Mitochondrial DNA variation of a natural population
advertisement

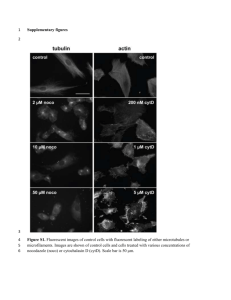
Parasitol Res (2007) 101:1439–1442 DOI 10.1007/s00436-007-0643-3 SHORT COMMUNICATION Mitochondrial DNA variation of a natural population of Gyrodactylus thymalli (Monogenea) from the type locality River Hnilec, Slovakia Charlotte Lindqvist & Laetitia Plaisance & Tor A. Bakke & Lutz Bachmann Received: 29 March 2007 / Accepted: 14 June 2007 / Published online: 12 August 2007 # Springer-Verlag 2007 Abstract The monogenean flatworm Gyrodactylus thymalli (Žitňan, Helminthologia, 2:266–269, 1960) is considered a harmless ectoparasite on grayling (Thymallus thymallus). The species is closely related to G. salaris Malmberg, 1957 that causes severe gyrodactylosis on Atlantic salmon (Salmo salar) in many Norwegian rivers. In this paper, we study the mitochondrial diversity of a G. thymalli population from one of the type localities Hrable on River Hnilec, Slovakia. By sequencing parts of the mitochondrial NADH dehydrogenase subunit 5 gene, we detected three haplotypes that differ from each other by 2.1–4.1%. The haplotype HnilecI was found most common. Our data suggest that River Hnilec has been colonized independently at least three times with G. thymalli. Introduction The monogenean flatworm Gyrodactylus thymalli was described from grayling (Thymallus thymallus) by Žitňan (1960) and is considered a harmless parasite. The closely related G. salaris Malmberg, 1957, however, has received C. Lindqvist (*) : L. Plaisance : T. A. Bakke : L. Bachmann Natural History Museum, Department of Zoology, University of Oslo, P.O. Box 1172 Blindern, 0318 Oslo, Norway e-mail: charlotte.lindqvist@nhm.uio.no Present address: L. Plaisance Scripps Institution of Oceanography, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0202, USA particular attention, as it is a severe pathogen on Atlantic salmon (Salmo salar) in many Norwegian rivers (Johnsen et al. 1999; Bakke et al. 2007). The parasite has been introduced to Norway via infected parr on at least three independent occasions by human activities (Hansen et al. 2003). There is a huge body of literature on G. salaris infecting Atlantic salmon in Norway, but still, very little is known about the parasite’s biology in natural populations that are not affected by human activities. In fact, it may be impossible to find G. salaris on undisturbed wild Atlantic salmon populations. In contrast, the closely related species G. thymalli occurs on relatively undisturbed host populations, and hence, studies of G. thymalli may offer deeper insights not only into the biology of this parasite–host association but into the biology of G. salaris as well. G. thymalli is widely distributed in Europe (Hansen et al. 2007; Bakke et al. 2007) and morphologically very similar to G. salaris (Shinn et al. 2004). Even the species’ internal transcribed spacers (ITS 1 and 2) of the nuclear ribosomal DNA cluster are identical (Ziętara and Lumme 2002). These molecular markers can otherwise discriminate the majority of Gyrodactylus species (Matejusová et al. 2001; Ziętara and Lumme 2002). In contrast, there is substantial mitochondrial DNA variation over the range of both G. salaris and G. thymalli (Hansen et al. 2003, 2006, 2007; Meinilä et al. 2004). Based on cytochrome oxidase I (COI) sequence data, 44 haplotypes are currently known that group into 11 well-supported clades (Hansen et al. 2007). In the Norwegian River Glomma for example, where until now eight mitochondrial haplotypes of G. thymalli have been detected, these haplotypes differ on average by 0.48% (Hansen et al. 2007). Along this river system, the different haplotypes were found at different sampling localities, but all belong to the same haplogroup (Hansen et al. 2006). However, very little is known about mitochondrial DNA 1440 Parasitol Res (2007) 101:1439–1442 Table 1 Sequence diversity between the NAD5 gene of the three mitochondrial haplotypes HnilecI, HnilecII, and HnilecIII of G. thymalli from the river Hnilec, Slovakia HnilecI HnilecII HnilecIII HnilecI HnilecII HnilecIII – 21 11a 4.14 – 16a 2.09 3.16 – of G. salaris (GenBank accession number: DQ988931; Huyse et al. 2007) and parts of the COI and NADH dehydrogenase subunit 5 (NAD5) genes have been identified as being among the most variable regions of the mitochondrial DNA. The p distance of the COI genes of these two mitochondrial genomes is 0.032 and that of the NAD5 genes is 0.030 (Plaisance et al. 2007). Parts of these two genes were sequenced for 43 G. thymalli specimens from 16 grayling host individuals; one parasite individual has been sampled from 11 fins, and from two fins, each six and eight parasite individuals were analyzed. DNA was extracted using the Qiagen DNeasy tissue kit (Qiagen) following the manufacturer’s instructions, except from the last elution step where DNA was eluted in 50 μl water. The following primer pairs were designed and used: Z1_F: 5′-TTATTTACTCTAGACCACAAGCG-3′ and Z1_R: 5′-CCCATATAACTATAGCAGTGTCCT-3′ amplify a 535-bp fragment of the COI gene, and Z2_F: 5′TTACTTCTCGTCAGCTGGGTA-3′ and Z2_R: 5′CTAAGCTTGATTCCCACCGGAA-3′ amplify a 530-bp fragment of the NAD5 gene. In a few cases, the ZMO primers from Hansen et al. (2003) were used to amplify the COI region. Polymerase chain reaction (PCR) was performed using either PuReTaq ready-to-go PCR beads (GE Healthcare) or Taq DNA polymerase (GE Healthcare), 1× buffer, 0.1 mmol/l of each diethylnitrophenyl thiophosphate, 0.4 μmol/l of each primer, and 5 μl of the extracted DNA in a 50-μl reaction volume. Thermal cycling conditions were the same for the two amplified mtDNA regions: 94°C for 3 min; 35 cycles of 94°C for 1 min, 50°C for 30 s, and 72°C for 45 s; and a final extension at 72°C for 5 min. PCR products were purified using 10× diluted exoSAP-IT (USB). Cycle sequencing, using the same primers as in the PCR reaction, was performed in 10-μl reactions using 2-μl BigDye terminator cycle sequencing ready reaction kit (Applied Biosystems), 2 μl 5× sequencing buffer, 10 pmol primer, and 3-μl cleaned PCR product. Sequencing products were purified with ethanol precipitation and analyzed using an ABI 3100 genetic analyzer (Applied Biosystems). DNA extracts have been deposited in the DNA/tissue collection of the Natural History Museum Oslo. Percent difference (above diagonal); number of substitutions (below diagonal). a The ambiguous positions at 14176 and 14278 in haplotype HnilecIII were not considered different. variation of Gyrodactylus within a population at a particular locality of a watercourse. According to the predictions of Price (1980), one would expect largely homogeneous parasite infra- and, to some extent, metapopulations due to the effect of genetic drift. However, other authors have documented mitochondrial DNA diversity within populations of helminth species that was at similar levels as in free-living animals (e.g., Blouin et al. 1992; Criscione and Blouin 2004). Due to the unique reproductive biology of Gyrodactylus, parasite offspring stay at first on the infected host individual, thereby creating rapidly growing infrapopupalations (Bakke et al. 2007), but emigration and direct or indirect transmission of parasites between host individuals may also be substantial in natural populations (Bakke et al. 1992). To understand the genetic patterns of a natural population of Gyrodactylus parasites and to test the former hypothesis, we studied mitochondrial DNA variation of G. thymalli on grayling in River Hnilec, Slovakia, one of the type localities according to Žitňan (1960). Materials and methods A total of 36 grayling (T. thymallus) were sampled at Hrable on River Hnilec, Slovakia on May 20th 2003. One complete mitochondrial genome of G. thymalli from the River Hnilec (GenBank accession number: EF527269) has recently been sequenced (Plaisance et al. 2007). This sequence has been compared to the mitochondrial genome Table 2 Nucleotide substitutions in the mitochondrial NAD5 gene of the three mitochondrial haplotypes HnilecI, HnilecII, and HnilecIII of G. thymalli from the river Hnilec, Slovakia Position 14110 14129 14136 14153 14176a 14191 14199 14212 14216 14236 14266 14278a HnilecI HnilecII HnilecIII A G . C T T T . C C T T G A R A G . T C . A . G T C . T C . G A A C T Y Parasitol Res (2007) 101:1439–1442 1441 Results and discussion In total, 59 sequences were determined, representing 22 COI and 37 NAD5 sequences. For 18 G. thymalli specimens, both COI and NAD5 sequences were determined. All obtained sequences of COI and NAD5 were identical to that of the recently sequenced mitochondrial genome of G. thymalli (Plaisance et al. 2007) at the exception of the sequences from two specimens. The most common haplotype, referred here to as HnilecI, was found in 41 (95.3%) of the studied parasites. The two other haplotypes, HnilecII (GenBank accession number: EU076809) and HnilecIII (GenBank accession number: EU076810), were rare, and each detected in only one G. thymalli specimen (2.3%). In our survey, all parasites sampled from the same fin had always the same mitochondrial haplotype. This was tested for 30 G. thymalli individuals (69.7% of the analyzed parasites) from five different grayling specimens (27.8% of the analyzed fins). The fins infected with G. thymalli haplotypes HnilecII and HnilecIII were only infected with one single parasite. The three haplotypes, HnilecI, HnilecII, and HnilecIII, differ from each other in the surveyed NAD5 regions as summarized in Tables 1 and 2. The substitutions are mainly transitions, which have been detected at 23 positions and two transversions (at positions 14416 and 14438; according to the G. thymalli complete mitochondrial; Plaisance et al. 2007). Such a strong transition/transversion (ti/tv) bias is commonly known and appears particularly pronounced in animal mitochondrial DNA (Brown et al. 1982). It has also been noted that at low levels of genetic divergence (<20% divergence), ti/tv appears to be high, whereas at high levels of genetic divergence, the two substitution types show equal frequencies (Yang and Yoder 1999). Of the total nucleotide substitutions, nine resulted in non-synonymous substitutions, all of which involved replacements of closely related amino acids. The difference between the NAD5 sequence of each of these three haplotypes is roughly of the same order of magnitude as the difference between either of them and the G. salaris NAD5 sequence in GenBank (accession number: DQ988931). The number of substitutions between this sequence and each of HnilecI, II, and III are 19, 20, and 15, respectively. The sequence dissimilarity between the NAD5 region of the haplotypes HnilecI, HnilecII, and HnilecIII is of the same range as that observed in COI sequences between G. thymalli and G. salaris belonging to different mitochondrial haplogroups (Huyse et al. 2007; Plaisance et al. 2007). Our data suggest that the G. thymalli population at the sampling locality, Hrable, on River Hnilec has been colonized independently at least three times with G. thymalli. The order of magnitude of NAD5 sequence variability between the three detected haplotypes (2.09– 4.14%) indicates that the three independent colonizations occurred through long-range dispersal, although the origin of these infections remains unknown. In other watercourses such as the Norwegian rivers Glomma or Trysil, sequence variation of G. thymalli haplotypes as estimated by COI sequences ranged clearly below 1% (Hansen et al. 2006, 2007). One can assume that this relatively low variability may have arisen after the initial colonization of the respective river systems. In contrast, the high genetic diversity of the three G. thymalli haplotypes at Hrable on River Hnilec indicates different evolutionary histories. Whether or not these putative independent colonizations can be related to human activities cannot be answered here. However, if one considered colonization through human activities such as, e.g., restocking or translocation of fish as a likely cause of the colonizations, then the present G. thymalli population in the river Hnilec cannot be considered natural any longer. G. thymalli in the river Hnilec has then provided yet another example on how easily pathogens can spread unnoticed. The results presented here support the importance of comprehensive species descriptions including both morphological and molecular parameters. It is important that DNA and/or tissue material of the type specimen(s) are deposited in scientific collections to allow for subsequent molecular identification or redescriptions of strains or species whenever necessary. The type material of G. thymalli is unfortunately not accessible for molecular approaches. Therefore, we cannot determine which of the mitochondrial haplotypes presented in this study, if any, at all, could be related to the specimens used by Žitňan (1960) in his original species description. Table 2 (continued) Position 14314 14353 14356 14394 14416 14425 14438 14454 14473 14480 14525 14540 14561 HnilecI HnilecII HnilecIII T C C C T . A . G T C C C A . A G . G C . T C C A G . A G . G A A T C . T . C Positions refer to the complete mitochondrial genome of G. thymalli from Hnilec, Slovakia, GenBank accession number: EF527269 (Plaisance et al. 2007). A dot (.) indicates an identical nucleotide when compared to haplotype HnilecI.a The ambiguous position could not be resolved for haplotype HnilecIII due to limited material. 1442 Acknowledgments We are greatful to Vladka Hanzelova for providing G. thymalli samples from Slovakia. The project was supported by the Norwegian Research Council Wild Salmon Programme (Project nr. 145861/720) and the National Centre for Biosystematics (Project nr. 146515/420), co-funded by the NRC and the NHM, University of Oslo, Norway. References Bakke TA, Harris PD, Jansen PA, Hansen LP (1992) Host specificity and dispersal strategy in gyrodactylid monogeneans, with particular reference to Gyrodactylus salaris Malmberg (Platyhelminthes, Monogenea). Dis Aquat Org 13:63–74 Bakke TA, Cable J, Harris PD (2007) The biology of gyrodactylid monogeneans: the “Russian doll-killers”. Adv Parasitol 64:161–460 Blouin MS, Dame JB, Tarrant CA, Courtney CH (1992) Unusual population genetics of a parasitic nematode: mtDNA variation within and among populations. Evolution 46:470–476 Brown WM, Prager EM, Wang A, Wilson AC (1982) Mitochondrial DNA sequences of primates, tempo and mode of evolution. J Mol Evol 18:225–239 Criscione CD, Blouin MS (2004) Life cycles shape parasite evolution: comparative population genetics of salmon trematodes. Evolution 58:198–202 Hansen H, Bachmann L, Bakke TA (2003) Mitochondrial DNA variation of Gyrodactylus spp. (Monogenea, Gyrodactylidae) populations infecting Atlantic salmon, grayling and rainbow trout in Norway and Sweden. Int J Parasitol 33:1471–1478 Hansen H, Martinsen L, Bakke TA, Bachmann L (2006) Incongruence of nuclear and mitochondrial DNA variation in the monogenean parasites Gyrodactylus salaris and G. thymalli: implications for diagnostics and taxonomy. Parasitology 133:639–650 Hansen H, Bakke TA, Bachmann L (2007) Mitochondrial haplotype diversity of Gyrodactylus thymalli (Platyhelminthes; Monogenea): extended geographic sampling in the United Kingdom, Parasitol Res (2007) 101:1439–1442 Poland, and Norway reveals further lineages. Parasitol Res 100:1389–1394 Huyse T, Plaisance L, Webster BL, Mo TA, Bakke TA, Bachmann L, Littlewood DTJ (2007) The mitochondrial genome of Gyrodactylus salaris (Platyhelminthes: Monogenea: Monopisthocotylea), a pathogen of Atlantic salmon (Salmo salar). Parasitology 134:739–747 Johnsen BO, Møkkelgjerd PI, Jensen AJ (1999) The parasite Gyrodactylus salaris on salmon parr in Norwegian rivers, status report at the beginning of year 2000. NINA Oppdragsmelding 617:1–129 (in Norwegian, English summary) Matejusová I, Gelnar M, McBeath AJA, Collins CM, Cunningham CO (2001) Molecular markers for gyrodactylids (Gyrodactylidae: Monogenea) from five fish families (Teleostei). Int J Parasitol 31:738–745 Meinilä M, Kuusela J, Ziêtara MS, Lumme J (2004) Initial steps of speciation by geographic isolation and host switch in salmonid pathogen Gyrodactylus salaris (Monogenea: Gyrodactylidae). Int J Parasitol 34:515–526 Plaisance L, Huyse T, Littlewood DTJ, Bakke TA, Bachmann L (2007) The complete mitochondrial DNA sequence of the monogenean Gyrodactylus thymalli (Platyhelminthes: Monogenea) a parasite of grayling (Thymallus thymallus) Mol Biochem Parasitol 154:190–194 Price PM (1980) Evolutionary biology of parasites. Princeton University Press, Princeton, New Jersey Shinn AP, Hansen H, Olstad K, Bachmann L, Bakke TA (2004) The use of morphometric characters to discriminate specimens of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol 51:239–252 Yang Z, Yoder AD (1999) Estimation of the transition/transversion rate bias and species sampling. J Mol Evol 48:274–283 Ziętara MS, Lumme J (2002) Speciation by host switch and adaptive radiation in a fish parasite genus Gyrodactylus (Monogenea: Gyrodactylidae). Evolution 56:2445–2458 Žitňan R (1960) Gyrodactylus thymalli sp. nov. aus den Flossen der Äsche (Thymallus thymallus). Helminthologia 2:266–269 (in German)