateral to the outer lobe (Figure 2K). Smed-hb9 expressing cells were observed among the laterally positioned serotonergic cells (Figures 2L and 2M). Hb9 family genes have been used as motoneuron-specific markers in vertebrates (Arber et al., 1999), C. elegans (Von Stetina et al., 2007), and Platynereis (Denes et al., 2007), but not Drosophila (Odden et al., 2002). Overall, the domain of serotonergic 193 neurons in the planarian brain was broader than the domain of serotonergic neurons during early differentiation of these neurons in the ventral vertebrate hindbrain. However, a parallel exists to annelid and vertebrate in that hb9+and serotonergic neurons in all cases are distributed between midline and sensory neuron domains (Figure 2N). Transcription factors expressed ventrally in the vertebrate neural tube are expressed in the medial planarian brain The M-L distribution of planarian cell types described above raised the question of whether similar nervous system patterning mechanisms might exist between planaria and vertebrates along the M-L/D-V axis. Homeodomain-containing transcription factors play crucial roles in nervous system patterning in multiple animal phyla (Mizutani and Bier, 2008). We therefore investigated the expression of a number of conserved genes encoding homeodomain-containing proteins in planarians. The nkx2.2 gene functions in establishing progenitor domains of the ventral spinal cord and hindbrain of vertebrates during embryogenesis (Briscoe et al., 1999), and nkx2-like genes are expressed medially in the neuroectoderm of Drosophila and Platynereis (Mizutani and Bier, 2008). A homolog of nkx2.2, Smed-nkx2, was expressed in the medial planarian brain, with expression densest in the space between the midline neurons and the outer lobe of the brain (Figures 3A-B). In the vertebrate trunk CNS, nkx2.2 is expressed in a domain ventral to the expression domain of the pax6 gene, and the expression domains of these genes form a well-defined boundary (Ericson et al., 1997). Complementarity of expression pattern has also been observed for these genes in Platynereis along the M-L axis (Denes et al., 2007). Two homologs of Pax6 have been identified in planarians, pax6A and pax6B (Pineda et al., 2002). Smed-pax6B, was expressed at its most medial position in the outer lobe, in a pattern approximately complementary to the expression domain of nkx2 (Figures 3C-D). At more dorsal levels pax6B expression became more 194 broadly distributed into the brain branches and the PNS (Figure 3C-D) and nkx2 was also observed in cells at lateral edges of the brain branches; no apparent nkx2* cells have been found in a similar (dorsal) region in the mouse spinal cord until postnatal stages (Qi et al., 2001). nkx6. I is another homeobox gene with important roles in patterning of the ventral vertebrate CNS (Sander et al., 2000) and an ortholog is expressed in the medial Platynereis neurectoderm (Denes et al., 2007). Smed-nkx6 is expressed in the medial planarian brain in a disparate population of cells (Fig 3E-F). Although expression was strongest near the midline, nkx6+ cells were also observed in the outer lobe (Figures 3G). The region where nkx6 and pax6 expression overlap, the ventral domain of the outer lobe (Figure 3H), is the same approximate M-L position where hb9' neurons are located (Figures 2L). In vertebrates, hb9+ motoneurons likewise arise from a region with nkx6. I/pax6 overlap and adjacent to the nkx2.2 expression domain (Sander et al., 2000). Expression of msx and dlx orthologs, and a function for bmp4 in the lateral planarian brain. In vertebrates, Dix family transcription factors function in positioning the neural crest domain at the (lateral) neural plate boundary prior to neurulation (McLarren et al., 2003), and are involved in specification of Rohon-Beard neurons, which are sensory neurons of the neural plate boundary and dorsal neural tube (Kaji and Artinger, 2004). Two Dix family homologs, Smed-dlx (Lapan and Reddien, 2011) and Smed-dlx-2, had expression in lateral sensory regions. dlx-2 expression was largely restricted to the rim of the head, and lateral to the brain branches (Figure 31-J). dlx was co-expressed with g-alpha in the brain branches (Figures 3K-L) but was also expressed in medial and intermediate regions. 195 In Drosophila, Platynereis, and vertebrates, Msx family transcription factors are expressed in dorsal/lateral regions of the neuroectoderm (Ramos and Robert, 2005). A homolog of msx, msh-2, was previously described in the planarian species Dugesia japonica with expression detected in scattered cells exclusively present during regeneration of the head from days 4-6 after amputation (Mannini et al., 2008). We cloned a full-length version of Smed-msh-2 and confirmed its homology to msx genes from other organisms (Figure SI). msh-2 was expressed in intact worms and within lateral pkd2* regions of the nervous system (Figures 3M-N). To further assess similarity between the lateral planarian brain and the dorsal vertebrate neural tube, the function of Bmp4, a secreted ligand of the TGF-P superfamily (Schmierer and Hill, 2007), was assessed by RNAi. Bmp4 is secreted from the roofplate in vertebrates and patterns dorsal regions of the spinal cord (Timmer et al., 2002). The Drosophila homolog of Bmp4, dpp, is expressed in the dorsal ectoderm of the Drosophila embryo and restricts the size of the dorsal (msx*) and intermediate (ind*) neural domains (Mizutani et al., 2006). Therefore, if the M-L axis of the planarian brain is homologous to the D-V axes of the developing nervous systems of these organisms, one prediction would be that Bmp4 primarily affects region size and/or patterning in the lateral regions of the planarian brain. In planarians, a bmp4-like gene, Smed-bmp4, is expressed on the dorsal side of the animal (Molina et al., 2007; Reddien et al., 2007). One round of regeneration following RNAi of bmp4 resulted in no gross defects in the size of the medial CNS. There was no significant change in the numbers of glutamatedecarboxylase' neurons in the medial brain (Figure 4A-C), nor was there a significant change in area of the single-minded* region (Figure 4F-G). The effects of bmp4 RNAi on lateral regions of the cephalic nervous system in seven day regenerating worms was assessed by examining expression of cintillo, trp-a, and trp-m. As reported (Molina et al., 2007), RNAi resulted in a broadening of the cintillo 196 expression domain, as well as an increase in the number of cintillo' cells (Figures 4A, B, and D). bmp4(RNAi) animals also displayed increase in the numbers of trp-m* cells (Figures 4H-K and P), with the greatest increase occurring on the dorsal surface. By contrast, trp-a* cells were reduced in number, particularly in the head rim of bmp4(RNAi) animals (Figures 4L-Q). The maintenance of a relatively normal distribution of trp-m+ cells in this area suggests that this loss was specific and not the result of elimination of the lateral-most region of neurons due to disruption of the D-V boundary. Thus, bmp4 is required for normal regeneration of lateral CNS regions. The function of Hedgehog signaling pathway components in planarians In vertebrates and Drosophila, Hedgehog ligands and Patched receptors have opposing activating and repressive roles, respectively, on Hedgehog pathway output (Hooper and Scott, 2005). Patched inhibits Smoothened, a membrane-bound effector of the Hedgehog signaling pathway, and Hedgehog binding alleviates this inhibition (Bijlsma et al., 2004). In order to investigate the function of Hedgehog signaling in the planarian brain, we used RNAi to reduce hh and ptc expression and observed the effect on head and tail regeneration. hh RNAi resulted in several defects including photoreceptors with ectopic or shortened axon tracts, reduced optic pigment cups, and reduced tail size (Figures 5A-B). ptc(RNAi) animals displayed fused photoreceptors (Figure 5A-B) and regenerated large tails (Figure 5A). Gli2 is a Hedgehog pathway effector and has an activating function in Hedgehog signaling in the spinal cord (Jacob and Briscoe, 2003). RNAi of Smed-gi2-1 caused a similar phenotype as did RNAi of hh with respect to photoreceptor and tail regeneration (Figure S2A). Similarly, Suppressor-of-fused (Sufu) is a protein with repressive pathway activity (Monnier et al., 1998). A gene encoding a homologous predicted protein, Smed-sufu, had a similar RNAi phenotype as did ptc, 197 including fused photoreceptors (Figure S2B). These results confirm similar recent findings from other groups (Rink et al., 2009; Yazawa et al., 2009). Tail phenotypes indicated that the Hedgehog pathway may have a role in patterning of the antero-posterior axis. Indeed, a number of ptc(RNAi) animals developed an ectopic pharynx at the anterior pole and a pointed, tail-like growth in place of a normal head (Figure 5C and S7A-B). Some of the ptc(RNAi) animals expressed the posterior marker wntP-1 at the anterior pole, confirming that ptc can regulate the ability to make anterior vs. posterior tissue during planarian regeneration (Figures 5D) (Rink et al., 2009; Yazawa et al., 2009). The function of Hedgehog signaling in the planarian brain We considered the possibility that Hedgehog signaling can affect both regeneration polarity and brain patterning. Whereas regenerating hh(RNAi) animals have a brain with no gross morphological defect, ptc(RNAi) animals can have greatly reduced and irregular brains (Figure 5E-F). However, this defect may simply be the result of posteriorization of the animal anterior. To circumvent the polarity defect that occurs following amputation, we analyzed the effect of perturbation of Hedgehog signaling in uninjured animals, which never form anterior tails under ptc RNAi conditions. We performed long-term RNAi of hh and ptc and analyzed the effect on global gene expression using RNA-seq. Because hh and ptc are expected to have opposite effects on gene expression, we looked for genes that were upregulated in ptc(RNAi) animals relative to the control, and downregulated in Hh(RNAi) animals relative to the control (Figure 6A). Following in situ hybridization screening with genes identified by RNA-seq, we identified numerous genes with expression in the medial intestine (Figure 6B). We also identified two genes expressed in the brain (Figure 6B). Smed-nf encodes an ortholog of neurofilament, and Smed-pcdhl9 encodes a protocadherin family gene. 198 These two genes were coexpressed in cells in the spongy region of the brain (Figure 6C). nf' and pcdh19' cells were relatively large, and contained numerous processes with detectable transcript, a feature not typical of planarian neurons (Figure 6D). We therefore examined whether this cell type lacks other features of neurons. These cells did not express netrin-2, a marker for neurons in the spongy region (Figure 6E). Planarian neurons express synaptotagmin and synapsin, and a large majority express chat. nf/pcdh19+ cells, however, did not express these markers (Figure 6F-G). Because of these properties, these cells may represent a non-neuronal CNS cell type and we refer to them as glia-like. However, the function of nf/pcdh19* cells is not yet known and further experiments are needed to determine the degree of similarity between these cells and vertebrate glia. nf/pcdh19* cells are located directly adjacent to the hh expression domain, but do not themselves express hh (Figure 7A). In embryogenesis, Patched is often in expressed in cells actively responding to Hedgehog signal (Marigo and Tabin, 1996), and we observed that most of the pcdh19* cells had strong ptc expression (Figure 7B). Therefore, Hedgehog signal may diffuse from the medial lining of the brain to regulate cell identity. To investigate this possibility, we examined expression of nf and pcdhl9 in hh(RNAi) and ptc(RNAi) animals. Remarkably, expression of both of these markers was nearly eliminated in all hh(RNAi) animals examined (Figure 7C). Furthermore, ptc RNAi resulted in a large increase in the number and distribution of cells expressing nf and pcdhl9 (Figure 7C). Typically, no pcdh19+ cells are observed along the lateral edges of the head (PNS region). However, in ptc(RNAi) animals, numerous pcdh19+ cells were observed in the head rim. Therefore, Hedgehog signaling prominently regulates a glialike cell type in the planarian brain. In vertebrates, Hedghog signaling has potent effects on the expression of orthologs of nkx2, nkx6, pax6, and sim. We did not observe changes in the expression of 199 the planarian orthologs these genes in our RNA-seq dataset. We verified this result by in situ hybridization. In hh(RNAi) and ptc(RNAi) animals, no observable defect was seen in the expression domains of these genes (Figures D-G). Therefore, under conditions of hh and ptc inhibition sufficient to cause major changes in the numbers of nf'*pcdh19' cells, the expression of several conserved transcription factors in the planarian brain is not detectably affected. DISCUSSION Comparing gene expression between the planarian brain and embryonic nervous systems Expression studies have revealed that the planarian brain possesses a wide variety of distinct cell types, and complex regional organization marked by differences in morphology and gene expression across the M-L axis (Umesono and Agata, 2009). Several major metaozoan signaling pathways such as Wnt, Fgf, and Bmp regulate the size and morphology of the planarian brain (Cebria et al., 2002; Kobayashi et al., 2007; Petersen and Reddien, 2008). However, the extent to which the planarian central nervous system is similar to other bilaterian nervous systems in structure and patterning mechanisms largely remains to be explored. We identified planarian orthologs of vertebrate genes encoding transcription factors that pattern the vertebrate CNS along the D-V axis. We found that expression of orthologs of Single minded (medial), Nkx2 (medial), Nkx6 (medial), Pax6 (intermediate), Msx (lateral) and DIx (lateral) were expressed in a planarian CNS in a manner consistent their D-V expression in vertebrates. Boundaries between transcription factor expression domains in the planarian brain are not as sharp as those observed during early stages of neural tube patterning. This likely reflects important differences in the structure of the tissues being compared. 200 The planarian brain probably does not form from an epithelial tissue, given that all known undifferentiated cells in planarians are mesenchymal. Second, although new cell formation occurs continuously in planarians, our analysis depends on comparing a largely differentiated adult structure to embryonic structures. Considering this fact, it is remarkable that substantive comparisons in gene expression can be made between these systems. Ongoing expression of developmentally important transcription factors in differentiated cells may relate to active tissue patterning in adult planarians. Persistent expression of fate-specifying transcription factors is observed in the CNS of adult axolotls, another highly regenerative organism. In these animals, regionalized expression of spinal cord transcription factors such as Msx1 and Pax6 is found in the adult spinal cord, as is ventral expression of Hedgehog (Schnapp et al., 2005). Despite the obvious complexities of comparing an adult planarian brain to an embryonic neural tube, evidence suggests important similarities exist between the M-L axis of the planarian brain and the D-V axis of the vertebrate neural tube. In addition to the distribution of transcription factors expression, this is indicated by the medial expression of orthologs of genes expressed at the ventral midline in the vertebrate CNS. Lateral localization of sensory cell types in planarians mirrors the dorsal localization of sensory interneurons and Rohon-Beard cells dorsally in vertebrates. Furthermore, the dorsal/lateral position of BMP and its role in lateral/dorsal neuron patterning represents another commonality with vertebrates. The role of Hh as a master patterning factor in nervous systems A striking similarity between vertebrates and planarians is the highly localized expression of Hedgehog at the ventral/medial position. Medial expression of Hedgehog is also observed in another invertebrate, the snail Patella vulgata (Nederbragt et al., 2002). Hedgehog has a well-studied role as a morphogen that is secreted from the 201 ventral neural tube, controlling expression of transcription factors that specify interneuron, glial, and motoneuron cell types (Briscoe et al., 2000). Despite the fact that orthologs of many of these transcription factors are expressed in planarians, Hedgehog signaling here does not appear to regulate their expression. Perturbation of Hedgehog signaling also does not cause dramatic morphological defects, which are characteristic of Hedgehog signaling pathway perturbations in vertebrates (Chiang et al., 1996). Therefore, while data in planarian and snail support a model in which medial/ventral expression of Hedgehog in the CNS is ancestral, no data currently exists to support a common role for Hedgehog in broadly orchestrating D-V/M-L pattern. Interestingly, despite the severity of defects stemming from Hedgehog signaling pathway mutations in vertebrates, much of the D-V pattern in the mouse spinal cord can be established in the absence of this signaling pathway. Combining Shh knockout with loss of Gi3, a repressive pathway regulator, can restore much of the pattern of the spinal cord (Litingtung et al., 2002; Persson et al., 2002). Therefore, other D-V patterning mechanisms must exist to create regionalized transcription factor expression. It will be of great interest to identify these mechanisms and investigate whether they are responsible for pattern formation in invertebrate nervous systems. Despite the widespread use of hedgehog in Drosophila development, this gene is not involved in D-V patterning of the neuroectoderm. The expression of nkx2lvnd, gsx/ind, and msx/msh along the D-V axis in Drosophila embryos depends upon dorsal Dpp and the NF-kB protein Dorsal as a ventral expressed morphogen (Mizutani and Bier, 2008). However, this mechanism is likely specific to insects because because Dorsal protein can only diffuse in syncytial embryos. We identified an important role for Hedgehog signaling in the formation of putatively non-neuronal cell type in the planarian brain. mRNA is not typically detected by in situ hybridization in neuronal processes using our standard histological methods; 202 however, Hedgehog-dependent cells displayed abundant mRNA signal in the vicinity of the cell body, and cell bodies often were attached to several visible processes. Furthermore, these cells were localized within nuclei-poor and axon-rich regions of the CNS, and did not express pan-neuronal genes in planarians. Based on these properties, these cells could be glial cells. It will be important to investigate this possibility further with a broad identification of the cellular gene expression profile and cell morphology studies. To date, no glial cell has been identified in planarians (Umesono and Agata, 2009). In many animals, glia are a critical component of the response to nervous system injury, with roles in sealing injury sites and phagocytosing necrotic cells (Robel et al., 2011). Because planarians have excellent capacity for repair following injury to the nervous system, it will be interesting to observe whether glial cell activity in planarians is important in regeneration. Hedgehog has a critical role in specification of oligodendrocytes in the vertebrates CNS (Nicolay et al., 2007). Oligodendrocyte development is under the control of Olig1 and Olig2, which are induced in a concentration-dependent manner by ventral Shh signaling. Furthermore, the boundaries of the Olig2* oligodendrocyte progenitor domain are determined by transcription factors such as Nkx2, Nkx6, and Pax6, which are controlled by Shh signaling. Ecoptic Shh expression via viral transfection can cause ectopic Olig2 expression and oligodendrocyte formation throughout the CNS (Lu et al., 2000; Nery et al., 2001). Indeed, the founding member of the vertebrate Gli family was identified in glioma tumours (Clement et al., 2007). In Drososphila nerve cord development, Hh signaling is required for specifying fate among midline glia, promoting the formation of posterior (non-ensheathing) glia over anterior (ensheathing) glia (Watson et al.). Further investigation into the mechanism by which Hedgehog regulates nf'/pcdh19' cells in planarian will yield insight into the common functions of this signaling pathway in diverse animal nervous systems. 203 MATERIALS AND METHODS Gene cloning Total RNA was purified from intact and regenerating S. mediterranea, and cDNA was made by RT-PCR using oligo dT (Invitrogen). Genes were cloned from cDNA library using gene-specific primers designed from EST databases and gene predictions (Genscan and Maker) (Cantarel et al., 2008). Complete gene sequence was determined using 5' and 3' RACE PCR (FirstChoice RLM-RACE, Ambion). For riboprobe synthesis, genes were cloned into pGEM (pGEM T-Easy, Promega) and then template for the transcription reaction was generated by PCR using primers with T7 promoter sequence. For RNAi experiments, gateway recombination was used to clone genes in pPR244, a dsRNA-expression vector, as described (Reddien et al., 2005). Histology and imaging Riboprobes were generated by in vitro synthesis with T7 enzyme. WMFISH was performed as described (Pearson et al., 2009) except that FITC-tyramide was used at 1:500, and HRP enzyme was inactivated using 4% formaldehyde in 0.1% PBST for 45 minutes instead of hydrogen peroxide. Tyramide was generated by conjugation of succinimidyl esters of rhodamine and FITC with tyramide-HCL (Roche). Colorimetric WMISH was performed as described (Reddien et al., 2007). For WMFISH labeling of RNAi worms, 7 day head blastemas were separated from the body prior to fixation and fixed within 30 minutes. Optical sectioning was performed using an Apotome, Axiocam digital camera, a Zeiss Axiolmager, and Axiovision software. Brightness, contrast, and gamma were adjusted as needed to improve visibility. RNAi 204 RNAi was performed by feeding intact animals with bacteria expressing dsRNA in a mixture of liver. Cultures were grown to an OD600 of 0.35-0.45, and then induced for two hours with 1 mM IPTG. Next, cultures were pelleted and resuspended in a volume of 70% liver/30% water equal to 1 /3 0 0 th of the original volume of the culture. Worms were at least seven-day starved at the time of the first feeding, and were fed three or four times, with at least 4 days separating each feeding. Amputation was performed on the day following the final feeding, and worms were fixed following one round of regeneration. RNA-seq and biofinformatic analysis >1ug of RNA was used to construct Illumina libraries with Illumina reagents and following the commercial protocol. Each library was analyzed by 36 bp single end sequencing on one lane of an Illumina Genome Analyzer II. Read quality analysis by FastQC showed good per base quality. Reads were aligned to a reference transcriptome (Adamidi et al., 2011) using Bowtie (Langmead et al., 2009), with only the best single alignment reported. Each condition (ptc, hh, control RNAi) was represented by two biological replicates. Enrichment across replicates was analyzed using DESeq (Anders and Huber, 2010). ACKNOWLEDGEMENTS Thanks to members of the Reddien lab for comments on the manuscript. We acknowledge support by the Keck, Searle, Rita Allen, and Smith foundations. PWR is supported by the Howard Hughes Medical Institute. 205 E A E 206 Figure 1. Expression of hedgehog and ptc in the planarian brain (A) hedgehog transcript is detected near the animal midline, and signal is also present in the nerve cord. (B) hedgehog is expressed in chat' cells of the medial brain. (C) hedgehog expression in day 4 regenerating worms at the anterior wound site. (D) Three day regenerating tail piece with an anterior spot of hedgehog expression. (E) ptc is broadly expressed, with signal strongest in the CNS. Scale bars, 100pm (A, E), 50pm (B, C, D). 207 N "di' Swnlory CNS sM 208 sr -~ Figure 2. Distribution of cell types in the planarian brain. Animals are viewed from the ventral side with anterior at the top of each image. All green cell labelings show chat* cells unless otherwise specified. (A) chat expression in the head of an intact animal, optically sectioned from ventral to dorsal. (B-D) Expression of genes in lateral brain branches. (E-G) Expression of TRP channels in the PNS lateral to the brain branches. (H) trp-a, pkd2, trp-m are sparsely expressed along the entire surface of the animal. Shown is ventral surface posterior to the brain. (1)netrin-1 and (J) single-minded are expressed in the medial brain. (K) tph is expressed in medial and intermediate regions, including the domain of hb9 expression (L). Arrows point to hb9 positive cells in the merged image. (M) Colorimetric in situ hybridization showing hb9 expression (peripheral staining is non-specific background). (N) Schematic reconstruction of a transverse section through one lobe of the brain. CG: cephalic ganglion, VNC: ventral nerve cord. All scale bars 100pm. 209 Q Serotonergic hb9+Sensory- Midline. . Serotonergic.. hb9+ 210 Figure 3. Expression of conserved transcription factors in the intact planarian brain. (A-F) nkx2, pax6B, and nkx6 expression in the brain is shown in z-stack projections with a chat counterstain. (G-H) Magnified view (corresponding to area outlined in (F)) of nkx6 expression in a single lobe of the brain shows the boundary with pax6b. (l-J) dlx-2 is expressed in the lateral head area. (K-L) dlx is expressed with g-alpha in the lateral brain branch region. (M-N) msh-2 is expressed in the pkd2' lateral head area. Inset is a higher magnification image showing colocalization of msh-2 and pkd2 signal. (O-P) Planarian orthologs of the ventral spinal cord genes lhx3 and bhlhb5 are also expressed in the medial CNS. (Q) Schematic reconstruction of a transverse section through one lobe of the planarian brain and mouse neural tube (rotated 90 degrees), incorporating data from spinal cord and hindbrain at -E10.5 of development. Dix expression is drawn to reflect the expression of Dix family genes at the neural plate boundary and in the neural crest based on data from zebrafish. All scale bars 100pm. 211 Control C bmp4 RNAi glutamate m decarboxylase E cintilo Sim so-01 040- 040 0 0 600 4o $s,0 10 C?1(0 COP Control O bmp4 RNAi F H L P Q Up-m ji trp-8 .310- I 100 I z 00 Z 0\U~ 212 Figure 4. Effect of bmp4 RNAi on the lateral versus medial nervous system in the head All images show anterior blastemas at 7 days of head regeneration. (A-B) Effect of bmp4 RNAi on regeneration of cintillo and glutamate decarboxylase domains. Side panels show broadening of the cintillo domain along the D-V axis in bmp4(RNAi) animals. (C-E) quantification of the effect of bmp4 RNAi on the number of glutamate decarboxylase' cells (C; number of animals, n>8), the number of cintillo' cells (D; n > 8, p=.0005) and the area in pm 2 of the single-minded domain of expression (E; n=5). (F-G) single-minded domain is not affected by bmp4 RNAi. trp-m' cells, viewed from dorsal surface (H-I) or in the lateral head domain (J-K) are more abundant in bmp4 RNAi. trp-a' cells, viewed from dorsal surface (L-M) or in the lateral head domain (N-0) are less abundant in bmp4 RNAi. (P-Q) quantification of the effect of bmp4 RNAi on the number of trp-m* (P; n=4, p<.05) and trp-a' (Q; n=10, p<.001) cells versus unc-22 RNAi control. Error bars in graphs show S.E.M. P value is from two-tailed t-test. All scale bars 100pm. 213 214 Figure 5. hedgehog and patched are expressed in the medial brain and control photoreceptor regeneration and antero-posterior patterning. (A-B) The effect of RNAi of hh or ptc is shown in animals at day 8 of regeneration. Photoreceptors in (B) are labeled with anti-Arrestin antibody. Control animals rarely display abnormal eyes or tails (abnormal pigment cups n=6/54, abnormal tails n=4/55). hh(RNAi) animals sometimes display reduced pigment cups and have abnormal tails (abnormal pigment cups, n=21/50, reduced tails n=28/46). ptc(RNAi) animals display enlarged/fused pigment cups and larger than normal tails (abnormal pigment cups n=29/37, enlarged tails n=12/12). (C) Some ptc(RNAi) animal have anterior tail-like growth (n=6/37). Arrow shows anterior tail. (D) Anterior regions of eight day regenerates showing expression of the posterior marker wntP-1. unc-22(RNAi) animals do not have wntP-1 expression in the anterior (n=0/3 with anterior expression), nor do ptc(RNAi) animals that have pigment cups (n=0/22). (Pigment cups are outlined in red based on pigment that is visible in magnified images.) wntP-1 staining was detected in a subset of ptc(RNAi) animals with no pigment cups (n=2/7). (E)-(F) ptc(RNAi) animals display severe brain defects at medial and lateral positions, as detected using sim and cintillo expression (n>5). All scale bars 100pm. 215 tON Gi) -I, m 0 1(1^ -/ , 0, I *____'___ '~ -- Fold enrichment CO ptc/control ptclhh hh/control > Figure 6. RNA-seq identifies a role for Hh signaling in regulating gene expression in the medial brain. (A) Fold difference in expression level of indicated genes as determined by ratio of BaseMean (DESeq) in RNA-seq dataset. Genes were selected for analysis that had expression in ptc RNAi that was higher than control, and expression in hh RNAi that was lower than in control. (B) Expression patterns for several genes identified by the criteria in (A). (C) pcdh19 and nf are expressed in the spongy region of the brain and overlap in expression. (D) pcdhI and nf are detected in projections in the vicinity of the cell body. (E) netrin-2 is also expressed in the spongy region of the brain, but netrin-2+ cells do not express pcdh19. (F-G) pcdh19* cells do not express chat or the pan-neuronal marker, synapsin. Scale bars 250 pm(B), 20pm (D), and 50pm (C and E-G). 217 00 sim pax6b nkx6 nkx2 DcdhlQ nf Figure 7. hh signaling regulates the nf'Ipcdh' cell type, but does not regulate expression of nkx2, nkx6, pax6b, or sim. (A) pcdh19* cells are adjacent laterally to the hh expression domain. (B) pcdh19* cells also express ptc. (C) After 4 weeks of RNAi, hh(RNAi) animals display greatly reduced expression of pcdh19 and nf (n=7/7). By contrast, ptc(RNAi) animals display an increase in the number of cells expressing these markers, as well as ectopic expression along the head rim (n=7/7). (D) RNAi of hh and ptc does not ovetly affect expression of the indicated transcription-factor encoding genes, (n>3 for all genes tested). Scale bars, 50pm. 219 - Hs_H89_NP_005506.3 O- GgjSonlicHhNP990152.1 fhs_onlciNP_000184.1 GL_Mnx_NP_990259.1 GgndHh_NP_99028.1 Dr_MnxNP_001009885.1 0.8 Rs 0.53 -CiHB9_NP_001122328.1 s - 0.57 57 PdHb9_AB093220.1 DesHhNP_066382.1 hdloHh NP 002172.2 Dr_IndHh__NP_001030165.2 DrSonctlh_NP_571138.1 NvMixABG67770.1 -Smed-Hedgehog sk_MnxAD97274.1 Bf_h_XP_002222723.1 0.& Bf_Mn__XP__002225703.1 Dm 0.99 0.66 HhB NP 001034065.1 -Tch_NP__001107837.1 Dm__exrexa_NP_648164.1 Sk__lbABD97267.1 Smed-Hb9 NvHhl_ ABX89897.1 - 0.77 Dm_cngaihedANP_523700.2 Ci _ Hhl NP 001027634.1 Cl__HU__NP__001027635.1 BfLenalledXP_002212355.1 - - sengrailedl -- 0.71 001417.3 NP GgMilN99 9.9 NvHedg&Ung_ABX8414.1 Nvh_ABX899&1 I Dr__Nk2-lbNP_57185L1 Hs oll - Hi_Msxl_NP__00239.2 - Dr__Msi:__NP_57134.1 Dr MsxD NP 571151 NP Nk24 Dr__Nk24_NP_001104636.1 - CA33M. -k2I m 2 149416.1 _Tfl__NP_001073136.1 med-Nkx2 HS MSX2 NP 002440.2 Dm__ScarecrowNP_010154 Gg_.Mx2 NP Dr 0.94 Hs 9I. Nk2-2_NP_0025M.1 Dr_ Nk22__NP_571497.1 MsxBNP571335.1 _ _ - Dr__MC__NP_571347.1 HiNk2 _NI _OS1lh.2 Dr_Nk2-9_NP_0109373.1 Sf__Nk2-2_XP_0222192.1 NV_Mix _1AG115.1 D. 039 SkMixABD97281., 0 f__Msx_XP_(02212819.1 NwNk2cMAbWA-W I Nv_Nk2b_ABb8642. Nv_Nk42a_A1A48.1 Nv_Nk2dABH6449. 07 Pd_Msx_CA13M101 3k_Nk2-2_AiW97281.1 Dm_ dropNP_ 4724.1 0.6; Smed-Msh-2 Mos_7425_NP_0037&. Dr_N2-5_NP_-57146. Sk_1k2-35 (2_Mx_CAD6691.1 ASD9727.1 r_Nk2-3.__NP_571498.1 -- Di_unman_.NP_._52403.1 lis__Nk2.4-_ __0743 HsNk2-3_NP__6632.2 8.1 s_.Mal Pd Vf Nk4 XP 002223325.1 Msx _NP_00439.2 CA138810.1 Nv _Ms_AGI598.1 220 Supplemental Figure 1. Orthology of genes. Branch nodes display posterior probabilities. 221 DrNk6NP001002475.1 -- 0.97 BfNk6.l_XP_002234335.1 -- 04 spatchcdl NP 00255.2 C. 0 jached_NP_ 510291. HsNk6_NP_006159.2 - i-__Nk6.2_NP_79374.1 Hs.pthcd2_NP_003729.3 0.52 L 0.85 Gg._Nk6_XP_421832.2 DrNk6.2_NP_001129256.1 L~~~td_NP57102.1 Q2.pachd_XV'__0021752.1 0.% CiNk6X_002127499.t l-l__..pLchdixI_431220949.i Dr_ Nk-3__XP_684904.1 30_patchbd_X_002219b79.1 NvNk6_ABG67784.1 0.62 Nv__patchcd_AAX89d96.1 Gg_Nk6-3_XP_428808.2 Ce cog-lNP 001022264.1 6 0.61 )zxpa110 5d_NO'_52361.2 PdNk6_A093211.1 0.5 Ce( -- Smed-Nkx6 0.93 3 NP 4941462 _jr 1_-49%6.2 Dk_1GTX-A_NP_652614.1 Smed-Patched Dm_HGTX-B__NP_001137954.1 - DrHox6-_NP_00110699.1 -- PdNkABQ0644.1 0. 01 ...pdcl Ni' 775766.2 l_lox6-1_NP_061815.2 BfNkx5_XP_002239995.1 (20_Dlxia_NP_5713W.1 --Hs5m2_NP_005060.1 - 1DrSi.2_NP_571911.1 Gg_Sim X 14_i~xi 0.G_ D 416724.2 . HsSital_NP_005059.2 0.6 03522.2 Ni 0i 401039307.2 Ni' 00i074359.i GIDx6 0.96 NJ' 043Di6_NP_025213.2 0.9( 0.53 l_XP_425565.2 Gapdc __ L D_-Dix6a_NP_57139.1I IDriNlMa_N_571375.1 7 Gg_SimlXP_41981 .2 CI-DI DrSiml__NP__835740.1 -N 001027821 1 Sk__Dix__AAP793AI Smed-Dlx-1 Bf.SimCAD44626.1 PdDxi__CA13799.1 PdSim_AB093219.1 (2.0m_1ixA_Ni'_523057.. 0.91 Ce_ch43 _N'P_497904.1 Sk_Sim._AD97275.1 IN4 I_ Smed-Single-minded 0.98 _Dlx 9DmSImANP_5243402 _AUG67787LI Ci_DliNi_00102771 XE _2212363.1 Smed-Dlx-2 Dm_S.B_NP_731771.3 Pd__sx _CAJ38810.1 ClSmXP002119436.1 SNvMsxKAGII98.1 . CNpas4_XP_002123352.1 .cXPNi_012439.2 t _ N4_X 002223325.1 0.98 HsNpas4_NP_849195.2 Dm H%_Npal_NP__00250M.2 222 ti2na NP 524431.1 Supplemental Figure 1, continued. Orthology of genes. Branch nodes display posterior probabilities. 223 IOWO 1 - !u Cl Cl Supplemental Figure 2. Phenotypes of gli2-1 and Sufu RNAi. (A) g1i2-1(RNAi) animals have similar abnormalities as hh(RNAi) animals (abnormal pigment cups: n=32/50 vs 9/31 for control. abnormal tail: 43/50 vs 2/29 for control). Images are of live worms, 8 days of regeneration. (B) sufu(RNAi) animals have similar abnormalities as ptc(RNAi) animals with respect to fused eyes (n=1 3/20 for sufu vs 0/10 for control). Images are of fixed worms, 8 days of regeneration. Scale bars, 1 OOpm. 225 REFERENCES Adamidi, C., Wang, Y., Gruen, D., Mastrobuoni, G., You, X., Tolle, D., Dodt, M., Mackowiak, S.D., Gogol-Doering, A., Oenal, P., et al. (2011). De novo assembly and validation of planaria transcriptome by massive parallel sequencing and shotgun proteomics. Genome research 21, 1193-1200. Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count data. Genome biology 11, R106. Arber, S., Han, B., Mendelsohn, M., Smith, M., Jessell, T.M., and Sockanathan, S. (1999). Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23, 659-674. Arendt, D., and Nubler-Jung, K. (1999). Comparison of early nerve cord development in insects and vertebrates. Development 126, 2309-2325. Bijlsma, M.F., Spek, C.A., and Peppelenbosch, M.P. (2004). Hedgehog: an unusual signal transducer. Bioessays 26, 387-394. Briscoe, J., Pierani, A., Jessell, T.M., and Ericson, J. (2000). A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101, 435-445. Briscoe, J., Sussel, L., Serup, P., Hartigan-O'Connor, D., Jessell, T.M., Rubenstein, J.L., and Ericson, J. (1999). Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398, 622-627. Cantarel, B.L., Korf, I., Robb, S.M., Parra, G., Ross, E., Moore, B., Holt, C., Senchez Alvarado, A., and Yandell, M. (2008). MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome research 18, 188-196. Cebrid, F., Guo, T., Jopek, J., and Newmark, P.A. (2007). Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Developmental biology 307, 394-406. Cebria, F., Kobayashi, C., Umesono, Y., Nakazawa, M., Mineta, K., Ikeo, K., Gojobori, T., Itoh, M., Taira, M., Sanchez Alvarado, A., et al. (2002). FGFR-related gene noudarake restricts brain tissues to the head region of planarians. Nature 419, 620-624. Cebrib, F., and Newmark, P.A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132, 3691-3703. 226 Chen, Y., and Struhl, G. (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553-563. Chiang, C., Litingtung, Y., Lee, E., Young, K.E., Corden, J.L., Westphal, H., and Beachy, P.A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413. Clement, V., Sanchez, P., de Tribolet, N., Radovanovic, I., and Ruiz i Altaba, A. (2007). HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell selfrenewal, and tumorigenicity. Curr Biol 17, 165-172. Denes, A.S., Jekely, G., Steinmetz, P.R., Raible, F., Snyman, H., Prud'homme, B., Ferrier, D.E., Balavoine, G., and Arendt, D. (2007). Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129, 277-288. Dessaud, E., McMahon, A.P., and Briscoe, J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489-2503. Dunn, C.W., Hejnol, A., Matus, D.Q., Pang, K., Browne, W.E., Smith, S.A., Seaver, E., Rouse, G.W., Obst, M., Edgecombe, G.D., et al. (2008). Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745-749. Ericson, J., Rashbass, P., Schedl, A., Brenner-Morton, S., Kawakami, A., van Heyningen, V., Jessell, T.M., and Briscoe, J. (1997). Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169-180. Fan, C.M., Kuwana, E., Bulfone, A., Fletcher, C.F., Copeland, N.G., Jenkins, N.A., Crews, S., Martinez, S., Puelles, L., Rubenstein, J.L., et al. (1996). Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Molecular and cellular neurosciences 7, 1-16. Harris, R., Sabatelli, L.M., and Seeger, M.A. (1996). Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17, 217-228. Hooper, J.E., and Scott, M.P. (2005). Communicating with Hedgehogs. Nat Rev Mol Cell Biol 6, 306-317. Hyman, L.H. (1951). The Invertebrates: Platyhelminthes and Rhynchocoela the acoelomate Bilateria, Vol 11 (New York, McGraw-Hill Book Company, Inc). 227 Jacob, J., and Briscoe, J. (2003). Gli proteins and the control of spinal-cord patterning. EMBO reports 4, 761-765. Kaji, T., and Artinger, K.B. (2004). dlx3b and dIx4b function in the development of Rohon-Beard sensory neurons and trigeminal placode in the zebrafish neurula. Developmental biology 276, 523-540. Kidd, T., Bland, K.S., and Goodman, C.S. (1999). Slit is the midline repellent for the robo receptor in Drosophila. Cell 96, 785-794. Kobayashi, C., Saito, Y., Ogawa, K., and Agata, K. (2007). Wnt signaling is required for antero-posterior patterning of the planarian brain. Developmental biology 306, 714-724. Langmead, B., Trapnell, C., Pop, M., and Salzberg, S.L. (2009). Ultrafast and memoryefficient alignment of short DNA sequences to the human genome. Genome biology 10, R25. Lapan, S.W., and Reddien, P.W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS genetics 7, e1002226. Litingtung, Y., Dahn, R.D., Li, Y., Fallon, J.F., and Chiang, C. (2002). Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979-983. Lowe, C.J., Wu, M., Salic, A., Evans, L., Lander, E., Stange-Thomann, N., Gruber, C.E., Gerhart, J., and Kirschner, M. (2003). Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853-865. Lu, Q.R., Yuk, D., Alberta, J.A., Zhu, Z., Pawlitzky, I., Chan, J., McMahon, A.P., Stiles, C.D., and Rowitch, D.H. (2000). Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25, 317-329. Mannini, L., Deri, P., Gremigni, V., Rossi, L., Salvetti, A., and Batistoni, R. (2008). Two msh/msx-related genes, Djmshl and Djmsh2, contribute to the early blastema growth during planarian head regeneration. The International journal of developmental biology 52, 943-952. Marigo, V., and Tabin, C.J. (1996). Regulation of patched by sonic hedgehog in the developing neural tube. PNAS 93, 9346-9351. McEwen, D.P., Jenkins, P.M., and Martens, J.R. (2008). Olfactory cilia: our direct neuronal connection to the external world. Current topics in developmental biology 85, 333-370. 228 McLarren, K.W., Litsiou, A., and Streit, A. (2003). DLX5 positions the neural crest and preplacode region at the border of the neural plate. Developmental biology 259, 34-47. Mitchell, K.J., Doyle, J.L., Serafini, T., Kennedy, T.E., Tessier-Lavigne, M., Goodman, C.S., and Dickson, B.J. (1996). Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 17, 203-215. Mizutani, C.M., and Bier, E. (2008). EvoDNo: the origins of BMP signalling in the neuroectoderm. Nature reviews. Mizutani, C.M., Meyer, N., Roelink, H., and Bier, E. (2006). Threshold-dependent BMPmediated repression: a model for a conserved mechanism that patterns the neuroectoderm. PLoS biology 4, e313. Molina, M.D., Sal6, E., and Cebrib, F. (2007). The BMP pathway is essential for respecification and maintenance of the dorsoventral axis in regenerating and intact planarians. Developmental biology 311, 79-94. Monnier, V., Dussillol, F., Alves, G., Lamour-Isnard, C., and Plessis, A. (1998). Suppressor of fused links fused and Cubitus interruptus on the Hedgehog signalling pathway. Curr Biol 8, 583-586. Nakazawa, M., Cebris, F., Mineta, K., Ikeo, K., Agata, K., and Gojobori, T. (2003). Search for the evolutionary origin of a brain: planarian brain characterized by microarray. Molecular biology and evolution 20, 784-791. Nederbragt, A.J., van Loon, A.E., and Dictus, W.J. (2002). Evolutionary biology: hedgehog crosses the snail's midline. Nature 417, 811-812. Nery, S., Wichterle, H., and Fishell, G. (2001). Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 128, 527-540. Nicolay, D.J., Doucette, J.R., and Nazarali, A.J. (2007). Transcriptional control of oligodendrogenesis. Glia 55, 1287-1299. Nishimura, K., Kitamura, Y., Inoue, T., Umesono, Y., Yoshimoto, K., Takeuchi, K., Taniguchi, T., and Agata, K. (2007). Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesiajaponica. Neuroscience research 59, 101-106. Odden, J.P., Holbrook, S., and Doe, C.Q. (2002). Drosophila HB9 is expressed in a subset of motoneurons and interneurons, where it regulates gene expression and axon pathfinding. J Neurosci 22, 9143-9149. Okamoto, K., Takeuchi, K., and Agata, K. (2005). Neural projections in planarian brain revealed by fluorescent dye tracing. Zoological science 22, 535-546. 229 Oviedo, N.J., Newmark, P.A., and Sinchez Alvarado, A. (2003). Allometric scaling and proportion regulation in the freshwater planarian Schmidtea mediterranea. Dev Dyn 226, 326-333. Pearson, B.J., Eisenhoffer, G.T., Gurley, K.A., Rink, J.C., Miller, D.E., and Sanchez Alvarado, A. (2009). Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238, 443-450. Persson, M., Stamataki, D., te Welscher, P., Andersson, E., Bose, J., Ruther, U., Ericson, J., and Briscoe, J. (2002). Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes & development 16, 2865-2878. Petersen, C.P., and Reddien, P.W. (2008). Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319, 327-330. Pineda, D., Rossi, L., Batistoni, R., Salvetti, A., Marsal, M., Gremigni, V., Falleni, A., Gonzalez-Linares, J., Deri, P., and Sblo, E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. Qi, Y., Cai, J., Wu, Y., Wu, R., Lee, J., Fu, H., Rao, M., Sussel, L., Rubenstein, J., and Qiu, M. (2001). Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128, 2723-2733. Ramos, C., and Robert, B. (2005). msh/Msx gene family in neural development. Trends Genet 21, 624-632. Reddien, P.W., Bermange, A.L., Kicza, A.M., and S nchez Alvarado, A. (2007). BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 134, 4043-4051. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and Sanchez Alvarado, A. (2005). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Rink, J.C., Gurley, K.A., Elliott, S.A., and Sanchez Alvarado, A. (2009). Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326, 1406-1410. Robel, S., Berninger, B., and Gotz, M. (2011). The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12, 88-104. Rosenbaum, J.L., and Witman, G.B. (2002). Intraflagellar transport. Nat Rev Mol Cell Biol 3, 813-825. Sander, M., Paydar, S., Ericson, J., Briscoe, J., Berber, E., German, M., Jessell, T.M., and Rubenstein, J.L. (2000). Ventral neural patterning by Nkx homeobox genes: Nkx6.1 230 controls somatic motor neuron and ventral interneuron fates. Genes & development 14, 2134-2139. Schmierer, B., and Hill, C.S. (2007). TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8, 970-982. Schnapp, E., Kragl, M., Rubin, L., and Tanaka, E.M. (2005). Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development 132, 3243-3253. Serafini, T., Colamarino, S.A., Leonardo, E.D., Wang, H., Beddington, R., Skarnes, W.C., and Tessier-Lavigne, M. (1996). Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001-1014. Stone, D.M., Hynes, M., Armanini, M., Swanson, T.A., Gu, Q., Johnson, R.L., Scott, M.P., Pennica, D., Goddard, A., Phillips, H., et al. (1996). The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature 384, 129-134. Thomas, J.B., Crews, S.T., and Goodman, C.S. (1988). Molecular genetics of the singleminded locus: a gene involved in the development of the Drosophila nervous system. Cell 52, 133-141. Timmer, J.R., Wang, C., and Niswander, L. (2002). BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459-2472. Tsujikawa, M., and Malicki, J. (2004). Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron 42, 703-716. Umesono, Y., and Agata, K. (2009). Evolution and regeneration of the planarian central nervous system. Development, growth & differentiation 51, 185-195. Venkatachalam, K., and Montell, C. (2007). TRP channels. Annual review of biochemistry 76, 387-417. Von Stetina, S.E., Fox, R.M., Watkins, K.L., Starich, T.A., Shaw, J.E., and Miller, D.M., 3rd (2007). UNC-4 represses CEH-12/HB9 to specify synaptic inputs to VA motor neurons in C. elegans. Genes & development 21, 332-346. Watson, J.D., Wheeler, S.R., Stagg, S.B., and Crews, S.T. (2011). Drosophila hedgehog signaling and engrailed-runt mutual repression direct midline glia to alternative ensheathing and non-ensheathing fates. Development 138, 1285-1295. Yazawa, S., Umesono, Y., Hayashi, T., Tarui, H., and Agata, K. (2009). Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. PNAS 106, 22329-22334. 231 232 Chapter 5 Discussion 233 This thesis describes discoveries pertaining to the biology of the planarian eye, and its usefulness as a system for exploring progenitor dynamics during regeneration. As an experimental system the planarian eye has many advantages. 1. Eyes are among the few organs in planarians that are highly localized. Many organs, such as the intestine, muscle, epidermis, and the excretory system, are diffusely distributed along the A-P axis. Precise localization facilitates application of surgical strategies that completely remove the eyes, allowing for observation of entirely de novo eye regeneration in numerous contexts. Moreover, a discrete position facilitates the definition of proximal and distal positions when describing progenitor dynamics. 2. The natural pigmentation of eyes facilitates macroscopic identification. Eye pigmentation is critical for allowing collection of eyes during purification, and allows precise targeting during surgical manipulations. Futhermore, pigmentation allows eye defects to be readily observed during routine screens of live animals (Reddien et al., 2005a), and this fact is responsible for the initial discovery of a number of eye regulators such as dlx. 3. The eye is not required for viability, allowing severe eye defects to be analyzed without confounding factors related to overall animal health and tissue integrity. Phenotypes affecting most other organs systems lead to lethal phenotypes, such as bloating, lesions, or an inability to eat (Scimone et. al., 2011 and unpublished observations). However, eyeless planarians are able to eat, and homeostatic maintenance of other organs is apparently normal. As an illustration of this, we were able to conduct ovo RNAi experiments for months with no adverse affects aside from eye loss. Beyond being a useful experimental system, the planarian eye is a remarkable structure. Although classically referred to as simple or prototypic, our understanding of 234 the complexity of this structure is growing. Data presented in this thesis and from other groups describe an unexpected variety of gene expression profiles among photoreceptor neurons (Collins et al., 2011). Two transcription factors have now been described that are required for specification of subsets of photoreceptor neurons (Gonzalez-Sastre et al., 2012). The significance of this heterogeneity, however, remains to be elucidated along with many other basic aspects of planarian eye biology and vision. What is the basic planarian phototransduction cascade? Is there a tissue similar to lens or cornea for condensing light? Does the pigment cup respond to light to generate a pupillary response? Do pigment cells phagocytose photoreceptive organelles, as in vertebrates? Our gene expression profile of the eye presents an entry point for addressing these and other questions, which will ultimately allow the planarian eye to be appreciated for its intricacy as well as its relative simplicity. I. Cross-species comparison of transcriptional regulation of eye formation cDNA sequencing of purified planarian eye tissue resulted in a dataset that included a high percentage of genes expressed in the eye while exhibiting low false discovery rates. None of the genes explicitly described in the literature to lack expression and function in the eye were represented by sequencing reads. Furthermore, all previously identified genes with expression and function in the eye were identified as highly enriched. The dataset may not be complete, as genes required only at early stages of eye lineage determination may not be detectable using the present eye purification scheme. Nonetheless, a large number of regulators of eye formation were identified such that meaningful comparisons to other organisms regarding the function of transcriptional regulators in eye formation can now be made. Expression data and functional analyses suggest a model wherein the transcription factors ovo, six-1/2, and eya are required for formation of all eye 235 progenitors from the earliest detectable stages. In addition, otxA is required specifically for formation of photoreceptor neurons, whereas sp6-9 and dlx are required for optic pigment cells. soxB and a recently described inhibitory smad (Gonzalez-Sastre et al., 2012) are required for formation of anterior photoreceptors specifically. Several aspects of this pathway are notable with respect to comparative transcriptional regulation of eye formation. First, planarians parallel Drosophila in that sine oculis (a Six-1/2 gene) and eyes absent are required for formation of the entire Drosophila eye, and orthodenticle is required only for photoreceptor neurons. This differs in subtle but important ways from vertebrate eye development, where Eya and Six3 (Oliver et al., 1995), not Six-1/2, are required for eye formation. Furthermore Otx2 is involved in both retinal pigment epithelium (RPE) formation and photoreceptor neuron specification in mouse (MartinezMorales et al., 2003). A further difference regarding RPE formation is that neither Drosophila nor planarians apparently rely on MitF, an important regulator of RPE specification, for formation of optic pigment cells (Hallsson et al., 2004). Therefore, despite the similarities we observe between planarian pigment cell function and RPE function with respect to solute transport and pigmentation (Chapter 3), there are key differences at the level of transcriptional regulation. The absence of a function for Pax6 orthologs in planarian eyes has been noted previously (Pineda et al., 2002). Our sequencing data and expression analysis in embryos support the argument that pax6A and pax6B are not expressed in planarian eyes. Pax6 has been described as an ancestral eye gene, and evidence for this claim comes from three principal sources. First, Pax6 genes have a prominent role in eye formation in mouse and Drosophila; second, mouse Pax6 can drive ectopic eye formation when expressed in Drosophila imaginal disc; third, Pax6 orthologs are expressed in a variety of animal eyes at distinct phylogenetic positions (Gehring, 2004). 236 There are several points to be made in reply to these observations (Harris, 1997). Although Pax6 genes are important for mouse and Drosophila eye development, their functions differs between these animals. For instance, pax6 in Drosophila is required for eye formation from the earliest stages of imaginal disc specification, whereas in the Pax6 knockout mouse, optic vesicles form and even become patterned to make RPE and neural retina (Ashery-Padan and Gruss, 2001). Second, the ability of mouse Pax6 to drive eye formation in Drosophila does not imply that these genes have the same set of targets in the eye. For instance, it may be sufficient for heterologous Pax6 to activate a handful of targets in Drosophila for eye development to be triggered (Harris, 1997). Third, the list of animals in which Pax6 orthologs are not expressed in the eye has grown considerably (Vopalensky and Kozmik, 2009). Finally, it is important to note the extent to which this gene is expressed in nervous systems. In the mouse, Pax6 is expressed and functions in a variety of brain regions and sensory placodes (Georgala et al., 2011; Li et al., 1994). It remains entirely possible that Pax6 genes functioned in the ancestral eye. However it is also apparent that they are not required to make an eye in many species. There are now several known instances in which global regulators of eye formation may be phylum-specific. Rax is required for eye formation in vertebrates (Furukawa et al., 1997), but orthologs are not required for Drosophila or planarian eye formation (Davis et al., 2003; Mannini et al., 2008). Furthermore, dachshund is required for formation of the Drosophila eye (Mardon et al., 1994), but not for the planarian or mouse eye (Davis et al., 2008; Lapan and Reddien). It remains to be determined whether ovo is another example of a phylum-specific eye regulator or whether its central function is conserved to other groups. 237 II. The cellular lineage of planarian eye cells This study opens many questions regarding the lineage and potency of planarian eye progenitors. It is not clear whether ovo+Isix-1/2+/eya+ progenitor cells represent self-renewing stem cells or merely transiently amplifying cells. It clearly is possible to generate these eye progenitors de novo during adulthood because eyes and eye progenitors can form in regenerating tissue fragments, such as the tail, that completely lack eye progenitors in intact animals. Furthermore, transplantation experiments demonstrate that pluripotent cells exist in planarians. Single pluripotent cells can give rise, over periods of weeks, to new eye tissue (Wagner et al., 2011). These experiments do not, however, preclude the possibility that long-term stem cells more restricted to the eye lineage exist in intact animals. Regardless of whether ovo+/six-1/2+/eya+ are stem cells or transit amplifying, the potency of cells in this population remains unknown. Nonoverlapping expression of otxA and sp6-9 in the eye progenitor population indicates that pigment cell and photoreceptor neuron progenitors exist as distinct populations in much of the progenitor trail. However, there may be a single lineage-restricted progenitor cell type that gives rise to both of these populations. Following the development of suitable transgenic tools, it may be possible to genetically label eye progenitors for fate mapping studies. Genetic labeling using expression of tamoxifen-inducible Cre under an ovo promoter could determine whether ovo+ progenitors are a self-renewing population. Furthermore, if labeling can be induced with clonal efficiency, it may be possible to determine whether individual cells within this population are potent to produce a variety of cell types. Another technical approach would be to develop a scheme for isolation of eye progenitors among the neoblasts by flow cytometry. Neoblasts are currently isolated by Hoechst staining and forward-side scatter (Reddien et al., 2005b), but this technique is not likely to allow identification of 238 eye progenitors specifically. Combining sorting with cell surface antibody combinations specific to eye progenitors may allow purification. Subsequently, single-cell transplantation (combined with a labeling strategy) can be used to identify clone forming ability, which might simultaneously address the question of self-renewal capacity and potency. II. Migration during planarian regeneration Some degree of migration is to be expected during planarian regeneration, and has long been thought to be important during blastema growth (Dubois, 1949). The neoblasts are completely absent from many tissue regions that experience cell turnover, and yet appear in these locations following injury (Wenemoser and Reddien, 2010). However, it has not been possible to correlate migration with the state of differentiation for a specific lineage. We observe cells with expression characteristic of the eye among the neoblasts, often far from the regenerating eye, while cells close to the eye express markers of more differentiated photoreceptor neurons and pigment cells. Eye growth following irradiation and BrdU pulse experiments indicate that differentiated eye cells have migrated from the neoblast population while undergoing differentiation. Migration of progressively differentiating cells may be a common feature of regenerating nervous tissues. For instance, glutamatergic neuron progenitors in zebrafish brain regeneration express the transcription factor Tbr in progenitors near the ventricular zone, and express the more differentiated state marker Vglut2.2 in Tbr+ progenitors deeper in the brain tissue, subsequent to a presumed migratory event (Marz et al., 2011). Our initial characterization of eye progenitors depended upon using genes found fortuitously or by candidate screening. RNA-seq using cDNA from purified eyes of intact animals succeeded in a finding numerous additional genes expressed in eye progenitors. It may be surprising that analysis of intact eyes resulted in identification of 239 genes that have relevance to the progenitor state. This could relate to two main factors. First, the active patterning experienced by planarians in adulthood possibly requires the perdurance of some patterning factors in intact eyes following differentiation. Second, RNA-seq was conducted on eyes from actively growing animals, which are expected to have newly incorporated cells that retain some features of the progenitor state. The new genes found to be expressed in trail cells will be important in understanding the events that guide progenitor maturation. Several genes with potential roles in migration were identified including an ortholog of robo, which encodes a receptor for Slit, and two orthologs of unc5, which encodes a netrin receptor. Slit has been demonstrated to function as a directional cue in rostral migratory stream migration (Nguyen-Ba-Charvet et al., 2004). We also identified a member of the KIf transcription factor gene family required for normal localization of eye progenitors. In k/f RNAi, photoreceptor neurons are ventrally displaced, as are a subset of ovo+ progenitors posterior to the abnormal photoreceptor aggregate; it will be important to investigate whether this phenotype is the result of a defect in guidance during migration. In order to form an assembled eye, migratory cells much switch from a mesenchymal state to an aggregated state. Photoreceptor neurons are clustered on one side of the eye, and pigment cells on the other. Therefore, mechanisms regulating homotypic aggregation are likely to be central to eye morphogenesis. A number of putative mediators of adhesion were identified in our screen, including several protocadherins expressed specifically in the pigment cells or in the photoreceptors. It is currently unknown whether extensive migration is unique to eye progenitors, or whether it is a common features of organ formation in planarians. The excretory system is a second planarian organ system for which a progenitor population has now been identified (Scimone et al., 2011). Excretory system progenitors - defined by genes such as pou2/3, sall, and six-1/2-2 - are found in the neoblast population of regenerating 240 animals. However, early excretory progenitors were primarily detected in the vicinity of aggregated excretory primordia undergoing differentiation, and evidence does not exist for extensive cell migration of these progenitors. The distribution of brain progenitors has not been described. Brain-expressed genes such as ap-2 and pax6 are expressed in dispersed populations around the wound early in regeneration (Wenemoser et al., 2012). However, without combinatorial transcription factor labeling it is difficult to ascertain whether these are indeed brain progenitors, as they may also be progenitors for other lineages that transiently activate a gene expressed in the differentiated brain. The formation of pcdhl9+/nf+ glial-like cells may be a good system for studying progenitor formation in the brain. These cells are not required for animal viability, they are localized to only one region of the brain, and the source of a key signaling factor required for their formation has already been identified. Identification of genes expressed cell-autonomously during specification of pcdhl9+/nf+ cells will be useful for determining the localization of progenitors for this cell type. IV. The progenitor response to injury An important question in regenerative biology is the extent to which different types of injuries cause differential regulation of the injury response. The ability to respond to specific injuries with distinct programs leads to more efficient response; for instance, it would be counterproductive to form brain progenitors following an amputation that leaves the brain intact. The cellular response to wounding in planarians has been defined with a variety of markers that reveal generic aspects of the responses to tissue loss. All types of amputations in planarians yield body wide increases in mitotic events, followed by a high density of mitosis at the wound site, and accumulation of neoblast descendants (defined by generic markers such as SMEDWI-1 and NB21.11e) (Wenemoser and Reddien, 2010). The ability of planarians to regulate the wound 241 response is revealed by the response to simple wounding (in contrast to wounds that cause tissue loss). In the case of simple wounding, a rapid body-wide mitotic increase is observed, but mitotic increase and progenitor accumulation at the wound site are not observed (Wenemoser and Reddien, 2010). In the absence of more specific progenitor markers, it has been difficult to ask whether neoblasts respond differently depending on the type of tissue removed by amputation. Using ovo as a marker for eye progenitors, we observe that animals lacking an anterior induce large amounts of new eye progenitors, whereas those lacking a posterior never induce ovo+ cells. Therefore, neoblasts are able to display distinctly different response to these vastly different injury types. The next phase of experimentation will require observing the progenitor response to more subtly different injuries. First, what is the range of injuries to which the animal will not respond by generating eye progenitors? Second, what is the smallest injury to the eye, or eyecontaining regions, that can elicit specific responses among progenitors? Questions of this nature are relevant to many neurogenic systems. Damage to the striatum induces neurogenesis in subventricular zone, but damage to the olfactory bulb - the normal destination of progenitors - does not (Arvidsson et al., 2002; Kirschenbaum et al., 1999). Furthermore, teleost retinal regeneration is triggered by the death of some cell types, but not others (Braisted and Raymond, 1993; Vihtelic and Hyde, 2000). The identification of progenitors that form planarian eyes, combined with the ability to surgically perturb the planarian body plan in diverse ways, introduces a highly relevant system for investigating the regulation of progenitor formation during regeneration. 242 REFERENCES Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z., and Lindvall, 0. (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature medicine 8, 963-970. Ashery-Padan, R., and Gruss, P. (2001). Pax6 lights-up the way for eye development. Current opinion in cell biology 13, 706-714. Braisted, J.E., and Raymond, P.A. (1993). Continued search for the cellular signals that regulate regeneration of dopaminergic neurons in goldfish retina. Brain Res Dev Brain Res 76, 221-232. Collins, J.J., 3rd, Hou, X., Romanova, E.V., Lambrus, B.G., Miller, C.M., Saberi, A., Sweedler, J.V., and Newmark, P.A. (2011). Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS biology 8, e1000509. Davis, R.J., Harding, M., Moayedi, Y., and Mardon, G. (2008). Mouse Dach1 and Dach2 are redundantly required for Mullerian duct development. Genesis 46, 205-213. Davis, R.J., Tavsanli, B.C., Dittrich, C., Walldorf, U., and Mardon, G. (2003). Drosophila retinal homeobox (drx) is not required for establishment of the visual system, but is required for brain and clypeus development. Developmental biology 259, 272-287. Dubois, F. (1949). Contribution A l'etude de la migration de cellules de regeneration chez les Planaires dulcicoles. . Bull Biol Fr Belg 83, 213-283. Furukawa, T., Kozak, C.A., and Cepko, C.L. (1997). rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. PNAS 94, 3088-3093. Gehring, W.J. (2004). Historical perspective on the development and evolution of eyes and photoreceptors. The International journal of developmental biology 48, 707-717. Georgala, P.A., Carr, C.B., and Price, D.J. (2011). The role of Pax6 in forebrain development. Developmental neurobiology 71, 690-709. Gonzalez-Sastre, A., Molina, M.D., and Salo, E. (2012). Inhibitory Smads and bone morphogenetic protein (BMP) modulate anterior photoreceptor cell number during planarian eye regeneration. The International journal of developmental biology 56, 155163. Hallsson, J.H., Haflidadottir, B.S., Stivers, C., Odenwald, W., Arnheiter, H., Pignoni, F., and Steingrimsson, E. (2004). The basic helix-loop-helix leucine zipper transcription factor Mitf is conserved in Drosophila and functions in eye development. Genetics 167, 233-241. 243 Harris, W.A. (1997). Pax-6: where to be conserved is not conservative. PNAS 94, 20982100. Kirschenbaum, B., Doetsch, F., Lois, C., and Alvarez-Buylla, A. (1999). Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J Neurosci 19, 2171-2180. Lapan, S.W., and Reddien, P.W. (2011). dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS genetics 7, e1002226. Li, H.S., Yang, J.M., Jacobson, R.D., Pasko, D., and Sundin, 0. (1994). Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Developmental biology 162, 181-194. Mannini, L., Deri, P., Picchi, J., and Batistoni, R. (2008). Expression of a retinal homeobox (Rx) gene during planarian regeneration. The International journal of developmental biology 52, 1113-1117. Mardon, G., Solomon, N.M., and Rubin, G.M. (1994). dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120, 3473-3486. Martinez-Morales, J.R., Dolez, V., Rodrigo, I., Zaccarini, R., Leconte, L., Bovolenta, P., and Saule, S. (2003). OTX2 activates the molecular network underlying retina pigment epithelium differentiation. The Journal of biological chemistry 278, 21721-21731. Marz, M., Schmidt, R., Rastegar, S., and Strahle, U. (2011). Regenerative response following stab injury in the adult zebrafish telencephalon. Dev Dyn 240, 2221-2231. Nguyen-Ba-Charvet, K.T., Picard-Riera, N., Tessier-Lavigne, M., Baron-Van Evercooren, A., Sotelo, C., and Chedotal, A. (2004). Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci 24, 1497-1506. Oliver, G., Mailhos, A., Wehr, R., Copeland, N.G., Jenkins, N.A., and Gruss, P. (1995). Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045-4055. Pineda, D., Rossi, L., Batistoni, R., Salvetti, A., Marsal, M., Gremigni, V., Falleni, A., Gonzalez-Linares, J., Deri, P., and Salo, E. (2002). The genetic network of prototypic planarian eye regeneration is Pax6 independent. Development 129, 1423-1434. Reddien, P.W., Bermange, A.L., Murfitt, K.J., Jennings, J.R., and Sanchez Alvarado, A. (2005a). Identification of genes needed for regeneration, stem cell function, and tissue 244 homeostasis by systematic gene perturbation in planaria. Developmental cell 8, 635649. Reddien, P.W., Oviedo, N.J., Jennings, J.R., Jenkin, J.C., and Sanchez Alvarado, A. (2005b). SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science 310, 1327-1330. Scimone, M.L., Srivastava, M., Bell, G.W., and Reddien, P.W. (2011). A regulatory program for excretory system regeneration in planarians. Development 138, 4387-4398. Vihtelic, T.S., and Hyde, D.R. (2000). Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. Journal of neurobiology 44, 289-307. Vopalensky, P., and Kozmik, Z. (2009). Eye evolution: common use and independent recruitment of genetic components. Philosophical transactions of the Royal Society of London 364, 2819-2832. Wagner, D.E., Wang, I.E., and Reddien, P.W. (2011). Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332, 811-816. Wenemoser, D., Lapan, S.W., Wilkinson, A.W., Bell, G.W., and Reddien, P.W. (2012). A molecular wound response program associated with regeneration initiation in planarians. Genes & development 26, 988-1002. Wenemoser, D., and Reddien, P.W. (2010). Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Developmental biology 344, 979991. 245