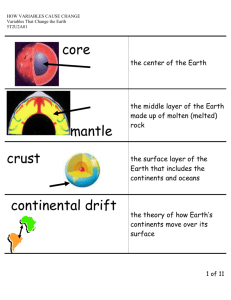
Density variations in the thickened crust as a function of... temperature, and composition
advertisement

Int J Earth Sci (Geol Rundsch) DOI 10.1007/s00531-010-0557-7 ORIGINAL PAPER Density variations in the thickened crust as a function of pressure, temperature, and composition Julia Semprich • Nina S. C. Simon Yuri Yu. Podladchikov • Received: 30 October 2009 / Accepted: 25 April 2010 Ó Springer-Verlag 2010 Abstract Constraints on density as a function of pressure, temperature, and composition are crucial to understand isostatic movements during geodynamic processes. Here, we provide a systematic series of density diagrams extracted from thermodynamic calculations for a variety of crustal compositions within a wide P–T range. We quantify systematic density changes in collisional settings for relevant compositional variations and attempt to simplify the density–composition relationships. Rock densities depend strongly on pressure, temperature, and composition. Densities at some selected pressure–temperature conditions increase linearly with increasing Al2O3 as well as MgO/ FeO contents in pelitic rocks. Al- and Fe-rich pelites yield the highest densities, which is mostly due to the formation of garnet but also depends on other minerals and changes of reactions. The effect of loading on densities is investigated, and we show that for deep burial, a meta-pelite rich in Fe and Mg yields much larger density changes than a dry basalt and that the burial of such a rock with a composition close to typical lower crust may result in significant negative buoyancy. Metamorphism of hydrous lower crust due to pressurization and heating thus leads to densification of thickened lower crust, while heating of dry crust leads to a decrease in density. Hence, water-loaded isostatic subsidence due to metamorphism of water-saturated lower crust is substantial and increases with the thickness and depth of the reacting layer, while dry compositions show much less or only transient densification and subsidence. The density change due to thermal expansion, an extensively used concept in geodynamic models, predicts uplift under the J. Semprich (&) N. S. C. Simon Y. Yu. Podladchikov Physics of Geological Processes, University of Oslo, Blindern, P.O. Box 1048, 0316 Oslo, Norway e-mail: julia.semprich@fys.uio.no same P–T conditions and is an order of magnitude smaller than the density variation calculated from petrologically consistent diagrams. Keywords Phase transitions Crustal densities Lower crust Basin formation Subsidence Mountain roots Introduction Crustal thickness commonly doubles in collisional settings causing a topography rise and the formation of deep foreland basins (Beaumont 1981; DeCelles and Giles 1996). The removal of topography by erosion does not necessarily lead to an isostatic rebound of crustal roots and basin basements (Kruse and McNutt 1988; Döring and Götze 1999). Based on analyses of gravity anomalies, mineral reactions in the lower crust were recently identified as the reason for the preservation of ancient crustal roots (Fischer 2002; James 2002). While past topography is difficult to constrain quantitatively, basin stratigraphy provides a highprecision record of vertical movements in collisional settings and potentially reflects the progress of metamorphic reactions in the lower crust. Such compressional basins are found in the foreland of mountain ranges (Beaumont 1981; DeCelles and Giles 1996), but may also be common in intra-continental settings, for example the Paleozoic intra-cratonic basins of North America (Sloss 1988). Intra-cratonic basins that are thought to be active today are the Hudson Bay, Chad, and Congo basins (Downey and Gurnis 2009). The Congo basin is currently under compressive stress (Ayele 2002) and is characterized by thick crust (Pasyanos and Nyblade 2007), as are for example the Williston basin in North America 123 Int J Earth Sci (Geol Rundsch) (Hamdani et al. 1994 and references therein) and the PreUralian Foredeep (Döring and Götze 1999). This implies that the crust-mantle boundary in these basins has been deepened due to thickening of the crust, and crustal material is exposed to upper mantle conditions. In some cases, this may lead to metamorphic phase transitions and densification, enhancing basin subsidence. Phase transitions have been suggested as a mechanism for basin subsidence since the late 1950s. Their relevance for basin formation has been extensively studied in a simplified approximation as one reaction line with a fixed density change Dq (Kennedy 1959; O’Connell and Wasserburg 1967; O’Connell and Wasserburg 1972; Haxby et al. 1976; Mareschal and Lee 1983; Mareschal 1987; Hamdani et al. 1994; Artyushkov 2005; Artyushkov 2010). However, phase transitions in natural rocks are complex and gradual, and their slope and position as well as the associated change in density depends on rock composition, in addition to pressure and temperature. Detailed studies of the influence of pressure, temperature, and composition (P–T-X) on densities have been provided in the context of extensional basins (Podladchikov et al. 1994; Yamasaki and Nakada 1997; Petrini et al. 2001; Kaus et al. 2005; Simon and Podladchikov 2008). In collisional settings, complex petrogenetic grids have been used to acquire density distributions in connection with the evolution of mountain belts (Bousquet et al. 1997; Le Pichon et al. 1997). Similar studies have been undertaken by for example Jull and Kelemen (2001), Tassara (2006), Massonne et al. (2007) and Hetényi et al. (2007) using thermodynamic calculations. To better understand the formation of intra-cratonic basins, foreland basins, and mountain belts, we need better constraints on the evolution of lower crustal densities in compressional settings as a function of pressure, temperature, and composition. Therefore, the main goal of this paper is to provide a systematic series of density diagrams extracted from thermodynamic calculations for a variety of crustal compositions. In addition, we examine which compositional changes are relevant, if they cause systematic density variations, and if and how we can simplify the density–composition relationships. Compositional variations in mixes with pelitic compositions are studied first; the results are compared to mafic rocks. We then demonstrate the relevance of the generated density diagrams by calculating subsidence as a consequence of loading. We discuss the application of our results to the preservation of orogenic roots and the formation of basins in compressional settings, with the intra-cratonic East Barents basin and the possibly related Uralian mountain range as examples. A more advanced model using fully dynamic 2-D thermo-mechanical finite element calculations including the realistic petrological P–T–density diagrams presented 123 here has been developed for the East Barents basin and will be published elsewhere (Gac et al. 2008 and Gac et al. manuscript in preparation). Methods and results Method of calculation All phase diagrams and petrologic densities were obtained with the Gibbs free energy minimization software Perple_X ‘07 (Connolly and Kerrick 1987; Connolly 1990; Connolly and Petrini 2002). Extensive documentation and downloads are provided by J. Connolly at http://www.perplex.ethz.ch. Perple_X uses the thermodynamic data set of Holland and Powell (1998) for minerals and aqueous fluids. Mineral abbreviations are listed in Table 1, and the solid solutions used for pelitic rocks can be found in Table 2. SiO2 polymorphs, aluminosilicate polymorphs (Ky, Sil, And), Kfs, Pg, Lws, Zo, and H2O are treated as pure phases. Solid solution models for amphiboles that would cause slight variations in the positions of the phase fields are ignored in the thermodynamic calculations for the pelitic compositions since the effect on the densities of these rocks is negligible. Pseudosections are calculated in the system K2O–Na2O– CaO–MgO–FeO–Al2O3–SiO2–H2O. For reasons of simplification, we initially assume that all iron is divalent and that all pelitic starting mixes are SiO2 and H2O saturated. MnO is ignored in the calculations since the MnO contents in the rock compositions used for our calculations are very low. However, increasing MnO would stabilize manganese-bearing minerals such as e.g., garnet over a wider P–T range (Symmes and Ferry 1992; Mahar et al. 1997; Tinkham et al. 2001). For a systematic discussion of compositional effects on rock densities, the P–T range is chosen between temperatures of 500–900°C and pressures of 0.05–3 GPa because we are mostly interested in deeply buried lower crustal rocks. The P–T range of a few selected compositions is then extended to a larger P–T window for the kinematic model (300–1,300°C and 0.6–4.5 GPa). Calculation of pelitic compositions In order to investigate the compositional variations in pelitic rocks, we calculate hypothetical pelitic mixes, which are composed of the following minerals: Qtz, Fsp, Ill, Chl, and Fe–Ti-oxides. The mineral compositions are listed in Table 3 and are taken from Massonne et al. (2007). Systematic variations of the whole rock compositions are generated by changing the proportions of these five minerals. Int J Earth Sci (Geol Rundsch) Table 1 List of mineral abbreviations and end-members used in the text Density diagrams of pelitic rocks Mineral Abbreviation Albite Ab Almandine Alm Amesite Ames Andalusite And Figure 2a shows the density distribution of mix1p (a pelitic starting composition, see Table 4) in P–T space. Densities vary between 2,600 and 3,300 kg/m3 at temperatures between 500 and 900°C and pressures between 0.05 and 3 GPa. The density diagram shows several density jumps, which are due to phase transitions (or reactions) in the rock. In order to see which minerals and ultimately which compositional variations are important for these differences in density, the major phase transitions and mineral reactions are plotted (Fig. 2b). Three major types of reactions occur: (1) polymorphic transitions such as quartz– coesite or sillimanite–kyanite, where the high-pressure polymorph has the denser structure; (2) dehydration reactions such as chlorite reacting to form minerals with less or no water in the structure, where the phases containing water have lower densities; (3) water-free reactions like Ab = Jd ? Qtz, which form minerals with higher densities and are rather pressure than temperature dependent. Except for the polymorphic transitions, many reactions are continuous, and the first appearance of a mineral does not necessarily cause a large difference in density. Plotting only the limits of mineral stability fields is not sufficient to investigate the effect of phase transitions on the rock density. Therefore, we plot the modal amounts of a mineral in the rock as a function of pressure and temperature. Figure 3 shows that continuous reactions can be easily detected by variations in mineral modes. While garnet (Fig. 3a) and phengite (Fig. 3c) are formed, biotite (Fig. 3b) and kyanite (Fig. 3d) are consumed. This continuous reaction is important for the density of pelitic rocks because it is a major garnet-forming reaction and can be formulated in KFASH as follows: Annite Ann Biotite Bt Carpholite Celadonite Cp Cel Chlorite Chl Chloritoid Cld Clinochlore Clin Clinopyroxene Cpx Cordierite Crd Daphnite Daph Eastonite East Feldspar Fsp Ferrosilite Fs Garnet Grt Illite Ill Jadeite Jd K-feldspar Kfs Kyanite Lawsonite Ky Lws Muscovite Ms Orthopyroxene Opx Paragonite Pg Phengite Phe Phlogopite Phl Plagioclase Pl Pyrope Prp Sanidine San Sillimanite Sil Staurolite St Talc Tlc Quartz Qtz Zoisite Zo See Kretz (1983) for most common minerals The starting mix (mix1p) has the following weight proportions: Qtz:Fsp:Ill:Chl:Fe–Ti-Ox = 25:12:40:20:3. We exclude the oxides which are not considered in the calculation and run a thermodynamic calculation with excess water (i.e., not constraining the water content in the rock). A pseudosection diagram of mix1p is shown in Fig. 1. Finally, a density grid is generated for each pelitic mix (see Connolly and Petrini 2002 for retrieval of rock properties). ðaÞ Ann þ 2Ky þ Qtz ¼ Alm þ Ms ðbÞ KFe3 AlSi3 O10 ðOHÞ2 þ2Al2 SiO5 þ SiO2 ¼ Fe3 Al2 Si3 O12 þ KAl3 Si3 O10 ðOHÞ2 ð1Þ In addition, the modal variations (Fig. 3) show at which P–T conditions a mineral has reached its maximum amount in the rock, which implies that all reactions forming this mineral have been completed. Quartz–Illite variations A systematic study of the effect of compositional variations on densities requires a parameter which allows a gradual change in the rock type. If we want to vary the composition between pelagic clay (poor in Ca but rich in Al) and sandstone, we mainly have to look at varying SiO2 and Al2O3 contents in the rock. To achieve this, we vary only 123 Int J Earth Sci (Geol Rundsch) Table 2 Solid solution models for the phases used in the pelitic mixes and mafic compositions Solid solution model End-members Formulas References Biotite (Bt) Phlogopite KMg2Mg[AlSi3O10](OH)2 Powell and Holland (1999) Annite KFe2Fe[AlSi3O10](OH)2 Eastonite KMg2Al[Al2Si2O10](OH)2 Ordered biotite KMg2Fe[AlSi3O10](OH)2 Carpholite (Cp) Chlorite (Chl) Chloritoid (Cld) Clinopyroxene (Cpx) Cordierite (Crd) Garnet (Grt) Phengite (Phe) Plagioclase (Pl) Staurolite (St) Talc (Tlc) Orthopyroxene (Opx) Amphibole (Amp) (clino) Magnesiocarpholite MgAl2[Si2O6](OH)4 Ferrocarpholite FeAl2[Si2O6](OH)4 Clinochlore Mg4MgAl[AlSi3O10](OH)8 Daphnite Fe4FeAl[AlSi3O10](OH)8 Amesite Mg4AlAl[Al2Si2O10](OH)8 Holland et al. (1998) Al-free chlorite Mg4MgMg[Si4O10](OH)8 Magnesiochloritoid Ferrochloritoid MgAl2O[SiO4](OH)2 FeAl2O[SiO4](OH)2 White et al. (2000) Green et al. (2007), Holland and Powell (1996), Zeh et al. (2005) Diopside CaMg[Si2O6] Hedenbergite CaFe[Si2O6] Jadeite NaAl[Si2O6] Ca-tschermaks CaAl[AlSiO6] Cordierite Mg2[Al4Si5O18] Ferrocordierite Fe2[Al4Si5O18] Hydrous cordierite Mg2[Al4Si5O18] H2O Pyrope Mg3Al2[Si3O12] Almandine Fe3Al2[Si3O12] Grossular Ca3Al2[Si3O12] Muscovite KAlAl[AlSiSi2O10](OH)2 Celadonite KAlMg[SiSiSi2O10](OH)2 Ferroceladonite KAlFe[SiSiSi2O10](OH)2 Mahar et al. (1997) Holland and Powell (1998) Powell and Holland (1999) Paragonite NaAlAl[AlSiSi2O10](OH)2 High albite Anorthite Na[AlSi3O8] Ca[Al2Si2O8] Newton et al. (1980) Holland and Powell (1998) Magnesiostaurolite Mg4Al18[Si7.5O44](OH)4 Ferrostaurolite Fe4Al18[Si7.5O44](OH)4 Talc Mg2Mg[SiSi3O10](OH)2 Ferrotalc Fe2Fe[SiSi3O10](OH)2 Tschermaks talc Mg2Al[AlSi3O10](OH)2 Enstatite MgMg[SiSiO6] Ferrosilite FeFe[SiSiO6] Magnesiotschermaks AlMg[AlSIO6] Tremolite Ca2Mg3Mg2[Si2Si6O22](OH)2 Ferrotremolite Ca2Fe3Mg2[Si2Si6O22](OH)2 Tschermakite Ca2Mg3Al2[Al2Si6O22](OH)2 Pargasite NaCa2Mg3MgAl[Al2Si6O22](OH)2 Glaucophane Na2Mg3Al2[Si2Si6O22](OH)2 the proportions of Qtz and Ill. The amount of Qtz significantly influences the total amount of SiO2 in the rock, while varying Ill proportions mainly has an effect on the Al2O3 and K2O contents (see compositions in Table 2). In addition, the Na2O, MgO, and FeO contents of the rock will also vary slightly. Table 4 shows the compositions 123 Same model as Cld Holland and Powell (1998) Holland and Powell (1996) Wei and Powell (2003) used for our calculations; P–T–density diagrams of a few of these theoretical mixes are shown in Fig. 4. The water content is just high enough to ensure water saturation of the rock under all conditions. Depending on the pressure and temperature conditions as well as on compositions, the water content in metapelites varies between 0.7 and 2.56 wt%. Int J Earth Sci (Geol Rundsch) Table 3 Composition of minerals used for the generation of hypothetical pelitic mixes (oxides in wt%) Oxides Quartz (Qtz) Feldspar (Fsp), An15 Illite (Ill) Chlorite (Chl) Fe–Ti-Oxides SiO2 100 67.5 47.5 26.0 – TiO2 – – 0.5 – 30.0 Al2O3 – 18.5 33.5 20.0 5.0 Fe2O3 – 0.5 – 5.0 40.0 FeO – – 2.0 20.0 16.0 MnO – – – – 3.0 MgO – – 1.5 16.0 2.0 CaO – 3.0 – – 4.0 Na2O – 10.0 1.5 – – K2O – 0.5 9.0 – – H2O – – 4.5 13.0 – (2) Ky, Cpx, and additional Grt-forming reactions in the high-pressure and high-temperature area, and (3) major garnet-forming reactions at lower pressure and intermediate temperature. Figure 5 shows the density diagram of mix1p with the most important reactions (see ‘‘Appendix’’ for detailed reactions). This reaction list is not complete. Especially in the lower temperature–lower pressure area, numerous reactions involving staurolite, aluminosilicate, chlorite, and biotite take place. For a complete petrogenetic grid, see Wei and Powell (2003), Wei and Powell (2004), and Wei and Powell (2006). Due to changes in composition, reactions might not only change their position but may also be replaced completely by another reaction (discussed below in more detail). Chlorite–Illite variations Fig. 1 Pseudosections of a meta-pelitic rock calculated for mix1p (see Table 4 for composition). For reasons of clarity, the small fields are not labeled. Mineral abbreviations are given in Table 1 The quartz–illite variation is shown from top to bottom of Fig. 4, where mix1p (pelagic clay or pelite composition sensu stricto) has the highest Al2O3 and the lowest SiO2 content while mix3p (intermediate between pelite and sandstone) has lower Al2O3 and higher SiO2 contents. The P–T–density diagrams in Fig. 4 show several significant density changes which are subject to varying composition and caused by the following reactions: (1) polymorphic transitions of quartz to coesite and sillimanite to kyanite, As a next step, we vary the composition of a pelite with less mafic to more mafic component which means changing FeO and MgO contents in the rock (but ignoring Ca variations). Therefore, only the illite–chlorite ratios are varied. Increasing chlorite and decreasing illite mainly results in an increase in whole rock FeO and MgO and a significant decrease in K2O, together with some reduction in Al2O3, Na2O, and SiO2 (Table 4). Figure 4 shows the whole rock FeO and MgO variation from left to right, with the lowest whole rock FeO and MgO contents in mix1p (pelite) and the highest in mix14p (FeO- and MgO-rich pelite). Understanding the influence of compositional variations on density In order to quantify and compare variations in mineralogy and consequently physical properties that are due to compositional changes, we compute mineral modes and densities at specific P–T conditions. The first point is chosen well within the high-P, high-T area at 800°C and 2.5 GPa 123 Int J Earth Sci (Geol Rundsch) Table 4 Starting compositions of hypothetical pelitic mixes (oxides in wt%): mix1p-mix10p; mix11p-mix14p; mix21p-mix23p; mix31pmix33p; MORB and Fjørtoft gneiss (Massonne et al. 2007), lower crustal Meta-pelite estimate (Schenk 1990) and average Lower Crust estimate (Rudnick and Fountain 1995; Rudnick and Gao 2003) mix1p mix2p mix3p mix4p mix5p mix6p mix7p mix8p mix9p mix10p mix11p SiO2 61.07 63.83 66.58 69.33 72.08 74.70 76.96 58.31 55.54 52.78 60.44 Al2O3 21.07 19.27 17.47 15.69 13.89 12.07 10.24 22.87 24.67 26.48 20.53 FeO 5.62 5.52 5.41 5.30 5.19 5.06 4.93 5.74 5.85 5.95 6.64 MgO CaO 4.11 0.51 4.03 0.51 3.95 0.52 3.86 0.51 3.79 0.51 3.70 0.51 3.59 0.50 4.20 0.51 4.28 0.51 4.36 0.51 4.93 0.52 Na2O 1.92 1.83 1.75 1.67 1.59 1.51 1.42 2.00 2.08 2.16 1.86 K2O 3.90 3.42 2.94 2.46 1.98 1.50 1.01 4.38 4.87 5.35 3.45 H2O 1.79 1.58 1.38 1.18 0.97 0.96 1.35 1.99 2.20 2.40 1.64 mix12p mix13p mix14p mix21p mix22p mix31p mix32p MORB Fjørtoft Meta-pelite Lower crust SiO2 59.81 58.93 57.65 63.22 62.58 65.99 65.06 51.40 55.37 56.40 53.96 Al2O3 19.97 19.33 18.57 18.72 18.14 16.90 16.24 16.66 24.38 21.00 17.08 FeO MgO 7.67 5.76 8.70 6.57 9.66 7.35 6.53 4.84 7.56 5.67 6.42 4.76 7.41 5.56 7.90 7.93 11.47 3.56 9.40 4.50 8.66 7.32 CaO 0.52 0.53 0.52 0.52 0.52 0.52 0.52 11.82 0.88 3.00 9.69 Na2O 1.79 1.72 1.63 1.78 1.71 1.69 1.62 2.84 0.41 2.00 2.68 K2O 2.99 2.52 2.02 2.96 2.51 2.48 2.00 0.09 2.53 2.50 0.62 H2O 1.49 1.70 2.59 1.43 1.31 1.23 1.58 1.37 1.39 Water contents are given at 500°C and 0.05 GPa; the water content varies with P and T. Water contents in Meta-pelite and Lower Crust compositions were varied from dry to water saturated, see Fig. 10 Fig. 2 a P–T-density diagram of mix1p (pelitic rock; densities in kg/m3). b Important phase transitions and mineral reactions. The label ‘Grt in’ means that the first garnet is formed at the curve; ‘Bt out’ implies that the last biotite breaks down. Large density changes are caused by hydration/dehydration reactions (e.g., ‘Chl in/out’) in addition to mainly pressure-dependent reactions (e.g., ‘Cpx in’ and quartz–coesite) (point in Fig. 5a), the second one is at 700°C and 1.2 GPa (point in Fig. 5b). Figure 6a shows the mineral modes in volume percent versus the whole rock Al2O3 content in 10 hypothetical mixes (see Table 4) at 800°C and 2.5 GPa. The stable phases at these conditions are Phe, Grt, Cpx (mostly jadeitic), Ky, and Qtz. As expected, the amount of Ky is decreasing with decreasing Al2O3 content in the rock. Compositions with approximately 12 wt% Al2O3 and lower (mix6p and mix7p) do not have enough Al2O3 to form kyanite in addition to the Al-bearing phases (Phe, Grt, Cpx). Since the density of Ky is quite high (&3,670 kg/ m3), the density of the rock is affected significantly. Cpx is 123 slightly increasing with Al2O3 (and Na2O), which also has an effect on the whole rock density. In addition, the amount of Phe is drastically increasing, while Qtz is decreasing. Since Phe has a higher density than Qtz (Phe: 2,900 kg/m3; Qtz: 2,690 kg/m3), this results in a higher rock density. The amount of Grt, the mineral with the highest density, is nearly constant over the whole compositional range. Additional Grt can only form when there is a sufficient amount of Al2O3 as well as FeO and/or MgO in the rock (Jull and Kelemen 2001). Since the FeO and MgO contents are almost constant, an increase in Al2O3 does not result in the formation of more Grt. Int J Earth Sci (Geol Rundsch) Fig. 3 Modal amounts of garnet (a), biotite (b), phengite (c), and kyanite (d) in volume% (varying scale). The ‘Grt in’ and ‘Bt out’ lines are added in order to show the continuous reaction as given by Eq. (1) A list of rock densities at those specific conditions is provided in Table 5. Figure 7a shows that there is a linear density increase with increasing whole rock Al2O3 content. If we compare the most silicic composition (mix7p, close to a sandstone) with the most aluminous rock (mix10p, pelagic clay), the density difference is 6.42% while the difference in Al2O3 content is 16.22 wt%. At 700°C and 1.2 GPa, the assemblage consists of the phases Grt, Pl, Ky, Bt, Phe, and Qtz (Fig. 6b). In the Al-poor mixes (mix6p and mix7p), Phe is not present at the given conditions and will be formed at higher pressure. Due to the decrease in whole rock Al2O3, Al in the Phe structure is gradually replaced by Si and Mg/Fe (celadonitic substitution), which is strongly dependent on pressure. Phe with a higher celadonitic proportion is stable at higher P. The mineral densities also differ from those at 800°C and 2.5 GPa, which is partly due to the different P and T values and partly due to the compositional change in the minerals (e.g., Grt has a higher Alm component, which is denser than Prp). All rock densities at 700°C and 1.2 GPa are listed in Table 5 and plotted in Fig. 7a. Again, an almost linear relationship exists between the density and the Al2O3 content of the rock. The difference in density between the most silicic rock (mix7p) and the most aluminous composition (mix10p) is 4.23%. We plotted the densities of two more points in Fig. 7a: 500°C and 1.5 GPa and 500°C and 0.7 GPa (see Table 5 for whole rock density data). While the density still increases linearly with aluminum content, the overall density change is lowered to 3.7 and 3.8%, respectively. The fact that Phe forms at higher pressure in Al-poor compositions also has an effect on the mineral reactions and the modes of the other minerals and therefore the density (Grt increases, Bt and Ky decrease, Fig. 6b). In Fig. 8, we plot the zero contour lines for these minerals for mixes 1p-7p (pelagic clay to sandstone). At higher temperatures, Bt decomposes at higher pressures with decreasing Al2O3 content (Fig. 8b) while Grt forms at lower pressures (Fig. 8a). Phe is also formed at higher pressures (Fig. 8c), and the Ky stability field is drastically reduced with decreasing Al2O3 in the whole rock (Fig. 8d). For Grt, we only consider reactions within the temperature interval from 600 to 900°C. In mixes 1p and 2p (pelites with Al-rich compositions), the first appearance of Grt on a prograde metamorphic path is due to reaction (1). Grt is also formed by reaction of biotite with sillimanite or cordierite (see reactions 10 and 11 in the appendix). In mixes 3p, 4p, and 5p (less Al-rich compositions), most Grt is formed by reaction (1). However, Grt is already formed at lower metamorphic conditions by the breakdown of Crd 123 Int J Earth Sci (Geol Rundsch) Fig. 4 P–T-density diagrams of mixes with different compositions. From top to bottom: increase in SiO2 and decrease in Al2O3; from left to right: increase in FeO and MgO. Absolute densities and density contrasts increase from lower left to upper right corner. Compositions are given in Table 4 Fig. 5 Important reactions responsible for significant increase in density in the pelitic composition (mix1p) at high P (a) and lower P–T (b). Black dots indicate the P–T conditions used for the comparison of modal amounts in Figs. 6 and 9. Numbers refer to reactions listed in the appendix except for reaction 1 on b which is given in the text (reaction 12 in appendix). Reactions (1) as well as (12) are also important Grt-forming reactions in mix6p and 7p (most silicic compositions); however, at lower P and T, Grt 123 is formed by a reaction of Opx and Crd (reaction 13 in the appendix). Although the first Grt is formed at lower metamorphic conditions with decreasing Al2O3, the Int J Earth Sci (Geol Rundsch) Fig. 6 Mineral modes in vol% plotted versus Al2O3 content (in wt%) in the hypothetical whole rock compositions mix1p-10p (pelitic to quartzitic composition; Table 4) at 800°C, 2.5 GPa (a) and 700°C, 1.2 GPa (b). Mineral densities at these conditions are also added. Garnet is the heaviest mineral but its modal amount is almost constant over the whole range of whole rock Al2O3 contents, but more (relatively dense) phengite and less (relatively less dense) quartz are stable in Al2O3-rich compositions Table 5 Densities in kg/m3 for hypothetical mixes at specified conditions (see Fig. 7) q at 800°C, 2.5 GPa mix1p mix2p mix3p mix4p mix5p mix6p mix7p 3100.80 3078.90 3057.30 3036.30 3015.50 2994.60 2977.30 q at 700°C, 1.2 GPa 2903.40 2888.80 2873.80 2859.40 2844.80 2834.30 2829.50 q at 700°C, 1.2 GPa dry 2960.60 2941.70 2923.10 2904.90 2887.00 2869.10 2852.40 q at 500°C, 1.5 GPa 2863.70 2851.70 2840.10 2828.30 2816.70 2805.10 2793.80 q at 500°C, 0.7 GPa 2767.70 2756.60 2745.30 2734.40 2723.30 2712.30 2701.80 mix8p mix9p mix10p mix11p mix12p mix13p mix14p q at 800°C, 2.5 GPa q at 700°C, 1.2 GPa 3122.90 2918.60 3145.30 2933.90 3167.80 2949.30 3139.60 2936.20 3180.90 2970.50 3223.00 3020.10 3268.50 3078.4 q at 700°C, 1.2 GPa dry 2979.60 2999.60 3020.90 3001.10 3043.60 3087.70 3134.3 q at 500°C, 1.5 GPa 2875.60 2887.50 2899.50 2773.10 2778.60 2784.10 2790.4 q at 500°C, 0.7 GPa 2779.10 2790.70 2802.00 2865.10 2866.20 2868.10 2869.3 Fig. 7 Density (in kg/m3) plotted versus whole rock Al2O3 content (in wt%) for mixes 1p-10p (pelitic to quartzitic compositions) (a) and versus whole rock FeO (in wt%) for mixes1p to 14p (FeO/MgO variations) (b) calculated at different P–T points: 800°C, 2.5 GPa, dry compositions at 700°C, 1.2 GPa, 700°C, 1.2 GPa, 500°C, 1.5 GPa, 500°C, 0.7 GPa. Note the difference in scales. Density increases almost linearly with increasing Al2O3 as well as FeO content in the rock. Whole rock densities at the specified P–T conditions are given in Table 5 amount of Grt in the rock is almost constant due to the lack of FeO/MgO. Changes in the Bt-forming reactions are mostly taking place in the range between 700 and 900°C. Ky and Bt breakdown according to reaction (1) in the pelitic compositions (mixes 1p and 2p). Ky is not stable anymore in intermediate compositions (mix3p to mix5p), and Bt breaks down according to reaction 14 (see ‘‘Appendix’’). This 123 Int J Earth Sci (Geol Rundsch) Fig. 8 Zero contour lines indicating the first or last formation of Grt (a), Bt (b), Phe (c), and Ky (d) in mixes mix1p7p. Compositional variations in the rock have a strong influence on the position of the mineral stability fields Fig. 9 Mineral modes in vol% plotted versus whole rock FeO content (in wt%) in the hypothetical compositions mix1p-14p at 800°C, 2.5 GPa (a) and 700°C, 1.2 GPa (b). Mineral densities at these conditions are also added. The modal amount of dense garnet increases significantly with increasing whole rock FeO, while phengite decreases reaction is displaced toward higher pressures with decreasing Al2O3 content in the rock. Figure 8 shows the complexity of the metamorphic reactions and the consequences for rock properties. A reduction of Al2O3 in the rock composition clearly reduces the amount of any additional aluminosilicate phase, which then has an influence on other mineral-forming reactions. Nevertheless, the first appearance of a mineral will not necessarily change the density significantly because the amount of the mineral may be small. We also compare the mineral modes at the two reference points at 800°C and 2.5 GPa and at 700°C and 1.2 GPa for the compositions with chlorite–illite variations. Here, whole rock FeO is plotted versus the modal amount of minerals in Fig. 9. At 800°C and 2.5 GPa (Fig. 9a), Qtz and Cpx modes are almost constant while kyanite and phengite decrease. Ky is not stable as a 123 phase in the compositions with low whole rock Al2O3, and Grt forms instead. Grt takes up the majority of FeO and MgO in the rock and yields the highest densities. From mix1p to mix14p, the FeO content increases by 4.2 wt% and the MgO content by 3.4 wt%, resulting in a density increase of 5.42% (see also Fig. 7b; Table 5). At 700°C and 1.2 GPa (Fig. 9b), Phe breaks down while the amounts of Qtz and Pl do not vary that much. Grt and Ky, however, increase at the expense of Bt after complete Phe breakdown, which is again caused by a change in reactions. The increase in density is 6.03% with whole rock FeO and MgO increasing by 4.2 wt% and 3.4%, respectively (Fig. 7b; Table 5). Figure 7b shows that densities increase approximately linearly with increasing whole rock FeO at higher temperatures, while the density is almost constant at 500°C, 1.5 GPa and 500°C, 0.7 GPa. Int J Earth Sci (Geol Rundsch) Fig. 10 P–T-density diagrams for meta-pelite (mix1p), Fe–Mg-rich meta-pelite (mix14p), and MORB (all compositions given in Table 4). Compositions are becoming more mafic from left to right, and water content is decreasing from top to bottom from water saturated to 1.5 wt% H2O to dry. The classical, mainly pressuredependent phase changes from gabbro to granulite to eclogite leading to large Dq are clearly visible in the dry MORB composition (lower right panel). However, water-saturated composition (upper panels) produce significant densification upon heating, and also the density distribution of the mafic compositions with just 1 wt% water is strongly influenced by temperature-dependent reactions (not shown) In summary, even small changes in whole rock composition can lead to significant changes in mineralogy and hence the density of the rock. Variations in the Al2O3 content of the rocks lead to an almost linear increase in density that is mostly due to the additional formation of aluminosilicates, while the amount of Grt is almost constant. This density increase is larger at higher P and T. An increased amount of FeO and MgO in the rock composition, however, significantly increases the amount of Grt. Grt also takes up most of the Al2O3 in the rock (Jull and Kelemen 2001). The formation of other Al-bearing phases is therefore suppressed with increasing FeO and MgO content. Since Grt is the densest mineral, only a few wt% of increase in FeO and MgO in the whole rock significantly increases the whole rock density. Varying water content In all calculations above, we assumed water to be an excess phase, which might be a good assumption for rocks in the upper crust. Lower crustal rocks, however, contain less water or may even be completely dehydrated (Austrheim 1991). In order to investigate the effect of H2O on density, we use the water-saturated mix1p (1.79 wt% H2O at 500°C and 0.05 GPa) as a starting point and compare it with the same composition containing approximately 1.5 wt% H2O and a dry mix1p. The density diagrams of the three mixtures are shown in Fig. 10. We also compare the densities at the two chosen P–T conditions. At 800°C and 2.5 GPa, the dry rock is 0.52% denser than the water-saturated rock. At 700°C and 1.2 GPa, the density difference is much 123 Int J Earth Sci (Geol Rundsch) higher (2.17%). The water content can change the density significantly at certain P–T conditions, especially at lower P and T, while the effect in the higher P, higher T field is not that pronounced. This is due to the fact that most hydrous phases decompose at relatively low temperatures, and the only water-bearing mineral in a water-saturated meta-pelite at high temperature and pressure is Phe. Comparison with the density of mafic rocks We also computed P–T–density diagrams for a mid-ocean ridge basalt (MORB, Table 4, composition from Massonne et al. 2007) to compare densities of a mafic rock with densities of different pelitic compositions (Fig. 10). Pseudosections are calculated in the system K2O–Na2O–CaO– MgO–FeO–Al2O3–SiO2–H2O as before, and all thermodynamic calculations involve divalent iron only. Most solid solutions used in the pelitic mixes are also relevant for the mafic rock; additional solid solutions, e.g., for amphibole and orthopyroxene, are given in Table 2. At 800°C and 2.5 GPa, the MORB yields a density of 3,427 kg/m3. The basalt only needs 0.04 wt% of water to be saturated at those conditions. If we compare this value to the density of mix1p, pelagic clay (3,101 kg/m3), the difference in density is 10.5%. However, if the basalt is compared to a more Fe- and Mg-rich composition, e.g., mix14p, the difference in density is reduced to 4.8%. Massonne et al. (2007) showed that pelitic rocks can get very dense at high to ultrahigh pressures, which is confirmed by our results. We also compute densities for a Fe- and Al-rich natural rock, the Fjørtoft gneiss (Fig. 11; Table 4), which was found to be the densest meta-pelite by Massonne et al. (2007) at ultrahigh-pressure conditions. The Fjørtoft gneiss yields 3,304 kg/m3 at 800°C and 2.5 GPa, and the density Fig. 11 P–T-density diagram of the Fjørtoft gneiss (Table 4) 123 difference between mafic and pelitic rocks is reduced to 3.7%. At 700°C and 1.2 GPa, the MORB has a density of 3,111 kg/m3 and a water content of 0.81 wt%. The MORB density is 7.17% higher than that of mix1p, while the density difference between the MORB and mix14p is only 1.07%. The Fjørtoft gneiss, however, is 3.54% denser than the MORB at these conditions. In accordance with the results of Massonne et al. (2007), we conclude that at certain conditions, a meta-pelitic rock can become denser than a meta-mafic rock if the metapelite has a significant amount of Al2O3 and FeO. The average lower crust is expected to be a mixture of felsic and mafic rocks as numerous xenolith analyses (e.g., Rudnick and Taylor 1987; e.g., Downes 1993; Rudnick and Fountain 1995) as well as exposed lower crustal sections (e.g., Fountain and Salisbury 1981; Schenk 1984; e.g., Schenk 1990) indicate. An extensively used representative average composition of the lower crust has been derived by Rudnick and Fountain (1995) on the basis of xenolith data and geophysical observations. Due to the fact that a majority of xenoliths is mafic, the calculated average composition yields phase relations and densities very similar to a MORB, yet with slightly higher silica and a lower CaO content and somewhat lower densities. Uncertainties of the density diagrams All calculated physical rock properties will be approximations of real rock properties due to the fact that we have to simplify the system, and some mineral solid solution models are not formulated with certain elements e.g., Ti or Fe3?. The same also holds true for the thermodynamic data since some minerals are thermodynamically less well known and stability fields might be under- or overestimated e.g., for biotite (Massonne and Schreyer 1989; Massonne et al. 2007). In addition, thermodynamic calculations imply perfect equilibrium, whereas metamorphic rocks do not always equilibrate instantly during prograde metamorphism (e.g., mineral zonation) and hardly during retrogression (metastability; Spear 1995). In the thermodynamic calculation, however, instant equilibrium is assumed. Another factor of uncertainty is the water content in the rock, which changes during metamorphism. In our calculations, we have to assume a certain water content of the initial rock, which will then be constant over the whole pressure and temperature range. Wherever H2O is present as an excess phase, however, it will not be included in the calculation of physical rock properties. Another simplification is the assumption that the fluid phase is only composed of H2O. The fluid may contain other components, and the amount as well as the composition of the fluid may change during different stages of metamorphism and may influence phase equilibria. Int J Earth Sci (Geol Rundsch) Despite the simplifications, the calculated phase diagrams and extracted densities correlate quite well with phase relations and densities of natural rocks (for discussions about the thermodynamic data and correlation with natural rocks see e.g., Sobolev and Babeyko 1994; Gerya et al. 2001; Tinkham et al. 2001; Wei and Powell 2003, 2004, 2006; Zeh and Holness 2003; Massonne et al. 2007). Our obtained density values are, within a 1% tolerance, very close to those calculated by Massonne et al. (2007) for a psammopelitic rock composition, the Fjørtoft gneiss, and a MORB at high to ultrahigh-pressure conditions and a meta-pelite at lower P–T conditions calculated by Gerya et al. (2001). In addition, the calculated P–T-dependent densities are a much more realistic approximation of the actual density distribution in the crust than the commonly used layers of constant, and P–T independent, average density, or densities that only depend on temperature via thermal expansion. In fact, feedback between tectonic processes and reactions that influence physical properties is expected, and P–T-dependent densities should therefore be incorporated in dynamic models (e.g., Gerya et al. 2001; Goffe et al. 2003; Gac et al. 2008; Simon and Podladchikov 2008). Partial melting Continental crust can melt by adding water to rocks at temperatures above their water-saturated solidi, by decompression due to dehydration-melting reactions and by an increased heat supply (Thompson and Connolly 1995). At temperature conditions of crustal orogenesis, water is required to generate melt, but the small amount of free water in lower crustal rocks will only produce negligible amounts. Dehydration-melting of muscovite and biotite at higher P–T conditions can produce up to 25 vol% of melt in meta-pelitic rocks (Thompson and Connolly 1995). Mafic compositions, however, produce an insignificant portion of melt. Independent of the amount of melt generated, partial melting of lower crustal rocks will remove the more felsic and less dense components of the crustal rocks and leave the more mafic and denser minerals as restite. According to Massonne (2009), Grt becomes a restite phase in granitic and dioritic rocks with rising temperature, and a significant increase in Grt content to more than 20 vol% is caused by the breakdown of Bt, Lws, and Phe. In fact, compositions such as the Fe- and Mg-rich pelite (mix14p) could represent such a restite. This is also in accordance with exposed crustal cross sections where the restitic Fe- and Mg-rich rocks constitute a significant and dense part of the lower crust while granites and granodiorites are found in the upper crust (Fountain and Salisbury 1981; Schenk 1984, 1990). Therefore, despite some expected melting in the pressure–temperature range of interest, we do not consider the formation of a silicate melt (melting is suppressed in the phase diagram calculations), and the phase relations calculated for the high-temperature field are metastable. Density along a continental shield geotherm We have shown here that phase transitions in natural rocks are complex and gradual, and their slope and position as well as the associated changes in density depend on rock composition. With our calculated density diagrams, more realistic densities can be extracted along a geotherm, and we are able to test the effect of phase transitions as a response to loading. Since the exact composition of the lower crust is not known, we cover a large range of possible compositions by using densities of meta-pelitic as well as meta-mafic rocks. We choose a linear geotherm for the lower crust and upper mantle which results in a temperature of approximately 300°C at 20 km and 1,300°C at 200 km depth, in accordance with reconstructed geothermal gradients for old continental shields (Jaupart and Mareschal 2007). In Fig. 12, this geotherm is plotted on top of the density diagram of the Fe- and Mg-rich pelitic rock (mix14p) to show that the chosen geotherm will pass through several significant density jumps. The densities for six different rock compositions are extracted along this continental shield geotherm: (1) the pelitic composition (mix1p), (2) a Fe- and Mg-rich pelite (mix14p), (3) the Fjørtoft gneiss as an Al- and Fe-rich end-member and representative of a naturally occurring rock, (4) a dry MORB, (5) a dry pelite Fig. 12 Continental shield geotherm (300°C at 20 km and 1,300°C at 200 km) plotted on top of the P–T-density diagram of mix14p (Fe–Mg-rich meta-pelite, Table 4). Arrows indicate the final P–T path that results for crustal burial from the steps of pressurization (DP = 1.2 GPa) and heating as described in the text for one point in the crust 123 Int J Earth Sci (Geol Rundsch) (mix1p), (6) a hydrated (water saturated) MORB, and (7) a hydrated lower crustal composition (Rudnick and Fountain 1995; Rudnick and Gao 2003). Figure 13 shows the densities of those seven compositions with increasing pressure along the geotherm. We chose the starting point to be well within the crust at approximately 20 km, which roughly corresponds to 0.6 GPa lithostatic pressure, and extend the density curves up to 4 GPa for completeness. As expected, there is a large variation in density depending on the rock type, with the more silicic compositions starting significantly lighter than the mafic rocks. Figure 13 also shows that phase transitions and therefore density jumps for the various compositions are not located at the same P–T conditions (except for the Qtz-Cs transition at approximately 3 GPa which is the same for all rock compositions). The most striking feature of Fig. 13 is that all water-saturated rocks show a larger density variation (Dq) along the P–T path than the dry compositions and that the Fe- and Mg-rich meta-pelitic rock has the largest Dq. While the dry MORB certainly yields the highest absolute densities under all conditions, the increase in density is small compared to all other compositions. Isostatic response to crustal phase transitions A simple 1-d isostatic model is developed to investigate the effect of loading or burial on densities and subsidence in a reacting crustal layer. The results are evaluated as a function of the pressure and temperature increase, the initial Fig. 13 Densities for seven different rock compositions extracted along the continental shield geotherm as shown in Fig. 12 and plotted against P and the approximate corresponding depth. Hydrated Fe– Mg-rich pelitic rocks show the highest density increase while MORB yields the highest density but only a small Dq. Dry rocks show a first step of densification around 0.5–1 GPa. The largest densification starts at slightly lower pressures in mafic compositions compared to meta-pelites, with the Fjørtoft gneiss being intermediate 123 depth and the thickness of the reacting layer, and the initial geotherm or thickness of the lithosphere. The water-loaded isostatic subsidence S is related to the density changes by the simple equation S ¼ z1 ðhq2 i hq1 iÞ qm hq2 i ðqm qw Þ ð2Þ where qm is the constant density of the mantle (3,300 kg/ m3), qw is the density of water (1,000 kg/m3), and z1 is the initial thickness of the reacting layer. hq1 i and hq2 i are the average densities of the reacting layer before and after the reaction, respectively. Since this paper is on the effect of crustal phase transitions, we assume that mantle density is constant. Hence, subsidence depends on the thickness of the reacting layer (z1) and the densities before and after reactions (Dq = hq2 i - hq1 i) scaled by hq2 i. In a simplified geometric approach, Dq depends on the rock type, the initial depth of the reactions, which is determined by the initial slope of the geotherm, and the angle between the slope of the geotherm and the phase transitions (Mareschal and Lee 1983; Simon and Podladchikov 2008). As shown above, however, reactions in crustal rocks are complex and cannot easily be reduced to single reaction lines and rigorously analyzed as done for mantle phase transitions in Simon and Podladchikov (2008). The burial and pressure increase may arise due to sediment or thrust loading, horizontal compression and lithospheric flexure and crustal thickening, or a combination of these processes, or by subduction or delamination. However, a discussion on the mechanism for pressure increase is beyond the scope of this paper, and we will merely investigate the density changes and subsidence due to some preset burial of the crust. Some discussion on the application of our results to common tectonic settings is provided below. Temperature rises in this model because the deepened lower crust heats back to the initial geothermal gradient. Additional heating may occur due to sediment blanketing, radiogenic heat production, or shear heating would increase the effect of thermal re-equilibration. An example for a schematic P–T path consisting of burial (pressurization) and thermal re-equilibration is given in Fig. 12 for one point in the crust. Figure 14 shows the density change Dq of a crustal layer with fixed initial thickness and position as a response to pressure (DP) and temperature increase (DT) for different bulk compositions with different water contents (see Fig. 10). The reacting layer is initially located at 20–40 km depth, corresponding to a lithostatic pressure interval of 0.56–1.2 GPa and a temperature of 300–476°C for an initially 140-km-thick lithosphere. Dq varies from -200 to ?350 kg/m3 in a complex and non-linear way, in particular for burial of wet lower crust. For the given conditions, these density variations Int J Earth Sci (Geol Rundsch) approximately correspond to water-loaded isostatic uplift or subsidence of -2,000 and 3,500 m, respectively. In dry or nearly dry rocks, the average density of the reacting layer generally increases as a consequence of increasing pressure and decreases due to heating for all compositions and conditions (Fig. 14d–i), with the most pronounced Dq increase caused by the granulite to eclogite facies transition (densely spaced contours in Fig. 14d–i). This transition is at lower absolute pressures for more mafic compositions (compare Fig. 14g, h and i; see also Fig. 10). In mafic wet lower crustal rocks, pressurization without significant heating may not affect densities or even lead to slightly decreasing average densities at some conditions (Figs. 14c, 10c). Heating of a completely dry lower crustal layer leads to a decrease in density and hence negative Dq. Hydrated crust, in contrast, may contract as a response to heating at pressures equivalent to or higher than those at normal continental Moho (Fig. 14a–c). In mafic rocks, significant heating-related densification occurs for water contents as low as 1 wt% (Fig. 14f). For DP and DT equivalent to a doubling of the crustal thickness (upper right corner of panels in Fig. 14), Dq is largest for the wet Fe–Mg-rich meta-pelite (up to 350 kg/m3). This is evident already from Fig. 13, where the densities along the continental geotherm are shown, and the Fe–Mg meta-pelite displays the largest density contrast of all compositions at around 2 GPa. The Fjørtoft gneiss and wet average lower crust behave similarly to the Fe–Mg-rich meta-pelite and wet MORB, respectively (not shown). Dq due to reactions in the lower crust and the waterloaded isostatic response depend critically on a range of parameters that will not be explored here. Figure 15 shows two examples for Dq and subsidence for two different P–T paths (i.e., trajectories through Fig. 14) and different initial lithosphere thicknesses (i.e., thermal states) for a set of rock compositions and pressurization and heating of a 20km-thick reacting layer initially located at 20–40 km depth. The density change and subsidence calculated using the widely used expression q ¼ q0 ð1 aDTÞ (q0 = 2,900 kg/m3, a = 3.28 9 10-5; McKenzie 1978) to obtain the Dq of this lower crustal layer are also shown for comparison. The cold and thick lithosphere (200 km) and moderate pressure increase (0.1 GPa; Fig. 15a) may be more relevant for cratonic interiors, whereas the large pressure increase (0.95 GPa) and hotter lithosphere (120 km, Fig. 15b) may describe the situation at a continental margin, e.g., in a foreland basin. All rock types densify when pressure increases, but while Dq is similar for wet and dry rocks with the same composition in a cold lithosphere (Fig. 15a), it is significantly larger for dry rocks than for wet rocks in a thinner and hotter lithosphere (Fig. 15b). After the 0.1 GPa pressure increase, the densities of all rock types except the wet average lower crust and wet MORB decrease as a response to heating, which would result in uplift. The wet mafic rocks have almost constant Fig. 14 Density contrast (Dq [kg/m3]) contoured as a result of pressurization (DP) and heating (DT) of a 20-km-thick lower crustal layer initially located at 20–40 km depth (Pinitial = 0.56–1.20 GPa, Tinitial = 300–476°C). Initial lithosphere thickness: 140 km, temperature at the base of the lithosphere: 1,300°C. For these model, parameters density contrasts of 351 and -197 kg/m3 correspond to a maximum isostatic waterloaded subsidence of 3,162 m and maximum uplift (no erosion) of 1,813 m, respectively. a hydrated meta-pelite, b hydrated Fe–Mg pelite, c hydrated MORB, d metapelite with 1.5 wt% water, e Fe–Mg pelite with 1.5 wt% water, f MORB with 1.5 wt% water, g dry meta-pelite, h dry Fe–Mg pelite and i dry MORB 123 Int J Earth Sci (Geol Rundsch) Fig. 15 Calculated mean Dq of the reacting layer and resulting water-loaded isostatic subsidence curves for selected compositions. Solely temperature-dependent density (q = q0 (1 - aDT)) model is shown for comparison. Reacting layer thickness and initial depth as in Fig. 14. a Typical cratonic conditions: initial lithospheric thickness = 200 km, small pressure increase by 0.1 GPa which may be caused by e.g., intra-plate stresses, flexure, or loading. Subsequent thermal re-equilibration follows the timescale of thermal conduction ([ 100 Ma). All compositions produce subsidence due to loading, but dry rocks expand and uplift upon heating. Dry MORB expands more than 5 times more than predicted by the commonly used thermal expansion coefficient over the temperature range of 173°C. Hydrated compositions contract or expand depending on P–T conditions. b Initially, 120-km-thick lithosphere and large pressure increase (0.95 GPa), which may be expected for large burial (orogeny, subduction) and/or strong compression (over-pressure). Dry rocks contract more during pressurization, but expand during heating, while wet compositions show protracted densification during heating, in particular the Fe–Mg-rich pelite. Near-linear scaling of subsidence with Dq breaks down for large Dq densities and expand slightly at DT [ 120°C. The solely temperature-dependent density approximates the density evolution of the wet pelites, but strongly underestimates the thermal expansion of dry MORB (Fig. 15a). The large pressure increase applied in Fig. 15b results in significant (500–1,350 m) water-loaded subsidence. Upon heating, the dry rocks then expand and uplift as predicted by 123 q ¼ q0 ð1 aDTÞ, while all hydrous compositions continue to contract and subside. The largest densification and subsidence (2,850 m) is reached by the Fe–Mg-rich pelitic composition for the given conditions (Fig. 15b). These results are remarkable given that most previous phase change models are based on reactions in dry basalt. If thermodynamic equilibrium is assumed, however, a dry basaltic layer at 20–40 km depth will already be very dense (in the garnet granulite and eclogite fields). The average density will increase significantly during burial, but decrease again during thermal equilibration, so subsidence is transient and not permanent. A wet meta-pelite that has lost a bit of silicic melt densifies due to pressure- and temperature-dependent reactions and may be a more suitable candidate for subsidence related to reactions. Moreover, our Fe–Mg-rich pelite composition is close to some estimates for average middle and lower crust and may constitute a large part of normal continental crust (Schenk 1984, 1990). Schenk (1990) calculated an estimated bulk composition for the meta-pelite unit as well as for the whole lower crust of the exposed lower crustal section in Calabria based on rock compositions and detailed mapping (both estimates are added to Table 4 for comparison). The lower crust is expected to be very heterogeneous with metabasic units as well as highly restitic pelitic rocks (Schenk 1990). The Fe–Mg-rich composition (mix14p) that we used for our calculation is close to the composition of these pelitic restites and may therefore be a good representative for a lower crustal bulk composition. Although most studies based on the composition of rare lower crustal xenoliths and seismic data suggest that the average composition of the lower crust is mafic (Pakiser and Robinson 1966; Rudnick and Fountain 1995; Rudnick and Gao 2003), we have to keep in mind that it is lithologically heterogeneous (Rudnick and Fountain 1995; Rudnick and Gao 2003). Furthermore, studies of exposed crustal cross sections reveal that the lower crust can be intermediate to silicic (Gray 1977; Fountain and Salisbury 1981) and that a major part of the lower crust may consist of meta-pelitic rocks with only the lowermost part being metabasic (Schenk 1990). In summary, we show that crustal phase transitions cause a significant densification of the crust as a response to burial. In contrast to expectations, the largest density changes are not caused by pressurization of (dry) basalts, but by heating (and pressurization) of initially hydrated meta-pelites. Negative buoyancy of a hydrous lower crust buried to approximately twice its initial depth will be significant (Dq * 300 kg/m3) and may result in 1–5 km of water-loaded subsidence, depending on initial depth and thickness of the reacting layer. Density change predicted by density as a function of thermal expansion only, in contrast, Int J Earth Sci (Geol Rundsch) shows the opposite behavior to our realistic density calculations (density decreases as a response to heating), and Dq is an order of magnitude smaller (*20–30 kg/m3, Fig. 15). Discussion and application Recently, Massonne et al. (2007) stated that our knowledge about precise density data as a function of rock composition and P–T conditions is very scarce. We are aware of only five previous studies on the density of crustal rocks at typical P–T and fluid conditions of crustal metamorphism computed by energy minimization methods. Jull and Kelemen (2001) investigated dry, mafic compositions at high temperatures ([800°C) only. Gerya et al. (2001) presented models using densities of fully hydrated crustal rocks at low pressure (\1 GPa). More recently, Hetényi et al. (2007) considered an averaged lower crustal bulk composition at three hydration levels: dry, with 1 wt% H2O and fully hydrated. Massonne et al. (2007) compared density diagrams of ten rock compositions at fully hydrated conditions, excluding the average lower crust composition studied by Hetényi et al. (2007). Although the studies of Hetényi et al. (2007) and Massonne et al. (2007) are complimentary, they are conducted using different software packages and solution models. Tassara (2006) compared extensive density calculations for anhydrous rocks using the approach of Sobolev and Babeyko (1994) and densities for three compositions characteristic of the lower crust under fully hydrated conditions computed by Perple_X. We presented here a more systematic comparison of the densities of lower crustal rocks at lithospheric P–T conditions and constructed ‘‘four-dimensional’’ P–T-q-X diagrams (Figs. 4, 10). The results of our calculations can be used to improve the interpretation of geophysical data (particularly gravity) and in kinetic and dynamic numerical models of basin formation, dynamics of mountain ranges and gravitational instabilities. In the following sections, we will discuss some geodynamic applications of our modeling results to settings where the lower crust is buried into the mantle, namely mountain ranges and intra-cratonic basins. Application to the preservation of orogenic roots The simplest model of mountain building involves thickening of the crust in a compressional setting, where the high topography of the mountain range is maintained because the density of the crustal root is lower than that of the surrounding mantle. With time, topography is removed by erosion, but the buoyant root leads to further uplift until the mountains are worn flat and the root is leveled again (e.g., James 2002). Alternative models propose eclogitization and delamination of the root, leading to collapse of the mountain range and eventually also to leveling of the topography and the crust-mantle boundary (e.g., Bird 1979). However, some old mountain ranges preserve their thick crustal root. Fischer (2002) argued that this preservation is due to metamorphic reactions that reduce the density contrast between the lower crust and the mantle. An alternative explanation based on the eclogitization and delamination model was provided by Leech (2001) who proposed that the Urals did not loose their crustal root because there was not enough fluid available to overcome kinetic boundaries and transform the lower crust from gabbro to eclogite. Both authors assumed that the lower crust consists of dry basalt and that the transformation from gabbro to eclogite is the only reaction that causes density changes. To get the Dq [ 300 kg/m3 that is needed for both hypotheses to work, Moho temperatures in the thickened crust initially have to be very high and then gradually cool to normal continental conditions. Fischer (2002) had to invoke Moho temperatures of 900–1,000°C during orogeny and cooling to 400°C in roughly 300 Ma in order to explain the gradual decrease in density contrast between lower crust and mantle and associated decrease in the ratio of mountain surface relief to crustal root thickness from active mountain belts to ancient orogens. We propose that our findings on the density variations in buried lower crust allow for re-evaluation of these hypotheses for the preservation of ancient crustal roots. We show that mafic rocks are not the only ones that produce large density contrasts during metamorphism. Moreover, most possible crustal compositions (with the exception of entirely dry rocks) experience the largest densification as a response to heating, in addition to pressure increase, and not due to cooling. According to our calculations, dry MORB densities increase by 220 kg/m3 for cooling from 900 to 500°C at 1.35 GPa, consistent with the assumptions of Fischer (2002). The same cooling at higher pressures would produce smaller Dq. However, sluggish kinetics will probably severely inhibit reactions in an entirely dry rock, especially at the lower temperatures. If the MORB is hydrated, which may help to speed up reaction kinetics, however, the net increase in density during the same cooling interval is close to zero (compare diagrams for wet and dry MORB in Fig. 10). In contrast, heating of a lower crust consisting of more felsic rocks, hydrated meta-mafic rocks, or a mixture of those by 300°C (e.g., from 400 to 900°C) at 1.35 GPa results in a density increase of 250–300 kg/m3 (Fig. 12). Considering absolute densities, dry metamorphic MORB (eclogite) at 1.35 GPa and 500°C has q = 3,400 kg/m3, increasing to 3,450 kg/m3 at 1.5 GPa. These densities are indeed higher than those of the mantle under these 123 Int J Earth Sci (Geol Rundsch) conditions and should be detectable due to higher seismic velocities (e.g., Mengel and Kern 1992). Moreover, the density contrast may indeed lead to delamination of the lower crust if the lithosphere is not rigid enough to hold this dense body. Fischer (2002) argues that isostatic equilibrium is maintained and isostatic uplift continues during erosion of the mountain range, which means that the crust and mantle are weak enough to deform. Meta-pelitic crust that is buried and heated, in contrast, displays Dq as large as the gabbro to eclogite transition, but has a density at 1–2 GPa that is lower or equivalent to that of the mantle, depending on temperature (Fig. 12). The real lower crust most likely consists of a mixture of mafic and more felsic components resulting in average crustal root densities intermediate between those of granulite/eclogite and metapelite under equilibrium conditions. Such a scenario could explain the observation of Fischer (2002) on the preservation of crustal roots, as well as the gradual decrease in density contrast with time. It may also be consistent with the observations of Leech (2001) since metamorphism of a crust that is not completely mafic will produce similar features to a dry, mafic lower crust that only partly reacted to eclogite. Of course, we cannot exclude metastability; the occurrence of incomplete reactions is well documented (e.g., Austrheim 1990). We hypothesize, however, that the metamorphic densification of crustal roots may be caused by conductive heating of the initially cold thickened lithosphere over more than 100 million years instead of conductive cooling of an initially hot orogen. Our hypothesis can be tested in future modeling studies, but the general arguments outlined here illustrate the importance of detailed studies of equilibrium density as a function of P–T-X. Application to large basins The phase change model for subsidence in intra-cratonic basins has been extensively studied using numerical and analytical models (Joyner 1967; O’Connell and Wasserburg 1967, 1972; Haxby et al. 1976; Middleton 1980; Sleep et al. 1980; Mareschal and Lee 1983; Mareschal 1987; Hamdani et al. 1991, 1994; Baird et al. 1995; Artyushkov 2005, 2010). Several models have focused on the detailed temperature evolution of the lithosphere loaded by sediments (Sleep et al. 1980; Mareschal and Lee 1983; Mareschal 1987) and/or subjected to a change in heat flow at the base of the lithosphere (Haxby et al. 1976; Hamdani et al. 1991, 1994), including the effect of latent heat of reaction (O’Connell and Wasserburg 1967) and sediment blanketing (O’Connell and Wasserburg 1972). The most investigated phase transition is the one from gabbro or granulite to eclogite which occurs close to continental Moho depths (Lovering 1958; Kennedy 1959; Joyner 1967; 123 Ahrens and Schubert 1975a, b; Mengel and Kern 1992; Artyushkov 2005, 2010), but the transition from greenschist to amphibolite facies in the mid-lower crust as a response to heating has also been invoked to explain the subsidence in the Cooper and Eromanga basins in Australia (Middleton 1980). Most models explored an increase in pressure due to loading of the lithosphere by sediments (O’Connell and Wasserburg 1967, 1972; Sleep et al. 1980; Mareschal and Lee 1983; Mareschal 1987), but a pressure increase due to compression or horizontal load has also previously been suggested as an important mechanism (Cloetingh and Kooi 1992; Hartz et al. 2007). Some authors proposed a metastable layer of gabbro at the base of the crust that converts directly to eclogite triggered by fluids or heating (Haxby et al. 1976; Datondji 1981; Artyushkov 2005, 2010). In all those models, the transition is approximated as one reaction line with a certain Clapeyron slope and fixed Dq. The East Barents Sea Basin is one of the world’s deepest basins with up to 20 km of sediments. As already mentioned, the formation mechanisms of intra-cratonic basins are not well understood. Consequently, previous studies do not agree on the underlying cause for the formation of the Barents Sea Basin (O’Leary et al. 2004; Ebbing et al. 2007; Ritzmann et al. 2007). One of the striking features of the Barents Sea is a generally flat Moho over large parts of the region at a depth of 35–37.5 km in the East Barents Sea area (Ebbing et al. 2007). In addition, the Moho depth does not reflect observed changes in the depth to the basement, whereas crustal thickness and Moho geometry should correlate according to simple crustal extension models for basin formation (McKenzie 1978). In summary, the depth of the Moho appears to be largely unaffected by the processes leading to the formation of the deep basin in the Eastern Barents Sea and the isostatic Moho is 8 km shallower than the seismic Moho (Ebbing et al. 2007). Moreover, there are large differences between the observed and modeled gravity field in conventional models. Since the structure of the upper crust is relatively better known, the additional mass needed to account for the observed gravity is assumed to reside in the lower crust or the mantle (Ebbing et al. 2007; Ritzmann et al. 2007). While Ebbing et al. (2007) argue for this excess mass to be in the mantle, Neprochnov et al. (2000) concluded that the high-velocity layers below the basin may represent a crust-mantle rock mixture in a zone of old rifting. Artyushkov (2005) excludes oceanic crust below the thick sedimentary cover and instead suggests a high-grade metamorphic lower crustal layer below the seismic Moho. He proposed a model where a mafic body of more than 20 km thickness with an initial density of 2,900 kg/m3 below the seismic Moho is transformed to an eclogite with a constant density of 3,500 kg/m3, in order to account for the observed Int J Earth Sci (Geol Rundsch) tectonic subsidence. Based on geophysical observations, he also argues that the East Barents Sea did not experience significant stretching and that the main subsidence occurred in a compressional setting in the Permo-Triassic. Even though this latter statement is controversial, it has been confirmed recently by Petrov et al. (2008) who interpret the East Barents basin as the foredeep caused by the Uralian collision and orogeny. Werner et al. (2010, in press) also state that the E. Barents region has been under compression since the Early Mesozoic. If we accept this interpretation, the E. Barents basin may represent a good test case for basin formation in a compressional setting due to crustal phase changes. Sediment loading amplifies subsidence, in particular in connection with phase changes, but an initial depression is needed which must have been caused by other processes. Hence, we suggest deflection of the lithosphere, so called buckling, in a compressional tectonic setting. Although such a lithospheric buckling should not take place if linear elasticity is assumed for the lithosphere, it has been reproduced by numerical models using more realistic lithospheric rheologies (Burg and Podladchikov 1999; Schmalholz and Podladchikov 2000; Schmalholz et al. 2002, 2005; Kaus and Schmalholz 2006; Burg and Schmalholz 2008; Burov and Cloetingh 2009). Burg and Podladchikov (1999) showed that a basic response of stratified lithosphere to compression is buckling independent of the thermal regime and that the folding of the lithosphere is mechanically preferable to homogeneous thickening. In addition, cold and therefore stronger lithosphere shows higher amplitude folding with a longer wavelength than hot lithosphere. Since the main explanation for the collapse of the buckles is the density contrast between the crust and the mantle at the crust-mantle boundary (Allen and Allen 2005), the densification of the lower crust by phase transitions may explain how buckles are maintained over geological timescales. A simplified cartoon model for the possible evolution of a basin formed by buckling and crustal phase changes is presented in Fig. 16. The initial setup consists of three major layers: the upper crust, the lower crust, and the mantle (Fig. 16a). The Moho is shown as the boundary between a lower density and a higher density rock, which may be the metamorphosed lower crust or the mantle. In a compressional setting, a horizontal force is applied to the initial rock column. This results in the buckling of the lithosphere (Fig. 16b). As a result of buckling, the crustmantle boundary is deepened, and lower crustal rocks are exposed to higher pressures. This pressure increase results in phase changes and a densification of the rocks, which is illustrated by the P arrow in the density diagram (Fig. 12), causing a water-loaded isostatic subsidence of up to 1,500 m (Figs. 14, 15). At the same time, sedimentation starts in the newly formed basin and additional subsidence is caused due to sediment loading. In addition to the increase in pressure, the lower crustal rocks are also Fig. 16 Conceptual model for subsidence due to compression and phase transitions, a initial condition; b stage 1: buckling of the lithosphere as a result of compression results in deepening of the crust-mantle boundary and therefore pressure increase. Additional loading due to sedimentation and densification of the lower crust due to pressure-dependent reactions leads to fast subsidence (depending on deformation and sedimentation rates); c stage 2: thermal equilibration and further density increase lead to more and prolonged subsidence on the timescale of thermal conduction. Relaxation of compressional stresses may lead to some pressure release and partial retrogression in the lower crust; d stage 3: stable state in isostatic equilibrium. See Fig. 12 for an approximate P–T path 123 Int J Earth Sci (Geol Rundsch) exposed to higher temperatures, but the thermal equilibration is supposed to be slower since it is governed by thermal diffusion through the lithosphere (Fig. 16c). An increase in T results in further reactions and density increase, as shown by the T arrow in Fig. 12. Our simple subsidence calculations yield 3,500 m of isostatic tectonic subsidence after pressurization and thermal equilibration (Fig. 14). Relaxation of compressional stresses may lead to some pressure release and partial retrogression in the lower crust (Gac et al. 2008, manuscript in preparation). The last stage represents complete isostatic re-equilibration with a lens of eclogite facies (but probably not eclogitic) rocks sitting between the crust and the mantle (Fig. 16d). This lens is denser than the former crustal rocks but still lighter than the mantle and may account for the additional mass required to explain the gravity signal in the E. Barents basin (Ebbing et al. 2007; Ritzmann et al. 2007). Due to the denser and seismically faster material in the crustal lens, the seismic Moho is also shifted to lower depths. In fact, crust-mantle transition zones characterized by Vp in the range of 7.0–7.9 km/s have been identified in many seismic profiles (Baird et al. 1995; Ziegler and Cloetingh 2004), and such a lens of intermediate velocities was also detected in the E. Barents Sea (Ivanova et al. 2006). The crustal compositions investigated here show major density jumps around 1.2–2.5 GPa, depending on temperature, and the more mafic pelite compositions, the Fjørtoft gneiss, and the dry and hydrous basalts reach densities close to or higher than those of the mantle at pressures higher than 1.5 GPa in a warm lithosphere and \2 GPa in a 200-km-thick cold lithosphere. While we propose that the E. Barents basin today is in a situation similar to that shown in Fig. 16d, Fig. 16b, c might be representative for other basins, where phase transitions did not go to completion; bending of the plate due to loading is not compensated by densification of the lower crust. An example of such a basin that did not reach isostatic equilibrium and displays a gravity anomaly is the Congo basin (Downey and Gurnis 2009). In contrast, a typical basin originally caused by plate bending, the PreUralian Foredeep, does not show a pronounced gravity anomaly, and the light sedimentary infill appears to be compensated by dense lower crustal material (Döring and Götze 1999). This may indicate that densification of lower crust as a response to burial is responsible for the preservation of both, the Uralian foreland basin and the thick crustal root of the Uralian Mountains. The situation in the Pre-Uralian Foredeep may be analogue to that of the E. Barents Sea and indeed Petrov et al. (2008) interpret the E. Barents basin as (another) foredeep related to the Uralian collision and orogeny. Our isostasy calculations show that a reacting layer with a minimum thickness of 30 km and a density contrasts 123 similar to that of our Fe–Mg-rich pelite model composition is needed to produce the isostatically balanced tectonic subsidence observed in the E. Barents basin during the Permo-Triassic, if the total load is around 1 GPa (Fig. 14). To test and validate our conceptual model, however, it has to be investigated how high the amplitude of the buckling needs to be to generate sufficient lower crustal burial and how much additional pressure is generated due to horizontal loading. The timescale of these processes depends on the speed of deformation, sedimentation, and thermal equilibration. The coupling of phase transitions with a fully coupled thermo-mechanical dynamic finite element model and application to the East Barents Sea basin is in progress (Gac et al. 2008, manuscript in preparation). However, because substantial extension and crustal thinning can be ruled out in a compressional setting, the lower crust is in any case buried to larger depth in such a sedimentary basin. Therefore, our general approach for the analyses of subsidence due to phase changes remains valid independent of the mechanism that leads to the deepening of the crust-mantle boundary. Most intra-cratonic basins, however, are much shallower than the E. Barents Sea and are characterized by a stepwise pattern of fast subsidence followed by long-term slow subsidence or uplift (e.g., Haddad et al. 2001; Armitage and Allen 2010). Our model predicts abrupt subsidence due to pressurization, followed by a period of extended slow subsidence or even uplift (Fig. 15). The phase transition model we propose may therefore explain the observed stepwise subsidence in intra-cratonic basins if compressional events occur repeatedly or periodically, similar to the model proposed by DeRito et al. (1983). The quantitative validation of our model, however, requires more advanced thermo-mechanical modeling and comparison with actual data. Conclusions We present systematic calculations of densities as a function of pressure and temperature for a large range of dry and hydrated compositions from pelitic to mafic using an internally consistent thermodynamic data set. Our calculations show that large density changes can occur due to pressure- and temperature-dependent reactions and hence that jumps in densities (and therefore also seismic velocities) do not necessarily imply a different rock composition. We show, however, that density also strongly depends on rock composition. Density increases linearly with increasing Al2O3 as well as MgO and FeO contents in the rocks. Moreover, Al- and Fe-rich rocks yield the highest absolute densities. Compositional variations can easily cause more than 6% density variation at fixed P–T conditions; at Int J Earth Sci (Geol Rundsch) certain conditions, meta-pelitic rocks can become denser than meta-mafic rocks. Our new density quantification is useful not only for the specific application to geodynamic settings where the crust is thickened, but in general also for e.g., improved gravity modeling, kinetic and dynamic basin models, and models involving gravitational instability. Densities extracted along a continental shield geotherm show that all hydrated rocks yield a larger Dq along the P–T path than the dry compositions and that a wet Fe- and Mgrich meta-pelite has the largest Dq. While dry MORB yields the highest absolute densities, the increase in equilibrium density (Dq) is small compared to many other compositions. In fact, all rocks that contain some hydrous minerals show densification as a response to heating, opposite to the behavior of dry meta-mafic rocks, which most studies investigating the geodynamic implications of lower crustal density changes used as representative compositions. By investigating the effect of lower crustal burial on densities and evaluating the resulting subsidence, we found that a wet meta-pelite rich in Fe and Mg consequently also yields much larger subsidence than a dry MORB. The density change due to thermal expansion, an extensively used concept in geodynamic models, is one order of magnitude smaller than Dq calculated from our diagrams for burial of lower crust. Moreover, the density predicted using q = q0(1 - aT) decreases, whereas in real crustal rocks, densities may increase due to pressurization and heating. For the Fe–Mg-rich pelite and wet average mafic lower crust compositions, water-loaded subsidence is substantial and increases with the thickness and depth of the reacting layer, while dry MORB shows much less subsidence. These results are remarkable given that most previous phase change models are based on reactions in dry basalt. Moreover, our Fe–Mg-rich pelite composition is close to estimates for average middle and lower crust and may constitute a large part of normal continental crust. Phase transitions and compositional variations in the mantle have a significant effect on geodynamics (Simon and Podladchikov 2008). Due to the wide compositional range and large P–T-X-dependent density variations in crustal rocks, the influence of the crustal density on geodynamic processes should be even bigger, provided the crust makes up a significant part of the lithospheric column. This is the case in stable continental shields and if continental crust is thickened, e.g., due to compression. Acknowledgments This research was funded through a Norwegian Research Council grant to J. S. and N. S. within the Petrobar Project. We thank our partners in the Petrobar project, in particular R. Huismans, S. Gac, and J. I. Falleide, and our colleagues at PGP, in particular J. C. Vrijmoed, for discussions and two anonymous reviewers for helpful criticism on a previous version of the manuscript. Two more reviews helped to condense and finalize the paper. Appendix For simplicity, reactions are formulated in the most relevant system, either MASH, FASH, or NASH. Cpx, Ky, and Grt-forming reactions at high-P, high-T conditions: ðaÞ 3MgCp þ Qtz ¼ 3Ky þ Tlc þ 5H2 O ðbÞ 3MgAl2 Si2 O6 ðOHÞ4 þSiO2 ð3Þ ¼ 3Al2 SiO5 þ Mg3 Si4 O10 ðOHÞ2 þ5H2 O ðaÞ 3FeCld þ 2Qtz ¼ Alm þ 2Ky þ 3H2 O ðbÞ 3FeAl2 SiO5 ðOHÞ2 þ 2SiO2 ¼ Fe3 Al2 Si3 O12 ð4Þ þ 2Al2 SiO5 þ 3H2 O ðaÞ Pg ¼ Ky þ Jd þ H2 O ðbÞ NaAl3 Si3 O10 ðOHÞ2 ¼ Al2 SiO5 þ NaAlSi2 O6 þ H2 O ð5Þ ðaÞ Ab ¼ Jd þ Qtz ð6Þ ðbÞ NaAlSi3 O8 ¼ NaAlSi2 O6 þ SiO2 Grt, Tlc, Cp, and Cld-forming reactions at lower P, intermediate-T: ðaÞ 3Clin þ 13Qtz ¼ 4Tlc þ 3MgCp þ 2H2 O ðbÞ 3Mg5 Al2 Si3 O10 ðOHÞ8 þ 13SiO2 ¼ 4Mg3 Si4 O10 ðOHÞ2 þ3MgAl2 Si2 O6 ðOHÞ4 þ 2H2 O ð7Þ ðaÞ 3Clin þ 10Qtz ¼ 3MgCld þ 4Tlc þ 5 H2 O ðbÞ 3Mg5 Al2 Si3 O10 ðOHÞ8 þ10SiO2 ¼ 3MgAl2 SiO5 ðOHÞ2 þ 4Mg3 Si4 O10 ðOHÞ2 þ 5H2 O ð8Þ ðaÞ 3Clin þ 8Qtz ¼ 3Prp þ 2Tlc þ 10H2 O ðbÞ 3Mg5 Al2 Si3 O10 ðOHÞ8 þ 8SiO2 ¼ 3Mg3 Al2 Si3 O12 þ 2Mg3 Si4 O10 ðOHÞ2 þ10H2 O ð9Þ ðaÞ 3Daph þ 3Ames þ 12Qtz ¼ 4Prp þ 5Alm þ 24H2 O ðbÞ 3Fe5 Al2 Si3 O10 ðOHÞ8 þ 3Mg4 Al4 Si2 O10 ðOHÞ8 þ 12SiO2 ¼ 4Mg3 Al2 Si3 O12 þ 5Fe3 Al2 Si3 O12 þ 24H2 O ð10Þ ðaÞ 31Daph þ 41Ms ¼ 41Ann þ 8FeSt þ 33Qtz þ 108 H2 O ðbÞ 31Fe5 Al2 Si3 O10 ðOHÞ8 þ 41KAl3 Si3 O10 ðOHÞ2 ¼ 41KFe3 AlSi3 O10 ðOHÞ2 þ 8Fe4 Al18 Si7:5 O44 ðOHÞ4 þ 33SiO2 þ 108 H2 O ð11Þ ðaÞ Ann þ Sil þ 2Qtz ¼ Alm þ San þ H2 O ðbÞ KFe3 AlSi3 O10 ðOHÞ2 þ Al2 SiO5 þ 2SiO2 ¼ Fe3 Al2 Si3 O12 þ KAlSi3 O8 þ H2 O ð12Þ 123 Int J Earth Sci (Geol Rundsch) ðaÞ 4Ann þ 3FeCrd þ 3Qtz ¼ 6Alm þ 4San þ 4H2 O ðbÞ 4KFe3 AlSi3 O10 ðOHÞ2 þ 3Fe2 Al4 Si5 O18 þ 3SiO2 ¼ 6Fe3 Al2 Si3 O12 þ 4KAlSi3 O8 þ 4H2 O ð13Þ ðaÞ 3FeCrd ¼ 2Alm þ 4Si þ 5Qtz ðbÞ 3Fe2 Al4 Si5 O18 ¼ 2Fe3 Al2 Si3 O12 þ 4Al2 SiO5 þ 5SiO2 ð14Þ ðaÞ FeCrd þ Fs ¼ 2Alm þ 3Qtz ðbÞ Fe2 Al4 Si5 O18 þ Fe2 Si2 O6 ¼ 2Fe3 Al2 Si3 O12 þ 3SiO2 ð15Þ ðaÞ Phl þ East þ 6Qtz ¼ Prp þ 2Cel ðbÞ KMg3 AlSi3 O10 ðOHÞ2 þ KMg2 Al3 Si2 O10 ðOHÞ2 þ 6SiO2 ¼ Mg3 Al2 Si3 O12 þ 2KAlMgSi4 O10 ðOHÞ2 ð16Þ References Ahrens TJ, Schubert G (1975a) Gabbro-eclogite reaction rate and its geophysical significance. Rev Geophys Space Phys 13:383–400 Ahrens TJ, Schubert G (1975b) Rapid formation of Eclogite in a slightly wet mantle. Earth Planet Sci Lett 27:90–94 Allen PA, Allen JR (2005) Basin analysis: principles and applications. Blackwell, Oxford Armitage JJ, Allen PA (2010) Cratonic basins and the long-term subsidence history of continental interiors. J Geol Soc 167:61–70 Artyushkov EV (2005) The formation mechanism of the Barents Basin. Russ Geol Geophys 46:700–713 Artyushkov EV (2010) The superdeep North Chukchi Basin: formation by eclogitization of continental lower crust, with petroleum potential implications. Russ Geol Geophysics 51:48–57 Austrheim H (1990) The granulite-eclogite facies transition: a comparison of experimental work and a natural occurrence in the Bergen Arcs, western Norway. Lithos 25:163–169 Austrheim H (1991) Eclogite formation and dynamics of crustal roots under continental collision zones. Terra Res 3:492–499 Ayele A (2002) Active compressional tectonics in central Africa and implications for plate tectonic models: evidence from fault mechanism studies of the 1998 earthquakes in the Congo basin. J Afr Earth Sci 35:45–50 Baird DJ, Knapp JH, Steer DN, Brown LD, Nelson KD (1995) Uppermantle reflectivity beneath the Williston basin, phase-change Moho, and the origin of intracratonic basins. Geology 23:431– 434 Beaumont C (1981) Foreland Basins. Geophys J R Astr Soc 65:291– 329 Bird P (1979) Continental delamination and the Colorado Plateau. J Geophys Res 84:7561–7571 Bousquet R, Goffe B, Henry P, LePichon X, Chopin C (1997) Kinematic, thermal and petrological model of the Central Alps: Lepontine metamorphism in the upper crust and eclogitisation of the lower crust. Tectonophysics 273:105–127 Burg JP, Podladchikov Y (1999) Lithospheric scale folding: numerical modelling and application to the Himalayan syntaxes. Int J Earth Sci 88:190–200 Burg JP, Schmalholz SM (2008) Viscous heating allows thrusting to overcome crustal-scale buckling: numerical investigation with application to the Himalayan syntaxes. Earth Planet Sci Lett 274:189–203 123 Burov E, Cloetingh S (2009) Controls of mantle plumes and lithospheric folding on modes of intraplate continental tectonics: differences and similarities. Geophys J Int 178:1691–1722 Cloetingh S, Kooi H (1992) Intraplate stresses and dynamical aspects of rifted basins. Tectonophysics 215:167–185 Connolly JAD (1990) Multivariable phase diagrams: an algorithm based on generalized thermodynamics. Am J Sci 290:666–718 Connolly JAD, Kerrick DM (1987) An algorithm and computer program for calculating composition phase diagrams. CALPHAD 11:1–55 Connolly JAD, Petrini K (2002) An automated strategy for calculation of phase diagram sections and retrieval of rock properties as a function of physical conditions. J Metamorph Geol 20:697–709 Datondji A (1981) The mechanical evolution of the Williston basin. Master’s Thesis, State University of New York DeCelles PG, Giles KA (1996) Foreland basin systems. Basin Res 8:105–123 DeRito RF, Cozzarelli FA, Hodge DS (1983) Mechanism of subsidence of ancient Cratonic Rift Basins. Tectonophysics 94:141–168 Döring J, Götze HJ (1999) The isostatic state of the southern Urals crust. Geol Rundsch 87:500–510 Downes H (1993) The nature of the lower continental-crust of Europe—petrological and geochemical evidence from Xenoliths. Phys Earth Planet In 79:195–218 Downey NJ, Gurnis M (2009) Instantaneous dynamics of the cratonic Congo basin. J Geophys Res 114. doi:10.1029/2008JB006066 Ebbing J, Braitenberg C, Wienecke S (2007) Insights into the lithospheric structure and tectonic setting of the Barents Sea region from isostatic considerations. Geophys J Int 171:1390– 1403 Fischer KM (2002) Waning buoyancy in the crustal roots of old mountains. Nature 417:933–936 Fountain DM, Salisbury MH (1981) Exposed cross-sections through the continental-crust—implications for crustal structure, petrology, and evolution. Earth Planet Sci Lett 56:263–277 Gac S, Huismans R, Simon NSC, Semprich J, Podladchikov Y (2008) Are phase transitions the origin of the large subsidence of the Barents Sea basin? Insights from dynamic modeling. 33rd International Geological Congress, Oslo Gerya TV, Maresch WV, Willner AP, Reenen DDV, Smit CA (2001) Inherent gravitational instability of thickened continental crust with regionally developed low- to medium-pressure granulite facies metamorphism. Earth Planet Sci Lett 190:221–235 Goffe B, Bousquet R, Henry P, Le Pichon X (2003) Effect of the chemical composition of the crust on the metamorphic evolution of orogenic wedges. J Metamorph Geol 21:123–141 Gray CM (1977) Geochemistry of Central Australian granulites in relation to chemical and isotopic effects of granulite facies metamorphism. Contrib Mineral Petrol 65:79–89 Green E, Holland TJB, Powell R (2007) An order-disorder model for omphacitic pyroxenes in the system jadeite-diopside-hedenbergite-acmite, with applications to eclogitic rocks. Am Mineral 92:1181–1189 Haddad D, Watts AB, Lindsay J (2001) Evolution of the intracratonic Officer Basin, central Australia: implications from subsidence analysis and gravity modelling. Basin Res 13:217–238 Hamdani Y, Mareschal JC, Arkanihamed J (1991) Phase-changes and thermal subsidence in intracontinental sedimentary basins. Geophys J Int 106:657–665 Hamdani Y, Mareschal JC, Arkanihamed J (1994) Phase-change and thermal subsidence of the Williston Basin. Geophys J Int 116:585–597 Hartz EH, Podladchikov YY, Medvedev S, Faleide JI, Simon NSC (2007) Force, energy and mass balanced basin models: new concepts and Arctic examples. Geophys Res Abstr 9:10468 Int J Earth Sci (Geol Rundsch) Haxby WF, Turcotte DL, Bird JM (1976) Thermal and mechanical evolution of Michigan Basin. Tectonophysics 36:57–75 Hetényi G et al (2007) Density distribution of the India plate beneath the Tibetan plateau: geophysical and petrological constraints on the kinetics of lower-crustal eclogitization. Earth Planet Sci Lett 264:226–244 Holland TJB, Powell R (1996) Thermodynamics of order-disorder in minerals: II. Symmetric formalism applied to solid solutions. Am Mineral 81:1425–1437 Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343 Holland T, Baker J, Powell R (1998) Mixing properties and activitycomposition relationships of chlorites in the system MgO-FeOAl2O3-SiO2-H2O. Eur J Mineral 10:395–406 Ivanova NM, Sakoulina TS, Roslov YV (2006) Deep seismic investigation across the Barents-Kara region and Novozemelskiy Fold Belt (Arctic Shelf). Tectonophysics 420:123–140 James D (2002) How old roots lose their bounce. Nature 417:911–913 Jaupart C, Mareschal JC (2007) Heat flow and thermal structure of the lithosphere. In: Schubert G (ed) Treatise on geophysics. Crust and Lithosphere, vol 6. Elsevier, Amsterdam, pp 217–251 Joyner WB (1967) Basalt-eclogite transition as a cause for subsidence and uplift. J Geophys Res 72:4977–4998 Jull M, Kelemen PB (2001) On the conditions for lower crustal convective instability. J Geophys Res 106. doi:10.1029/2000jb 900357.10.1029/2000jb900357 Kaus BJP, Schmalholz SM (2006) 3D finite amplitude folding: implications for stress evolution during crustal and lithospheric deformation. Geophys Res Lett 33. doi:10.1029/2006GL02 6341 Kaus BJP, Connolly JAD, Podladchikov YY, Schmalholz SM (2005) Effect of mineral phase transitions on sedimentary basin subsidence and uplift. Earth Planet Sci Lett 233:213–228 Kennedy GC (1959) The origin of continents, mountain ranges, and ocean basins. Am Sci 47:491–504 Kretz R (1983) Symbols for rock-forming minerals. Am Mineral 68:277–279 Kruse S, McNutt M (1988) Compensation of paleozoic orogens—a comparison of the urals to the appalachians. Tectonophysics 154:1–17 Le Pichon X, Henry P, Goffe B (1997) Uplift of Tibet: from eclogites to granulites—implications for the Andean Plateau and the Variscan belt. Tectonophysics 273:57–76 Leech ML (2001) Arrested orogenic development: eclogitization, delamination, and tectonic collapse. Earth Planet Sci Lett 185:149–159 Lovering JF (1958) The nature of the Mohorovicic discontinuity. Trans Am Geophys Union 39:947–955 Mahar EM, Baker JM, Powell R, Holland TJB, Howell N (1997) The effect of Mn on mineral stability in metapelites. J Metamorph Geol 15:223–238 Mareschal JC (1987) Dynamic behaviour of a phase boundary under non-uniform surface loads. Geophys J R Astr Soc 54:703–710 Mareschal JC, Lee CK (1983) Initiation of subsidence in a sedimentary basin underlain by a phase change. Geophys J R Astr Soc 74:689–712 Massonne HJ (2009) Hydration, dehydration, and melting of metamorphosed granitic and dioritic rocks at high- and ultrahighpressure conditions. Earth Planet Sci Lett 288:244–254 Massonne HJ, Schreyer W (1989) Stability field of the high-pressure assemblage talc ? Phengite and 2 new Phengite barometers. Eur J Mineral 1:391–410 Massonne H-J, Willner AP, Gerya T (2007) Densities of metapelitic rocks at high to ultrahigh pressure conditions: what are the geodynamic consequences? Earth Planet Sci Lett 256:12–27 McKenzie D (1978) Some Remarks on development of sedimentary basins. Earth Planet Sci Lett 40:25–32 Mengel K, Kern H (1992) Evolution of the petrological and seismic moho—implications for the continental-crust mantle boundary. Terra Nova 4:109–116 Middleton MF (1980) A model of intracratonic basin formation, entailing deep crustal metamorphism. Geophys J R Astr Soc 62:1–14 Neprochnov YP et al (2000) Comparison of the crustal structures of the Barents Sea and the Baltic Shield from seismic data. Tectonophysics 321:429–447 Newton RC, Charlu TV, Kleppa OJ (1980) Thermochemistry of the high structural state plagioclases. Geochimica et Cosmochimica Acta 44:933–941 O’Connell RJ, Wasserburg GJ (1967) Dynamics of motion of a phase change boundary to changes in pressure. Rev Geophys 5:329– 410 O’Connell RJ, Wasserburg GJ (1972) Dynamics of submergence and uplift of a sedimentary basin underlain by a phase-change boundary. Rev Geophys Space Phys 10:335–368 O’Leary N et al (2004) Evolution of the Timan-Pechora and South Barents Sea basins. Geol Mag 141:141–160 Pakiser LC, Robinson R (1966) Composition and evolution of continental crust as suggested by seismic observations. Tectonophysics 3:547–557 Pasyanos ME, Nyblade AA (2007) A top to bottom lithospheric study of Africa and Arabia. Tectonophysics 444:27–44 Petrini KATE, Connolly JAD, Podladchikov YY (2001) A coupled petrological–tectonic model for sedimentary basin evolution: the influence of metamorphic reactions on basin subsidence. Terra Nova 13:354–359 Petrov OV et al (2008) Palaeozoic and Early Mesozoic evolution of the East Barents and Kara Seas sedimentary basins. Norw J Geol 88:227–234 Podladchikov YY, Poliakov ANB, Yuen DA (1994) The effect of lithospheric phase transitions on subsidence of extending continental lithosphere. Earth Planet Sci Lett 124:95–103 Powell R, Holland TJB (1999) Relating formulations of the thermodynamics of mineral solid solutions: activity modeling of pyroxenes, amphiboles and micas. Am Mineral 84:1–14 Ritzmann O et al (2007) A three-dimensional geophysical model of the crust in the Barents Sea region: model construction and basement characterization. Geophys J Int 170:417–435 Rudnick RL, Fountain DM (1995) Nature and composition of the continental crust: a lower crustal perspective. Rev Geophys 33:267–309 Rudnick RL, Gao S (2003) The composition of the continental crust. In: Holland HD, Turekian KK (eds) Treatise on geochemistry, vol 3: The Crust. Elsevier-Pergamon, Oxford, pp 1–64 Rudnick RL, Taylor SR (1987) The composition and petrogenesis of the lower crust—a xenolith study. J Geophys Res 92:13981– 14005 Schenk V (1984) Petrology of Felsic Granulites, Metapelites, Metabasics, Ultramafics, and Metacarbonates from Southern Calabria (Italy)—Prograde Metamorphism, uplift and cooling of a former lower crust. J Petrol 25:255–298 Schenk V (1990) The exposed crustal cross section of southern Calabria, Italy: structure and evolution of a segment of Hercynian crust. In: Salisbury MH, Fountain DM (eds) Exposed crosssections of the continental crust. Kluwer, Amsterdam, pp 21–42 Schmalholz SM, Podladchikov YY (2000) Finite amplitude folding: transition from exponential to layer length controlled growth. Earth Planet Sci Lett 179:363–377 Schmalholz SM, Podladchikov YY, Burg JP (2002) Control of folding by gravity and matrix thickness: implications for largescale folding. J Geophys Res 107. doi:10.1029/2001JB000355 123 Int J Earth Sci (Geol Rundsch) Schmalholz SM, Podladchikov YY, Jamtveit B (2005) Structural softening of the lithosphere. Terra Nova 17:66–72 Simon NSC, Podladchikov YY (2008) The effect of mantle composition on density in the extending lithosphere. Earth Planet Sci Lett 272:148–157 Sleep NH, Nunn JA, Chou L (1980) Platform Basins. Annu Rev Earth Planet Sci 8:17–34 Sloss LL (1988) Tectonic evolution of the craton in Phanerozoic time. In: Sloss LL (ed) The geology of North America, vol D2, Sedimentary Cover of the North American Craton. Geological Society of America, Boulder, pp 25–51 Sobolev SV, Babeyko AY (1994) Modeling of mineralogical composition, density and elastic-wave velocities in anhydrous magmatic rocks. Surv Geophys 15:515–544 Spear F (1995) Metamorphic phase equilibria and pressure–temperature–time paths. Mineralogical Society of America Monograph. Mineralogical Society of America, Washington Symmes GH, Ferry JM (1992) The effect of whole-rock MnO content on the stability of garnet in pelitic schists during metamorphism. J Metamorph Geol 10:221–237 Tassara A (2006) Factors controlling the crustal density structure underneath active continental margins with implications for their evolution. Geochem Geophys Geosyst 7. doi:10.1029/2005GC 001040 Thompson AB, Connolly JAD (1995) Melting of the continentalcrust—some thermal and petrological constraints on anatexis in continental collision zones and other tectonic settings. J Geophys Res-Sol Ea 100:15565–15579 Tinkham DK, Zuluaga CA, Stowell HH (2001) Metapelite phase equilibria modeling in MnNCKFMASH: the effect of variable Al2O3 and MgO/(MgO ? FeO) on mineral stability. Geol Matl Res 3:1–42 123 Wei C, Powell R (2003) Phase relations in high-pressure metapelites in the system KFMASH(K2O-FEO-MGO-Al2O3-SiO2–H2O) with application to natural rocks. Contrib Mineral Petrol 145:301–315 Wei C, Powell R (2004) Calculated phase relations in high-pressure metapelites in the system NKFMASH (Na2O–K2O–FeO–MgO– Al2O3–SiO2–H2O). J Petrol 45:183–202 Wei C, Powell R (2006) Calculated phase relations in the system NCKFMASH (Na2O–CAO–K2O–FeO–MgO–Al2O3–SiO2–H2O) for high-pressure metapelites. J Petrol 47:385–408 Werner SC, Ebbing J, Litvinova TP, Olesen O (2010) Structure of the Barents and Kara Sea regions based on gravity and magnetic data. In: Spencer AM, Gautier D, Stoupakova A, Embry A, Sørensen K (eds) Arctic petroleum geology. Geol Soc Memoirs (in press) White RW, Powell R, Holland TJB, Worley BA (2000) The effect of TiO2 and Fe2O3 on metapelitic assemblages at greenschist and amphibolite facies conditions: mineral equilibria calculations in the system K2O-FeO-MgO-Al2O3-SiO2-H2O-TiO2-Fe2O3. J Metamorph Geol 18:497–511 Yamasaki T, Nakada M (1997) The effects of the spinel–garnet phase transition on the formation of rifted sedimentary basins. Geophys J Int 130:681–692 Zeh A, Holness MB (2003) The effect of reaction overstep on garnet microtextures in metapelitic rocks of the Ilesha Schist Belt, SW Nigeria. J Petrol 44:967–994 Zeh A, Holland TJB, Klemd R (2005) Phase relationships in grunerite–garnet-bearing amphibolites in the system CFMASH, with applications to metamorphic rocks from the Central Zone of the Limpopo Belt, South Africa. J Metamorph Geol 23:1–17 Ziegler PA, Cloetingh S (2004) Dynamic processes controlling evolution of rifted basins. Earth Sci Rev 64:1–50