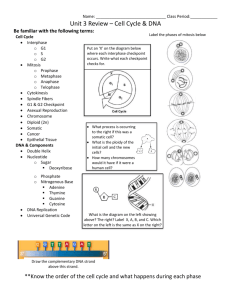
Identification of TICRR, a novel checkpoint and replication regulator Nelly Cruz
advertisement

Identification of TICRR, a novel checkpoint and replication regulator MASSACHUSETTS INSTITJTE OF TECHNOLOGY by MAY 25 2011 Nelly Marie Cruz LIBRARIES B.S. University of Puerto Rico Mayaguez, PR 2005 ARCHIVES SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTORATE OF PHILOSOPHY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2011 C 2011 Massachusetts Institute of Technology All rights reserved Signature of Author.... Nelly M. Cruz Department of Biology May 9, 2011 Certified by........... Jacqueline A. Lees Professor of Biology Thesis Supervisor Accepted by...... ........................................ Robert T. Sauer Professor of Biology Chair, Biology Graduate Committee Identification of TICRR, a novel checkpoint and replication regulator by Nelly M. Cruz Abstract The eukaryotic cell cycle refers to a sequence of events by which a cell duplicates its genomic DNA and divides into two daughter cells. Deregulation of the cell cycle can cause aberrant cell proliferation, as well as genomic and chromosomal instability, events that contribute to the development of cancer. Along with the machinery that promotes cell cycle progression, cells have evolved surveillance mechanisms, or checkpoints, that protect the cells from DNA lesions. Understanding the molecular mechanisms by which checkpoints act is of clinical relevance, since mutations in checkpoint components are often associated with human developmental disorders and cause a predisposition to cancer. A collection of 336 zebrafish (Danio rerio) lines, each carrying a recessive embryonic lethal mutation caused by a retroviral insertion, was screened for novel genes required for the ionizing radiation-induced G2/M checkpoint. Among the mutant lines that displayed a defect in checkpoint activation, two carry insertions in a novel gene that we have named ticrr (for TopBP1 interacting, checkpoint and replication regulator). The loss of ticrr impairs DNA replication and disrupts the S/M checkpoint, leading to premature mitotic entry of cells with partially replicated genomes and mitotic catastrophe. Therefore, Ticrr is a novel cell cycle regulator essential for genomic integrity with roles in replication as well as in the S/M and G2/M checkpoints. We have identified the human ortholog of Ticrr and showed that both the human and zebrafish Ticrr proteins associate with TopBP1, a protein with known roles in checkpoints and replication. We hypothesized that TICRR is required for pre-initiation complex assembly during replication initiation, in an analogous manner to the TopBP1 yeast ortholog Dpbl 1 and the yeast protein Sld3. Consistent with this model, we show that ticrr-deficiency disrupts chromatin association of pre-initiation complex, but not prereplication complex, components in the zebrafish. The work described in this thesis demonstrates the utility of zebrafish for performing genetic screens for cell cycle regulators. We have used human cell lines to complement our studies in the zebrafish and showed that taking advantages of the strengths that each system offers represents a powerful strategy to elucidate gene function. Thesis Supervisor: Jacqueline A. Lees Title: Professor of Biology Acknowledgments I owe my deepest gratitude to my advisor Jackie Lees, for her guidance, continuous support, and encouragement, which have been invaluable to my growth as a scientist over the years. I would also like to thank the members of my thesis committee, Frank Solomon, Angelika Amon and Steve Bell; as well as Nancy Hopkins for their insightful comments and contributions to my research. I am grateful to my friends and colleagues in the Koch Institute, specially past and present members of the Hopkins and Lees lab, for sharing reagents and their knowledge with me and making the Koch Institute a great place to work. I would like to particularly thank Chris Sansam, who helped me greatly throughout my graduate career and Crystal Lee, who has been an excellent classmate, benchmate and friend. I would also like to show my gratitude to Simona, Keren, Mindy, Tiziana and Amy, for great companionship and all the positive energy they bring to the lab. I am indebted to my family and friends for their unconditional love and support. The encouragement of many friends has been indispensable and I would like to particularly acknowledge Grisel, Gisselle, Ingrid, Ricky and Francis for always being there for me. I would also like to mention my grandparents Nelida, Jose M., Merida and Guillermo, who have been a true inspiration for me. I am very grateful to my brother Jose G., my sister Cristina, my boyfriend Jose Luis and my parents Jose E. and Ana for innumerable things, especially for always believing in me and supporting all my endeavors. They have been a driving force for me through my graduate studies and to them I dedicate this thesis. Table of Contents A bstra ct ............................................................................................................................ 2 Acknowledgments ...................................................................................................... 3 CHAPTER 1: Introduction ........................................................................................... 9 O ve rv iew .......................................................................................................................... 10 The eukaryotic cell cycle ............................................................................................. 11 Cyclin-dependent kinases and the regulation of the cell cycle .................... 11 Regulation of replication initiation in eukaryotes .......................................................... 15 Pre-replication complex (pre-RC) assembly............................................................. 17 Pre-initiation complex (pre-IC) assembly................................................................. 20 Mechanism for prevention of re-replication 26 ............................. Cell cycle checkpoints ................................................................................................ 28 ATM and ATR: initiators of the signaling cascade in response to DNA damage.........31 The G1/S checkpoint .............................................................................................. 34 DNA-damage induced G2/M arrest ............................................................................. 35 Checkpoints during S-phase .................................................................................. 36 The zebrafish as a model system for the study of cell cycle regulation....................... 39 R e fe re nc e s ...................................................................................................................... 41 CHAPTER II: A zebrafish screen for novel DNA damage response genes identifies TICRR..............................................................................................................................51 Results............................................................................................................................. A genetic screen for zebrafish mutants with a defective IR response 55 ........... 55 Insertional mutations in zebrafish that affect normal mitotic progression................60 Disruption of de novo purine nucleotide synthesis in zebrafish abrogates IR-induced cell cycle arrest............................................................................................................62 An insertional mutation in a novel gene that abrogates IR-induced cell cycle arrest in th e z e b ra fish ................................................................................................................ 63 Disc ussio n ....................................................................................................................... What role do the identified genes play in normal cell cycle progression? ............... What role do the identified genes play in the DNA damage response? ......... . ....... . . 71 71 72 Are the identified genes required for normal cell cycle progression and checkpoints in ...... .............. .............. ... ........ . . 74 other vertebrates? ..................................................... Materials and methods ................................................................................................ Zebrafish maintenance G2/M checkpoint assays ....................................... 75 ..... 75 .......................................... 75 Genotyping and expression analysis of zebrafish lines........................................... 75 In Vitro Kinase Assay .............................................................................................. 76 FACS Analysis............................................................................................................. 77 Acknow ledgem ents .................................................................................................... 77 References ...................................................................................................................... 77 CHAPTER III: TICRR associates with TopBP1 and is required for replication initiation..........................................................................................................................80 S um m a ry ......................................................................................................................... 81 Intro duction ...................................................................................................................... 82 Re sults ............................................................................................................................. 85 Disruption of ticrr impairs S-phase progression and causes premature chromatin condensation ............................................................................................................... 85 TICRR binds TopBP1 BPI............................................ .................................................. 90 Ticrr-deficiency inhibits pre-IC form ation ................................................................. 98 Disc us sio n ..................................................................................................................... 100 TICRR in DNA Replication ........................................................................................ 100 TICRR in the SIM and G2/M Checkpoints................................................................. 102 Materials and methods .................................................................................................. Human cell culture and chromatin fractionation . Immunoprecipitation ....... .............. ......... 105 ................107 ....................... 107 Zebra fish chrom atin preparation................................................................................108 Antibodies..................................................................................................................108 Acknow ledgem ents ....................................................................................................... 108 R efe re nce s .................................................................................................................... 109 CHA PTER IV: Discussion ........................................................................................... 112 Key conclusions.............................................................................................................113 A screen in zebrafish identifies Ticrr....................................113 Ticrr is required for DNA replication and functions in a similar manner to Sld3 ............ 114 The role of CDK activity in regulating TICRR function in DNA replication.....................117 Other proteins with functional similarity to TICRR.........................................................118 Possible roles of TICRR in the maintenance of genome stability..................................119 R e fe re nc e s .................................................................................................................... 12 2 APPENDIX A: Characterization of the functional significance of the TICRRTopBP1 interaction......................................................................................................125 Re sults a nd Discussion ................................................................................................. 126 The TopBP1 interaction domain lies within the C-terminal half of TICRR ................. 126 Two putative CDK phosphorylation sites are highly conserved in TICRR ................. 127 A TICRR mutant that localizes to PCNA and RPA foci ............................................. Mate rials and methods .................................................................................................. 133 13 8 Human cell culture and transfections ........................................................................ 138 Immunoprecipitation .. .......................................................................... 139 Immunofluorescence 139 ............................................ A n tib o dies .................................................................................................................. 14 0 A cknow le dg eme nts ....................................................................................................... 14 0 R e fe re nce s .................................................................................................................... 14 0 Figures and Tables CHAPTER I: Introduction ........................................................................................... 9 Figure 1. Overview of the CDK-Cyclin complexes and the periods of the cell cycle at w hich they are active................................................................................................ . . 13 Figure 2. Regulation of Rb and E2F activities in mid-late G1............ ...... 14 Figure 3. Sequential assembly of pre-RC proteins to origins leads to loading of the MCM2 -7 helicase ............................................................................................................. 20 Figure 4. Recruitment of additional proteins to origins leads to formation of the pre-IC and subsequent activation of the MCM2-7 helicase............................. ...................... 25 Figure 5. General scheme of the signal transduction pathways that are activated in .... 30 .................... response to DNA damage or stalled replication forks. Figure 6. A Simplified scheme of DNA damage-induced cell cycle arrest and apoptosis. ......................................................................................................................................... 36 CHAPTER II: A zebrafish screen for novel DNA damage response genes identifies T ICRR..............................................................................................................................51 Figure 1. Zebrafish can be used to screen for IR-induced G2 checkpoint response ge ne s............................................................................................................................... 56 Figure 2. A zebrafish screen for novel DNA damage response and cell cycle regulators. ......................................................................................................................................... 58 Figure 3. hi1487 homozygous mutants show an aberrant accumulation of pH3 positive cells in the absence of exogenous DNA damage........................................................62 Figure 4. Mitosis after irradiation phenotype in hi923 mutants. ............. ..... 64 Figure 5. hil 573 and hi3202A zebrafish embryos show a Mitosis After Irradiation (MAI) phe noty pe........................................................................................................................ 65 Figure 6. The checkpoint defect results from disruption of a novel gene, ticrr. .......... 67 Figure 7. Cdc2 kinase activity remains high in ticrr mutants after IR treatment. ......... 68 Figure 8. Cells in ticrr homozygous mutants continue to enter mitosis after IR treatment. ......................................................................................................................................... 69 Table 1. Insertional mutants from the Hopkins zebrafish collection that showed an increase in pH3 staining after IR treatment. . .................. ....... ......... 61 Table 2. Conservation between D. rerio Ticrr and orthologs identified by psiBLAST......70 CHAPTER III: TICRR associates with TopBP1 and is required for replication initiation..........................................................................................................................80 Figure 1. Ticrr is required for normal DNA replication. ............................ Figure 2. Ticrr is required for S/M checkpoint function. .................. 86 ..... 88 Figure 3. A DNA replication defect but not premature mitotic entry occurs in 24 hpf ticrr muta nts ............................................................................................................................ 90 Figure 4. Human TICRR is a chromatin-associated protein.... ................ 93 Figure 5. Human TICRR interacts with TopBP1..........................................................94 Figure 6. Human TopBP1 interacts with zebrafish TopBP1. ............... Figure 7. TICRR interacts with the first two BRCT domains in TopBP1 ..... 95 ............ 96 Figure 8. TICRR can bind TopBP1 in a CDK independent manner.......... .... 98 Figure 9. Ticrr is essential for the chromatin association of the pre-IC component Psfl.99 CHA PTER IV: Discussion ........................................................................................... 112 APPENDIX A: Characterizing the functional significance of the TICRR-TopBP1 interactio n .................................................................................................................... 125 Figure 1. Residues 976-1261 of TICRR protein are required and sufficient for TopBP1 bindin g........................................................................................................................... 127 Figure 2. Sequence alignment of conserved CDK consensus motifs in the TICRR pro te in ............................................................................................................................ 129 Figure 3. Mutation of Thr 969 and Ser 1001 in the TICRR protein causes a reduction in T o pBP1 bin din g ............................................................................................................. 13 0 Figure 4. Loss of TICRR in human cells causes a replication defect. ........................... 131 Figure 5. Ticrr is required for S/M checkpoint function in human ce is..... ..... 132 Figure 6. TICRR knockdown induces cellular senescence in U2OS cells.....................133 Figure 7. GFP-TICRR' 565-19 1 0 localizes Figure 8. GFP-TICRR' 565~1 91 0foci to foci within the nucleus.......... do not colocalizes with 5H2AX. .............. Figure 9. GFP-TICRR 1 565-1910 foci colocalizes with RPA and PCNA. ...... 134 135 .............. 137 Figure 10. Expression of GFP-TICRR 1565-1910 impairs S-phase progression..................138 CHAPTER 1: Introduction Overview The cell cycle refers to a sequence of events by which a cell duplicates its hereditary material and divides into two daughter cells. This process is the basis for growth and development of all living organisms. The cell cycle is divided into four phases: G1, S, G2 and M phase. G1 refers to a gap during which the cell prepares for DNA synthesis occurring during S phase. The replication of chromosomal DNA is followed by a gap called G2 during which the cell prepares for mitosis, or M phase. During M phase the replicated chromosomes are segregated and cells divide. There is a decisive moment in G1, referred to as the restriction point in mammalian cells and start in yeast, in which the cell commits to enter the cell cycle. Under specific circumstances, cells in G1 can enter a resting state called GO before committing to DNA replication. Cells in GO account for the majority of the non-proliferating cells in the human body (Nurse, 2000). Deregulation of the cell cycle can cause aberrant cell proliferation, as well as genomic and chromosomal instability, events that contribute to the development of cancer (Malumbres and Barbacid, 2009). Therefore, cell cycle events are under precise temporal control. Along with the machinery that promotes cell cycle progression, cells have surveillance mechanisms, or checkpoints, that ensure the correct ordering of events and trigger cell cycle arrest in response to DNA damage or errors. The work described in this thesis has led to the identification of TICRR, (for TopBP1 interacting, checkpoint and replication regulator), a novel protein essential for normal cell cycle progression. In the following introduction, I will review the regulatory mechanisms of the eukaryotic cell cycle with a particular focus on replication initiation, a process in which TICRR plays an important role. I will then discuss the checkpoints that ensure the fidelity of cell division and protect the cell from the deleterious effects of DNA damage. Finally, I will discuss the advantages of using zebrafish as a model organism for the identification of novel cell cycle and checkpoint regulators. The eukaryotic cell cycle Cyclin-dependent kinases and the regulation of the cell cycle Cyclin dependent kinases (CDKs), best described as the drivers of eukaryotic cell cycle progression, are proline-directed serine/threonine protein kinases that phosphorylate substrates appropriate for the phase of cell cycle that the kinase regulates. In yeast, cell cycle progression is controlled by a single CDK, called Cdc28 in Saccarhomyces crevisiae and Cdc2 in Schizosaccharomyces pombe (Forsburg and Nurse, 1991). This mechanism is more complicated in vertebrates, with four CDKs (CDK1, 2, 4 and 6) playing a role in regulating the cell cycle (Satyanarayana and Kaldis, 2009). CDKs are regulated in several ways to ensure that their activity is limited to specific stages. Cyclins, the CDK binding partners, comprise a diverse family of proteins first identified in sea urchin eggs and later found to be conserved from yeast to humans (Evans et al., 1983; Lew and S, 1992). Their levels oscillate throughout the cell cycle, accumulating at a specific stage where they bind and promote CDK activity. Like yeast, mammalian cells express multiple cyclins. In the current model for the mammalian cell cycle, each phase is driven by specific CDK-cyclin combinations (Figure 1)(Malumbres and Barbacid, 2009). Mitogenic signals induce expression of the D-type cyclins (D1, D2 and D3) that preferentially bind and activate CDK4 and CDK6 during G1 (Sherr, 1995). Key substrates of the G1 CDKcyclin complexes are the pocket proteins, pRb, p107 and p130, which repress E2F activity by directly binding to them (Figure 2). The E2F family of transcription factors activate genes encoding proteins involved in cell cycle regulation and DNA synthesis, including the late-G1 cyclin E and the S-phase cyclin A and CDK2. Phosphorylation of the pocket proteins at multiple sites prevents their association with E2Fs, thereby allowing the transcription of genes required for S entry (Figure 2). This is one of the principal events for passage through the restriction point (Trimarchi and Lees, 2002). In turn, E-type cyclins bind and activate CDK2, which further phosphorylate the pocket proteins, leading to their complete inactivation. CDK2-cyclin E drives the G1/S transition (Tsai et al., 1993). CDK2 is subsequently bound by cyclin A during late stages of DNA replication to drive the transition to G2. Finally, CDK1 is thought to be activated by cyclin A at the end of G2 to facilitate the onset of mitosis. Following nuclear envelope breakdown, cyclin A is degraded, facilitating formation of the CDK1-cyclin B complex responsible for driving cells through mitosis. Regulatory mechanisms of CDK activity As stated above, the binding of a cyclin subunit is required for CDK activity. In this section, I will provide a brief description of the additional mechanisms of CDK regulation. All these regulatory mechanisms act in concert at the right time to promote the transition from one phase of the cell cycle to the other. Phosphorylation Full activation of the CDK-cyclin complex usually requires phosphorylation of a conserved threonine residue located on the activation loop of the kinase domain (Thr161 in CDK1, Thr-60 in CDK2, and Thr-172 in CDK4). These phosphorylations induce conformational changes and enhance the binding of cyclins (Jeffrey et al., 1995). On the other hand, CDK1 activity can be inhibited by phosphorylation of residues within the ATP-binding pocket of the CDK (Thr-14 and Tyr-15 in CDK1) by Weel and Myt1 kinases. Dephosphorylation at these sites by the Cdc25 family of phosphatases is necessary for activation of CDK1 and further progression through the cell cycle (Lew and Kornbluth, 1996) (Figure 1). Cdc25A CDK4/6 cyclin D1/2/3 CDK2 Cyclin E T Cdc25A/B/C CDK2 Cyclin A S sil CDK1 Cyclin A G2 CDK1 Cyclin B MI Restriction Point Figure 1. Overview of the CDK-Cyclin complexes and the periods of the cell cycle at which they are active. Mammalian cells use several CDKs and cyclins to regulate passage through the cell cycle. The activity of mammalian CDK-cyclin complexes is induced by Cdc25 phosphatases, which catalyze the removal of inhibitory phosphorylations on the CDK subunit. Adapted from Trimarchi and Lees, 2002. CDK inhibitors There are two families of CDK inhibitors that have been defined on the basis of their primary sequence and biochemical properties (Figure 2). The INK4 family includes p15, p16, p18, and p19, which specifically inactivate the G1 CDKs (CDK4 and CDK6). These form stable complexes with the CDK before cyclin binding, preventing association of cyclin D. The Cip/Kip family includes p21, p27 and p57, and they inhibit a broader spectrum of CDK-cyclin complexes, including CDK2-cyclinE, CDK2-cyclin A, CDK1cyclin A and CDK1-cyclin B. They modulate CDK activity by interacting with both subunits of the CDK-cyclin complex. p21 also inhibits DNA synthesis by binding to and inhibiting PCNA, a protein that acts as a processivity factor for DNA polymerase (Waga S 1994). These inhibitors are regulated by a variety of mechanisms; for example, the expression of p21 is under the transcriptional control of the p53 tumor suppressor (Sherr and Roberts, 1995). INK 4 CycD CDK4 CIP/KIP (v3E CVA CDK2 CDK2 G1 S Figure 2. Regulation of Rb and E2F activities in mid-late G1. The pocket protein Rb represses E2F activity by directly binding to E2F. CDK4/6-Cyclin D complexes phosphorylate Rb, releasing some E2F, which stimulates transcription of cyclin E and CDK2. The CDK2- Cyclin E complex further phosphorylates Rb, resulting in rapid increase of E2F and increased CDK2 activity drives passage through the restriction point. Two types of CDK inhibitors contribute to cell cycle control in mammals, the INK4 and CIP/KIP family of proteins, which act by binding to CDK and inhibiting its activity. Adapted from Trimarchi and Lees, 2002. Intracellular localization The intracellular localization of different cell cycle-regulating proteins also contributes to correct cell cycle progression. Cyclin B is actively exported from the nucleus until the beginning of prophase. The 14-3-3 group of proteins regulate the intracellular trafficking of various proteins. For instance, the CDK activating phosphatase Cdc25 is kept in the cytoplasm during interphase through interaction with 14-3-3 proteins (Wilker and Yaffe, 2004). Role of protein degradation in cell cycle control The activity of CDKs is also controlled by the ubiquitin-mediated proteolysis of key regulators such as cyclins and CDK inhibitors. Two E3 ubiquitin ligases, the SkplCULl-F-box (SCF) complex and the anaphase-promoting complex/cyclosome (APC/C) mediate the specific ubiquitination of these regulators (Nakayama and Nakayama, 2006). Two specific events are very well characterized; specifically, the SCF regulates entry into S-phase by targeting CDK inhibitors and G1 cyclins for degradation (Ang and Wade Harper, 2005). The APC/C is active from mid-mitosis to the end of G1 and facilitates exit from mitosis by targeting the mitotic cyclins, A and B, for degradation. The APC/C also plays a major role in controlling the onset of anaphase and is the key target of the spindle assembly checkpoint (Wasch and Engelbert, 2005). Regulation of replication initiation in eukaryotes DNA replication is the process by which cells duplicate their genome prior to cell division. To duplicate their genomes in a timely manner, eukaryotes have many replication start sites, or origins of replication. Origins in the budding yeast S. cerevisiae contain an essential 11-bp consensus sequence and a non-conserved region that is also important for origin activity (Marahrens and Stillman, 1992). In contrast, no evident consensus sequence has been found in origins of other eukaryotes and it is thought that other factors may facilitate the selection of specific segments in the genome, such as DNA topology, chromatin structure, gene transcription and nucleosome formation (Masai et al.). Despite the apparent lack of conservation for origin sequences, the proteins involved in origin selection are conserved among eukaryotes. DNA replication initiation is tightly controlled so that each origin is fired only once and re-replication does not occur. Most of our understanding on the mechanism underlying replication initiation comes from studies in yeast, a powerful model for studying molecular genetics and biochemical function. In vitro and in vivo studies in metazoans have revealed that the key players and events required for replication initiation are highly conserved among eukaryotes. It has become clear that replication initiation is a very dynamic and coordinated process involving recruitment of various protein complexes to origins. The process is divided into two steps, characterized by the formation of two major complexes, the pre-replication complex (preRC) and the preinitiation complex (prelC). To ensure that DNA replication is restricted to a single round per cell cycle, these steps are temporally regulated and they take place during different stages (Diffley et al., 1994). Assembly of pre-RC can only occur during late M and early G1, when CDK activity is low (Dahmann et al., 1995). When cells enter S phase, the activity of CDK and Cdc7-Dbf4 (DDK), promote the recruitment of additional proteins to the pre-RC and the subsequent activation of the replicative helicase MCM2-7 (Labib, 2010). Additionally, CDK plays an important role in inhibiting re-assembly of pre-RC in origins, an important mechanism to prevent re-replication (discussed in following sections)(Arias and Walter, 2007). Below, I will describe the proteins required for replication initiation in eukaryotes and mechanisms by which these proteins are recruited to the origins and DNA replication is initiated. I will also describe the mechanisms by which the cell regulates this process to ensure that the entire genome is duplicated only once. I will focus on studies in S. cerevisiae, since they provide the framework for the current model of replication initiation. I will also review relevant findings in metazoans that expand our understanding of this process in more complex organisms. Pre-replication complex (pre-RC) assembly The first step in the initiation of DNA replication is the selection of sites that will act as origins of replication. This is mediated by the sequential assembly of ORC, Cdc6, Cdtl and MCM2-7 proteins onto origins to generate a pre-RC (Figure 3) (Bell and Dutta, 2002). Pre-RC formation directs the loading of the Mcm2-7 helicase onto origin DNA, a process also known as origin licensing. The loaded MCM2-7 helicase remains inactive until S-phase entry when two kinases, CDK and DDK, promote its activation (discussed in the following section). Recent in vitro studies in budding yeast, combined with in vivo genetics and biochemical approaches, have revealed details on the mechanism by which the pre-RC is formed. A key feature is the ATP regulation of various steps. The origin recognition complex (ORC) is the first pre-RC component to associate with origins and is essential for the recruitment of other factors (Figure 3B)(Bell, 2002). It consists of six different subunits, Orcl-6, first identified in budding yeast for its ability to bind origins in an ATP dependent manner (Bell and Stillman, 1992). Orc1, Orc4 and Orc5 are members of the AAA+ family of ATPases, while Orc2 and Orc3 are distantly related to this class of proteins (Erzberger and Berger, 2006). However, only the ATP binding and hydrolysis by the largest ORC subunit, Orc1, has been shown to be required for ORC function in preRC assembly (Bowers et al., 2004; Klemm et al., 1997). Specifically, Orc1 binding to ATP promotes association of the complex with origin DNA (Klemm et al., 1997), while hydrolysis occurs after the initial loading of the MCM2-7 helicase (Randell et al., 2006). Studies in Drosophila and in Xenopus in vitro system (using recombinant human ORC) indicate that the requirement of Orc1 ATPase activity for replication is conserved in metazoans (Chesnokov et al., 2001; Giordano-Coltart et al., 2005). Like Orc1, Cdc6 is a member of the AAA+ family of ATPases whose ATP binding and hydrolysis is necessary for pre-RC assembly (Perkins and Diffley, 1998; Randell et al., 2006; Weinreich et al., 1999). The current model for pre-RC assembly proposes that ORC recruits ATP-bound Cdc6 to origins, and this complex promotes the recruitment of a pre-formed Cdtl-Mcm2-7 complex to origins (Figure 3C) (Randell et al., 2006; Tsakraklides and Bell, 2010). Cdtl exists in a complex with Mcm2-7 (Tanaka and Diffley, 2002) and these two components associate to DNA with similar kinetics at equimolar ratios (Randell et al., 2006), suggesting that they are recruited to the origins as a complex. This is most likely mediated through Cdtl binding to Orc6 (Chen et al., 2007). The next step is the hydrolysis of ATP by Cdc6, which promotes the dissociation of both Cdc6 and Cdtl from the complex, and triggers tight binding of the MCM2-7 complex with DNA (Figure 3D)(Randell et al., 2006; Tsakraklides and Bell, 2010). Two recent studies, using an in vitro loading system with purified budding yeast proteins, show that the inactive helicase is loaded onto double stranded DNA as a head-to-head double hexamer (Evrin et al., 2009; Remus et al., 2009). The common view is that the helicase is active as a single hexamer. Indeed, a study in the Xenopus egg extract in vitro system provides evidence supporting a model in which the two Mcm2-7 complexes uncouple upon activation and travel away from one another, and therefore function autonomously (Yardimci et al., 2010). Currently, it is not clear how these two complexes dissociate and move away from origins to form two replication forks. The steps described above appear to be repeated multiple times, yielding preRCs containing multiple MCM2-7 complexes (Bowers et al., 2004; Randell et al., 2006). ORC ATP hydrolysis occurs after Cdc6 ATP hydrolysis and is required for multiple rounds of MCM2-7 loading (Figure 3E, F)(Randell et al., 2006). The function of the extra Mcm2-7 complexes at origins is not well understood, but studies in human cells suggest that they rescue collapsed replication forks upon replication stress (Ge et al., 2007; Ibarra et al., 2008). Although previous data indicate that the budding yeast ORC associates with origins throughout the cell cycle, a recent study involving in vitro reconstitution of preRCs shows that it is released after Mcm2-7 loading and ATP hydrolysis (Tsakraklides and Bell, 2010). It remains to be seen if release of ORC upon Mcm2-7 loading also occurs in vivo. The fact that Cdc6, Cdtl and possibly ORC are released from the origins once the helicase is loaded is consistent with in vitro studies in Xenopus egg extracts indicating that ORC, Cdc6 and Cdtl are not required to maintain Mcm2-7 association with the origin after loading is complete (Hua and Newport, 1998; Jares and Blow, 2000; Rowles et al., 1999). Although it is not known whether the described mechanism operates in the same fashion in other organisms, the sequential association of ORC, Cdc6, Cdtl and Mcm2-7 at origins appears to be conserved in other eukaryotes, including S. pombe and Xenopus. A /XXXX0000000000(\ QRC B /=0OOOOOO O OOC Cdc6 CF Mcm2-7-Cdtl Cdtl 9 Cdc6 ATP E Figure 3. Sequential assembly of pre-RC proteins to origins leads to loading of the MCM2-7 helicase. (A, B) ATP-bound ORC binds to origin DNA; red star represents ATP. (C, D) Association of Cdtl and MCM2-7, which are recruited to origins as a complex, follows the association of ATPbound Cdc6. (E) ATP hydrolysis by Cdc6 leads to the loading of MCM2-7 complex and release of Cdc6 and Cdtl. (F) ATP-hydrolysis by ORC allows re-initiation of this cycle for further loading of MCM2-7 helicase complexes onto origins (Adapted from Masai H et al. 2010) Pre-initiationcomplex (pre-IC) assembly Initiation of replication requires the recruitment of a number of factors to the existing pre-RC in a CDK and DDK dependent manner (Figure 4). The large multiprotein complex that is formed at this step is referred to as the pre-initiation complex (pre-IC). The main purpose of the pre-IC is to activate the Mcm2-7 helicase. Recent studies provide insights into how this may occur. GINS and Cdc45 act as cofactors of the helicase, and these proteins form a complex at the onset of replication initiation. This complex was first co-purified in Drosophila, but has also been co-purified from S. cerevisiae and there is evidence for its existence in humans and Xenopus (Aparicio et al., 2009; Gambus et al., 2006; Moyer et al., 2006; Pacek et al., 2006). Importantly, the binding of Cdc45 and GINS has been shown to activate Mcm2-7 helicase activity in vitro (Ilves et al., 2010). The structure of the Cdc45-Mcm2-7-GINS complex has been recently solved using single-particle electron microscopy and provides insightful details into how Cdc45 and GINS may promote helicase activation (Costa et al., 2011). This study proposes that the Mcm2-7 complex loaded onto origins during pre-RC formation exist in a ring-like open configuration, with a gap between Mcm2 and Mcm5 subunits. This structure alternates between two conformations and Cdc45 and GINS binding stabilizes one of the structures by bridging the gap. This is followed by ATP binding, which promotes ring closure around DNA. Formation of the Cdc45-Mcm2-7-GINS complex and subsequent helicase activation requires CDK and DDK activity. In the following section, I will describe our current knowledge on how CDK and DDK regulate DNA replication initiation. I will focus on studies that have been performed in the budding yeast, where currently this process is best understood. The role of DDK The S phase kinase DDK is required for recruiting Cdc45 and other pre-IC components to origins (Sclafani et al., 2002; Zou and Stillman, 2000). Studies in a number of organisms indicate that MCM2-7 is the major target of DDK phosphorylation (Labib, 2010; Masai and Arai, 2002). All budding yeast Mcm2-7 subunits, with the exception of Mcm5, are phosphorylated by DDK in vitro. The major targets are the amino terminal tails of Mcm2, Mcm4 and Mcm6 (Labib, 2010). The exact mechanism by which DDK acts in replication initiation has been unclear, however, new details are starting to emerge. An interesting study reveals that DDK phosphorylation of Mcm4 alleviates an inhibitory activity of its N-terminal region (Sheu and Stillman, 2010). Removing the Nterminal domain of Mcm4 allows DDK-independent initiation of DNA replication, suggesting that Mcm4 is the minimum target of DDK for replication initiation. However, in the absence of DDK these cells grow slowly and the Cdc45-Mcm2-7 complex is defective, suggesting that DDK has additional roles during replication (Sheu and Stillman, 2010). Further characterization of the functional significance of other DDK targets is needed in order to gain a complete picture of the role of this essential kinase in replication. The role of CDK The protein Dpbl 1 has a dual role in replication initiation and in DNA damage checkpoints. To further understand Dpbl 1's function, Kamimura et al. identified factors interacting with Dpbl 1 by isolating synthetically lethal mutants in S. cerevisiae (Kamimura et al., 1998). The pre-IC components SId2 and Sid3, the major CDK targets during replication initiation, were identified in this screen. Sld2 is phosphorylated by CDK in at least 7 sites, which triggers Sid2 binding to Dpbl 1 and this interaction is essential for replication initiation (Masumoto et al., 2002; Tak et al., 2006). Dpbl1 contains four BRCT domains, which often function as motifs for the binding of phosphopeptides (Glover et al., 2004). Sld2 interaction was mapped to the C-terminal pair of the four BRCT domains in Dpbl1 (Tak et al., 2006). In budding yeast, SId3 forms a complex with Cdc45 and their recruitment to origins is mutually dependent (Figure 4A, B) (Kamimura et al., 2001). SId3 is phosphorylated by CDK at three sites, and this promotes its association with the Nterminal pair of BRCT domains in Dpbl 1 (Tanaka et al., 2007; Zegerman and Diffley, 2007). Like Sid2, this interaction is essential for replication. Furthermore, the role of CDK activity in replication can be bypassed by using phospho-mimetic mutations in Sid2 together with a fusion of SId3 to Dpbl 1, suggesting that SId2 and SId3 are the minimal CDK targets required for replication initiation (Zegerman and Diffley, 2007). The mechanism by which these proteins activate DNA replication has not been elucidated. By treating cells with a cross-linking reagent it was found that GINS, DNA polymerase e, Dpbl 1 and phosphorylated Sld2 could all form a complex as cells enter S phase, dependent on CDK activity but not DDK activity (Muramatsu et al., 2010). This complex has been called the preloading complex. Based on these and previous findings, a model has been proposed in which Sld3-Cdc45 complex is recruited to origins and helps to recruit the pre-loading complex through direct interaction of SId3 with Dpbl1 in a CDK dependent manner (Figure 4C). GINS binds Mcm2-7 and, together with Cdc45, activate the helicase. Sid2, Sld3 and DpB1 1 are released from origins through an unknown mechanism while the other factors become part of the replisome (Figure 4D). Conservation in metazoans It seems likely that the mechanism by which DDK promotes activation of the Mcm2-7 helicase and initiation of replication will be fundamentally similar between yeast and other eukaryotes. There is evidence indicating that the N-termini of Mcm2 and Mcm4 are phosphorylated by DDK in human cells, consistent with studies of their yeast counterparts (Masai et al., 2000; Masai et al., 2006; Montagnoli et al., 2006). Additionally, DDK and CDK activity is required for formation of the Cdc45-Mcm2-7-GINS helicase complex in human cells (Im et al., 2009). However, the major CDK targets during replication remain elusive in higher eukaryotes. This may be in part because, until recently, orthologs of SId2 and Sld3 had not been identified in metazoans. The primary sequence of SId3, DpB1 1 and Sld2 have diverged greatly during evolution even between budding yeast species, making the search for orthologs much more difficult than for many other replication factors. Orthologs of Dpb11 have been identified in metazoans (TopBP1/Mus101/Cut5), all containing BRCT domains although the number of repeats varies between species (Garcia et al., 2005). Importantly, all orthologs are required for the initiation of DNA replication in the organisms examined (Garcia et al., 2005). An ortholog of Sld2 has been recently identified in higher eukaryotes, the RecQL4 DNA helicase (also called RecQ4) that is mutated in the human genetic disease Rothmund-Thomson syndrome, which predisposes patients to cancer (Sangrithi et al., 2005). Mutations causing human Rothmund-Thomson syndrome often cause truncations that leave the N-terminal portion of the protein intact. Strikingly, this part of the protein shows homology with yeast Sld2, although it is relatively limited (Sangrithi et al., 2005). Depletion of RecQL4 in Xenopus egg extracts blocks chromosome replication and this can be rescued by the N-terminus portion carrying homology with Sld2 (Matsuno et al., 2006). Moreover, the Xenopus RecQL4 N-terminus binds Cut5/TopBP1 (ortholog of Dpbl 1), suggesting that RecQL4 has a related role to yeast SId2 during the initiation of replication (Matsuno et al., 2006). Subsequent studies revealed that there are clear differences between RecQL4 and Sld2, as well as human and Xenopus RecQL4. Although Xenopus RecQL4 is phosphorylated by CDK in vitro, the binding to Cut5/TopBP1 can occur even after dephosphorylation of RecQL4, suggesting that, unlike SId2's interaction with Dpbl 1, phosphorylation is not absolutely required for the interaction. RecQL4 also seems to be acting at a later step, the recruitment of DNA polymerase a. However, similarly to yeast SId2, human RecQL4 is required for assembly of the Cdc45-Mcm2-7-GINS complex and is not required for elongation (Im et al., 2009; Xu et al., 2009). The reasons for the differences between Xenopus and human RecQL4 are unclear, but these studies suggest that the precise mechanism of action of RecQL4 in replication initiation has diverged among different organisms. An ortholog of Sid3 remains elusive. Inthis thesis I will describe the identification of a metazoan protein that has the core characteristics of SId3. It associates with TopBP1 and is essential for initiation of chromosome replication, specifically for pre-IC formation. We propose that this protein, TICRR, is the metazoan ortholog of the Sid3 protein. A DDK SId3-Cdc45 CDK@ Dpbll, Sld2, Po E,I GINS Dpbll, Sld2, Sld3 C D Figure 4. Recruitment of additional proteins to origins leads to formation of the pre-IC and subsequent activation of the MCM2-7 helicase. (A) Sld3 and Cdc45 associate with the pre-RC in a mutually dependent manner. (B) A complex consisting of Dpb 11, Sld2, DNA polymerase c (pol e) and GINS is assembled in a CDK dependent manner and subsequently recruited to origins through a CDK-dependent interaction between Sld3 and Dpbl1. (D) Dpbll, Sld2 and Sld3 are released through an unknown mechanism while Cdc45 and GINS form a stable complex with MCM2-7, activating the helicase. (Adapted from Araki, H 2010) Mechanism for prevention of re-replication Eukaryotes have evolved multiple mechanisms to ensure that replication is initiated from each origin only once during each cell cycle (Arias and Walter, 2007; Diffley, 2004). The temporal separation of events (i.e. MCM loading during G1 phase and activation during S phase) is key for preventing re-replication. Thus, replication initiation is tightly coordinated with cell cycle progression, whose major feature is the oscillation of CDK activity. Pre-RC formation can only occur during G1, when CDK activity is low. The ubiquitin ligase APC/C plays a major role in ensuring low CDK activity during G1 and promoting pre-RC formation (Wasch and Engelbert, 2005). The APC/C is active from mid mitosis to the end of GI and mediates proteolysis of mitotic cyclins and the accumulation of CKIs throughout G1 phase, suppressing CDK activity. In addition, it promotes degradation of the metazoan-specific inhibitor of Cdtl, Geminin (McGarry and Kirschner, 1998). APC/C also regulates DDK activity by targeting the kinase regulatoy subunit, Dbf4, for proteasomal degradation during G1 (Masai and Arai, 2002). Therefore, the APC/C is responsible for inactivation of CDK and DDK as cells enter G1 and setting up the right environment for pre-RC formation. All additional mechanisms that prevent re-replication inhibit the first step of replication initiation, pre-RC assembly. Once MCM2-7 is loaded, ORC, Cdc6 and Cdtl are not required for initiating replication indicating that their primary role is to deliver MCM2-7 to origins (Duncker et al., 1999; Hua and Newport, 1998; Rowles et al., 1999). This feature allows for the inactivation of these pre-RC components outside of G1 phase, without compromising initiation of replication from already licensed origins. In the budding yeast, all mechanisms known to date to ensure that DNA is replicated only once per cell cycle depend on CDK activity. CDK phosphorylates all preRC components and these modifications have distinct consequences on each target. CDK phosphorylates two ORC subunits, Orc2 and Orc6 (Nguyen et al., 2001). In addition, direct binding of the S phase cyclin, Clb5, to an RXL motif in Orc6 also contributes to preventing rereplication (Wilmes et al., 2004). Recently, it was shown that CDK phosphorylation of Orc2 and Orc6 and possibly the interaction of Orc6 and Clb5, block Cdtl binding to Orc6, preventing MCM2-7 helicase loading (Chen and Bell, 2011). Cdc6 is inhibited by CDK in three ways: phosphorylation of Swi5 (Cdc6 transcriptional activator) prevents Cdc6 nuclear import, Cdc6 phosphorylation promotes its proteolysis, and direct association with mitotic CDK inhibits its association with origins (Mimura et al., 2004; Moll et al., 1991). CDK also promotes the nuclear export of Mcm2-7, leading to its exclusion from the nucleus in G2 and M phases (Nguyen et al., 2000). Other factors are involved in regulating the inhibition of pre-RC formation in metazoans and CDK plays a less important role. Cdtl seems to be the major target for inhibition. Cdtl destruction by Cul4-Ddbl-Cdt2 ubiquitin ligase during S phase is a highly conserved mechanism (Arias and Walter, 2007). Another well-conserved mechanism among metazoans is the inhibition of Cdtl by Geminin, a protein that is cell cycle regulated. Geminin was originally identified in Xenopus in a screen for APC/C substrates (McGarry and Kirschner, 1998). It is destroyed during mid-mitosis to G1 and reaccumulates during late G1 when APC is inactivated. Geminin binds Cdtl, inhibiting MCM2-7 binding to Cdtl and subsequent loading of the MCM2-7 complex onto origins (Fujita, 2006). In mammals, Cdtl is targeted by the SCF E3 ubiquitin ligase for proteolysis in a CDK dependent manner (Fujita, 2006). Although the mechanisms by which ORC is inhibited are fundamentally conserved, there are notable differences from organism to organism (DePamphilis, 2005). In general, chromatin binding is regulated and one or more ORC subunit dissociate from chromatin after pre-RC assembly. Like in yeast, CDK phosphorylation plays an important role in regulating ORC activity. It has been shown that mitotic Cdk1 phosphorylates one or more ORC subunits, and this blocks pre-RC assembly until mitosis is complete. Finally, in flies and mammals, Orc1 is targeted for proteolysis. Cdc6 and Mcm2-7 do not seem to be major targets of negative regulation in metazoans (Arias and Walter, 2007). Cell cycle checkpoints The integrity of genomic DNA can be extensively altered by errors occurring during DNA replication and by reacting with molecules in normal cellular environments, such as reactive oxygen and nitrogen species. In addition, cells are constantly exposed to DNA damage caused by environmental sources such as UV and ionizing radiation as wells as a myriad of chemical agents. (Jackson and Bartek, 2009). The repair of this damage is essential for the survival and proper function of all eukaryotic cells. Eukaryotic cells have developed several mechanisms to counteract the potentially deleterious effects of DNA damage, which are collectively known as the DNA damage response. Inthe following section I will focus on one mechanism, the cell cycle checkpoints. Checkpoints comprise a complex network of pathways that cause arrest or delay of cell cycle progression after DNA damage, allowing additional time for repair to take place before the cell enters the next phase of the cell cycle (Lukas et al., 2004). When damage is irreparable, those signaling pathways can permanently prevent proliferation of damaged cells by mediating apoptosis or senescence. These control mechanisms are crucial for the maintenance of genomic integrity because they minimize the risk of DNA lesions to be converted into inheritable mutations. Defects in the DNA damage checkpoints lead to accumulation of mutations and chromosomal aberrations, which in turn increase the probability of developing genetic disorders and diseases including cancer (Jackson and Bartek, 2009). Checkpoints can be described as signal transduction systems consisting of sensors that detect DNA lesions, mediators that amplify the signal and transducer and effector kinases that transmit the signal to downstream targets (Figure 5) (Harper and Elledge, 2007). DNA damage-induced checkpoints have been identified at the G1/S and G2/M boundaries as well as during S phase of the cell cycle. Many DNA damage sensors and signal transducers are integral components of all three checkpoints. ATM and ATR are two protein kinases of the phosphatidylinositol 3-kinase (P13K)-like family that are critical for the initiation of the signaling cascade in response to DNA damage (Figure 5). ATM and ATR have similar structures; they both contain FAT and FATC domains and a catalytic P13K domain. In addition, they preferentially phosphorylate serine or threonine residues followed by glutamine. There are also clear differences between these kinases; ATM is activated primarily by double strand breaks, while ATR responds to a broader range of DNA lesions and stalled replication forks (Shiloh, 2003). Transgenic mice lacking ATM are viable, while loss of ATR causes early embryonic lethality, suggesting that ATR plays essential roles during normal cell cycle regulation (Brown and Baltimore, 2000; de Klein et al., 2000; Elson et al., 1996). A group of proteins, including Claspin, 53BP1, Mdcl, and BRCA1, mediate the transmission of checkpoint signaling to two major downstream targets, Chk1 and Chk2 kinases. Activation of Chk1 is ATR-dependent, while Chk2 is an important target of ATM (Harper and Elledge, 2007). The final targets of the ATR/Chk1 and ATM/Chk2 pathways depend on the cell cycle phase at which damage is sensed and include components of the cell cycle, apoptotic, and DNA repair machinery (Figure 5). Importantly, there is considerable redundancy amongst pathways comprising the eukaryotic DNA damage checkpoints and a simple linear pathway targeting a single substrate can rarely be identified. The growing number of cell cycle and DNA repair regulators that are modified in response to checkpoint activation underscores the complexity of this mechanism and how numerous proteins work in concert to successfully execute the checkpoint response (Matsuoka et al., 2007). In the following sections, I will provide an overview of the mammalian DNA damage checkpoints by outlining the general principles of the key signaling pathways orchestrated by ATR and ATM. In addition, I will review how the final response is determined depending on the cell cycle phase at which damage is detected by these signaling pathways. Genotoxic stress /)00000000000000OO\ Sensor proteins Transducers ATR and ATM Effectors Chk1 and Chk2 Cell cycle arrest Senescence DNA repair Apoptosis Figure 5. General scheme of the signal transduction pathways that are activated in response to DNA damage or stalled replication forks. DNA damage or stalled replication forks are sensed by a group of proteins, leading to the activation of the ATM and ATR kinases which in turn activate the Chk1 and Chk2 kinases. ATM and ATR function in combination with Chk1 and Chk2 to phosphorylate a number of targets leading to a variety of cellular outcomes. (Adapted from Kastan MB and Bartek J 2004). A TM and A TR: initiators of the signaling cascade in response to DNA damage ATM activation ATM was identified as the product of the gene mutated in the autosomal recessive disorder Ataxia telangiectasia, characterized by cerebellar degeneration, immunodeficiency, chromosomal instability and radiation sensitivity (Savitsky K 1995). In mammalian cells, ATM has critical roles in cellular responses to double strand breaks (DSBs), which can arise from exposure to ionizing radiation and genotoxic chemicals, as well as from cellular processes such as DNA replication, meiosis and V(D)J recombination (Sun et al., 1989; Ward, 1988). Early studies on ATM indicated that its kinase activity is induced very rapidly in response to DSBs, suggesting it acts at an early stage of the signal transduction cascade (Banin et al., 1998; Canman et al., 1998). Experimental evidence indicates that without cellular stress, ATM forms dimers that are catalytic inactive. Very rapidly after DSBs have occurred, one ATM molecule phosphorylates residue Serine 1981 on an interacting ATM molecule, leading to disruption of the complex and release of active ATM monomers (Bakkenist and Kastan, 2003). Two additional autophosphorylation sites, S367 and S1893, are also important for ATM activation in response to DSBs (Kozlov et al., 2006). Interestingly, this mechanism of ATM activation appears to be less important for the activity of mouse ATM, where mutation of the S1987 site (equivalent to S1981 in the human protein) does not affect ATM activity (Pellegrini et al., 2006). It is not clear what signal triggers the autophosphorylation of ATM, but there is evidence suggesting that such signal could be the result of changes in higher-order chromatin structure caused by DSBs (Bakkenist and Kastan, 2003; Kim et al., 2009; Kruhlak et al., 2006). In addition, several proteins are required for ATM activity (as discussed below) and may play a direct role in inducing autophosphorylation and subsequent activation of ATM. Much attention has been focused on understanding the early events that take place between DSB formation and ATM activation. One of the hallmarks of the response to DSBs is the rapid accumulation of several checkpoint signaling and repair proteins at the location of the lesion to form distinct foci. The recruitment of proteins to DSBs is thought to facilitate repair of the lesion and amplification of the checkpoint signal. In support of this concept, localization of checkpoint signaling components to chromatin is sufficient to trigger activation of the checkpoint in the absence of DNA damage (Soutoglou and Misteli, 2008). Imaging technology in live mammalian cells has elucidated the temporal order in which the initial proteins are recruited to DSBs (Lukas et al., 2005). Mrell, Rad50 and Nbs1, components of the MRN complex, are the first proteins to bind to the sites of DSB formation (Lisby et al., 2004; Lou et al., 2006; Lukas et al., 2004) and thus have been implicated in the initial detection of DSBs and transduction of the DNA damage signal. Their binding is independent of all proteins that have been tested, including ATM. Consistent with the notion that MRN acts as a sensor of DSBs, it was shown that the MRN complex mediates recruitment of ATM to sites of DSBs and stimulates ATM kinase activity (Lee and Paull, 2004). The recruitment of ATM occurs through a protein-protein interaction between the MRN component Nbsl and ATM (Lee and Paull, 2004; You et al., 2005). Another key event in the response to DSBs is the phosphorylation of the H2A histone variant H2AX by ATM (Burma et al., 2001). H2AX is phosporylated very rapidly after DSB formation, and form foci at the sites of DSBs that can be detected within 1 min after exposure to ionizing radiation (IR) (Rogakou et al., 1999). Phosphorylation of H2AX provides a high-affinity binding site for the mediator protein MDC1, which in turn orchestrates the recruitment of downstream factors to the sites of DSBs (Stucki et al., 2005). 53BP1, a protein proposed to function as a co-activator of ATM, is one of the factors recruited to foci. Depletion of 53BP1 by siRNA decreases ATM autophosphorylation in response to IR, as well as the phosphorylation of ATM substrates (DiTullio et al., 2002; Wang et al., 2002). The mechanism by which 53BP1 contributes to ATM activity is unclear. Numerous ATM substrates have been identified with functions in a wide range of processes (Kastan and Lim, 2000; Matsuoka et al., 2007). Chk2 is an effector kinases rapidly phosphorylated by ATM in response to DNA damage (Ahn et al., 2000; Matsuoka et al., 1998; Matsuoka et al., 2000). Chk2 is not immobilized at sites of DNA damage after IR treatment, suggesting that it acts as a signal distributor (Lukas et al., 2003). Functionally, Chk2 can activate both apoptosis and cell cycle arrest pathways. ATR activation ATR is activated in response to a number of lesions, including stalled replication forks, UV-induced dimmers, and double strand breaks. Activation of ATR requires its association with ATRIP, TopBP1, the Rad17-RFC2-5 complex and the Rad9-Radl-Husl (9-1-1) complex (Flynn and Zou). ATR forms a stable complex with ATRIP, which regulates the localization of ATR to sites of replication stress and DNA damage and is essential for ATR signaling (Cortez et al., 2001). Single stranded DNA is the primary trigger for ATR activation. When single stranded DNA is generated, it is coated by replication protein A (RPA) (Wold and Kelly, 1988). ATR is recruited to ssDNA via its stable partner ATRIP, which binds to RPA-coated ssDNA (Namiki and Zou, 2006; Zou and Elledge, 2003). The Rad17-RFC2-5 complex is independently recruited to ssDNA (Zou et al., 2002). The presence of a dsDNA-ssDNA junction stimulates this complex to load a second complex to the sites of DNA damage, the Rad9-Radl-Husl (9-1-1) complex (Zou et al., 2003). Recruitment of the 9-1-1 complex by Rad17 enables substrate selection by ATR. TopBP1 is a BRCT-domain containing protein that functions in replication initiation. TopBp1 is also recruited to the sites of DNA damage, where it directly stimulates ATR kinase activity. A region sufficient for ATR activation, called the ATR activation domain (AAD), has been identified between BRCT domains VI and VII of the TopBP1 protein (Kumagai et al., 2006). Studies in human cells and Xenopus egg extracts showed that TopBP1 interacts constitutively with Rad9 and identified a phosphorylation site in the C-terminus of Rad9 that is required for the interaction (Lee et al., 2007; Makiniemi et al., 2001; St Onge et al., 2003). The region of TopBP1 that binds to Rad9 has been mapped to the first two BRCT domains (Lee et al., 2007). The functional significance of this interaction appears to be to recruit TopBP1 to sites of damage, where ATR has been independently recruited, to induce activation of ATR (Lee and Dunphy, 2010). Chk1 is the major downstream effector kinase of ATR, which propagates and amplifies the checkpoint signal (Guo et al., 2000; Liu et al., 2000; Zhao and PiwnicaWorms, 2001). A major role of Chk1 is to inhibit the cell cycle phosphatases Cdc25A, Cdc25B and Cdc25C (Sanchez et al., 1997; Sorensen et al., 2003; Zhao et al., 2002) thereby preventing them from promoting progression through S phase or entry into mitosis (Figure 6). The G1/S checkpoint CDK2, which is essential for entry into S-phase, is the key target of the G1/S DNA damage checkpoint. The rapid induction of the checkpoint is mediated by the inhibition of Cdc25A, a phosphatase that activates CDK2. Chk1/Chk2 phosphorylation of Cdc25A promotes its degradation through the ubiquitin-proteasome system in the presence of DNA damage (Donzelli and Draetta, 2003). A p53-dependent pathway can also arrest cell cycle progression at the G1/S transition. p53 is phosphorylated by ATR/ATM and Chk1/Chk2 in response to DNA damage, causing its dissociation from Mdm2, an E3 ubiquitin ligase that targets p53 for degradation (Figure 6) (Wahl and Carr, 2001). p53 upregulates the transcription of proapoptotic and cell cycle inhibiting genes such as the CDK inhibitor p21, which inhibits CDK2 (Figure 6). The response caused by this pathway takes longer to occur because it requires the transcription and accumulation of newly synthesized proteins (Bartek and Lukas, 2001). Another mechanism to arrest the cell cycle at the G1/S transition is the DNA damage-induced degradation of CDT1, a licensing factor essential for the initiation of replication. It has been shown that CDT1 also undergoes ubiquitination and proteolysis in response to DNA damage (Higa et al., 2003; Hu et al., 2004). DNA-damage induced G2/M arrest The G2/M checkpoint can be divided into two phases known to control mitotic entry upon DNA damage. The early phase is ATM dependent, transient, and is reflected by an abrupt reduction in mitotic index. This early G2/M checkpoint is activated within one hour after damage and causes the arrest of cells that were in G2 at the time of damage. The arrest results from phosphorylation of Cdc25C, a phosphatase required for activation of CDK1, the mitotic CDK. Cdc25C phosphorylation leads to its sequestration from the nucleus by 14-3-3. The late phase is ATM independent and reflected by an accumulation of cells in G2 (Xu et al., 2002). It causes the arrest of cells that had been in earlier phases of the cell cycle at the time of damage, but had continued progressing to the G2 phase. The p53 pathway is known to contribute to the late phase of the G2/M checkpoint through transcriptional activation of p21 and 14-3-30, which results in increased inactivation and cytoplasmic sequestration of CDK1 (Figure 6) (Bunz et al., 1998; Hermeking et al., 1997). DNA Damage/Replication Stress Figure 6. A Simplified scheme of DNA damage-induced cell cycle arrest and apoptosis. ATM/ATR Chk1/Chk2 I MDM2 N CDC25 14-3-3 p53 CDK activity is inhibited by DNA damage checkpoints in several ways including inhibition of Cdc25 phosphatases and activation of p21. p5 3 controls the transcription of genes involved in cell death and cell cycle arrest. (Adapted from Kastan MB and Bartek J 2004). p21 CDKs Apoptosis Cell cycle progression Checkpoints during S-phase The genome is particularly susceptible to mutation during S phase, where replication errors or replication stress can result in the stalling of replication forks, replication fork collapse and subsequent DNA damage that is potentially deleterious to the cell. Thus, monitoring DNA replication is essential for maintaining the integrity of the genome. Two checkpoints have been described during S phase, the intra-S phase and the S/M checkpoint (Bartek et al., 2004). The intra-S checkpoint prevents origin firing in the presence of DNA damage or stalled replication forks, leading to a slowing in S-phase progression. In addition, it is important for stabilizing stalled forks, allowing proper resumption of DNA synthesis once the damage is repaired. The S/M checkpoint monitors DNA replication and prevents mitotic entry until the genome is fully replicated. These two responses converge at the same effector protein kinases and a clear distinction is often difficult to make. The intra-S phase checkpoint ATR plays a particularly important role in S phase checkpoints in response to DNA damage. Collapsed or stalled replication forks in S phase activate ATR, leading to phosphorylation of Chk1 and the subsequent inhibition of Cdc25A phosphatase activity. This prevents initiation of new replication origins, causing a reversible delay in cell cycle progression (Bartek and Lukas, 2001). There is a second branch of the intra-S phase checkpoint involving SMC1, NBS1 and BRCA1 and FANCD2. However, the specific roles of these proteins remain unclear. The S/M checkpoint The S/M checkpoint is a surveillance mechanism that prevents initiation of mitosis until the genome is fully and accurately replicated. In the absence of a functional S/M checkpoint, cells enter mitosis prematurely and chromosomal fragmentation is prone to occur. Mammalian cell fusion studies showing that fusion of a S-phase cell with a G2-phase cell inhibits mitosis demonstrated the existence of such a checkpoint (Rao and Johnson, 1970). Mitosis in the absence of DNA synthesis is observed when mammalian cells are treated with caffeine, an inhibitor of the ATM and ATR kinases (Brinkley et al., 1988; Schlegel and Pardee, 1986). These studies suggested that regulators maintain the normal order of cell cycle events by detecting the completion of DNA synthesis. Accumulating evidence suggests that the ATR-Chk1 pathway may fulfill this function. A role for ATR in normal cell cycle progression was first implied by the fact that ATR and other components of the ATR signaling pathway, such as Chk1, are essential for mammalian development and viability (Brown and Baltimore, 2000; Takai et al., 2000). Mouse blastocysts lacking ATR accumulate chromosome breaks, a phenotype attributed to loss of the S/M checkpoint (Brown and Baltimore, 2000). Furthermore, several components of the ATR pathway associate with replicating chromatin during Sphase in the absence of exogenous DNA damage (Dart et al., 2004; Hekmat-Nejad et al., 2000). Activation of CDK1-cyclin B is the key event required for mitosis to initiate. Chk1 has been shown to localize to interphase centrosomes but not mitotic centrosomes during unperturbed cell cycle progression, preventing premature activation of CDK1cyclin B by negatively regulating Cdc25B (Kramer et al., 2004). There is also evidence for a role of Chk1 during an unperturbed S-phase. Specifically, it is required for maintaining replication fork stability and regulates activation of late origin firing by phosphorylating Cdc25A in the absence of DNA damage. (Sorensen et al., 2003; Zhao et al., 2002). Depletion of Chk1, Rad9, Hus1, ATR or Claspin in human cells leads to an accumulation of Cdc25 in the absence of external DNA damage (Sorensen et al., 2004) suggesting that these proteins are involved in the regulation of Cdc25A during replication. Furthermore, it was shown that inhibition of ATR and Chk1 increases origin firing and loading of the replication factor Cdc45 in undamaged cells (Syljuasen et al., 2005). Together, these studies provide evidence supporting a model in which the ATR-Chk1 pathway is active at a low level during normal S-phase due to intrinsic lesions arising from DNA replication and activation of checkpoints then further activates the pathway. In addition, the ATR-Chk1 pathway maintains DNA replication through structurally unfavorable stretches of DNA, termed fragile sites in mammalian cells (Casper et al., 2002; Cha and Kleckner, 2002). The zebrafish as a model system for the study of cell cycle regulation In the early 1970's, Dr. George Strisinger identified the zebrafish as a model system for the study of vertebrate biology (Grunwald and Eisen, 2002). For the following decades, zebrafish studies focused on developmental biology largely due to the advantages that this organism offers. Zebrafish have a short generation time, around 34 months, and large numbers can be housed in a small space at relatively low cost. In addition, fertilization of oocytes occurs externally, permitting easy manipulation of the embryos. There are two particular characteristics that make the zebrafish a very attractive model for developmental studies: the embryos (100-200 per clutch) are transparent, and their development is very rapid. In fact, cleavage divisions, gastrulation, morphogenesis and organogenesis all occur within 24 hours (Driever et al., 1996; Kimmel et al., 1995). Therefore, scientists could follow in real time the behavior of single cells or group of cells in the embryo at different stages of development. Soon it became evident that the zebrafish represented a powerful tool for studying genes that regulate specific developmental processes and large-scale screens were developed. In particular, two groups used the methylating agent ethylnitrosourea (ENU) as a mutagen and isolated mutants with morphological developmental phenotypes (Driever et al., 1996; Haffter et al., 1996). The advantage of this process is that many mutants can be isolated in a relatively fast manner, but the cloning of the affected genes is quite laborious. High throughput gene and EST mapping projects revealed extensive conserved syntety between the zebrafish and human genomes and assisted in the identification of mutated genes of interest by positional cloning (Barbazuk et al., 2000). Nowadays, chemical mutagenesis is widely used for performing screens and isolating mutants with phenotypes of interest. The Hopkins lab developed an alternative approach for large-scale screens using a retrovirus as an insertional mutagen. Although this method is less efficient, its advantage relies on providing a feasible way of identifying the mutated gene due to the insertion of a tag at the site of the lesion. In this screen, 525 embryonic lethal mutant zebrafish were isolated, that represent lesions in 390 different cell essential genes. It was estimated that this screen unveiled approximately 25% of all genes required for embryonic and early larva development (Amsterdam et al., 2004). These mutants can be classified into two categories based on their gross morphological phenotypes; one class of mutants have developmentally specific phenotypes, whereas the other class display non-specific phenotypes such as extensive cell death or general growth defects. The latter class of mutants includes housekeeping genes such as genes involved in transcription, RNA processing and translation. Mutants for known cell cycle regulators, such as polo-like kinase, Cyclin B1 and Aurora B kinase, also fall into this category (Amsterdam et al., 2004). Importantly, 20% of the genes were novel or poorly characterized at the time they were cloned (Amsterdam et al., 2004). We predicted that among these novel genes were regulators of the cell cycle. Our lab designed an assay for screening the zebrafish insertional mutant library for a defect in the ionizing radiation induced checkpoint response in order to identify novel components required for the DNA damage response. In the following chapters, I will describe how such a screen was performed, leading to the discovery of a novel protein, Ticrr (for TopBP1 -interacting, checkpoint and replication regulator). I will also describe how the analysis of the defects in ticrr zebrafish mutants gave insight into the function of this protein, which was later complemented with the characterization of the human ortholog. References Ahn, J.Y., Schwarz, J.K., Piwnica-Worms, H., and Canman, C.E. (2000). Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res 60, 5934-5936. Amsterdam, A., Nissen, R.M., Sun, Z., Swindell, E.C., Farrington, S., and Hopkins, N. (2004). Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A 101, 12792-12797. Ang, X.L., and Wade Harper, J. (2005). SCF-mediated protein degradation and cell cycle control. Oncogene 24, 2860-2870. Aparicio, T., Guillou, E., Coloma, J., Montoya, G., and Mendez, J. (2009). The human GINS complex associates with Cdc45 and MCM and is essential for DNA replication. Nucleic Acids Res 37, 2087-2095. Arias, E.E., and Walter, J.C. (2007). Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev 21, 497-518. Bakkenist, C.J., and Kastan, M.B. (2003). DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499-506. Banin, S., Moyal, L., Shieh, S., Taya, Y., Anderson, C.W., Chessa, L., Smorodinsky, N.I., Prives, C., Reiss, Y., Shiloh, Y., et al. (1998). Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281, 1674-1677. Barbazuk, W.B., Korf, I., Kadavi, C., Heyen, J., Tate, S., Wun, E., Bedell, J.A., McPherson, J.D., and Johnson, S.L. (2000). The syntenic relationship of the zebrafish and human genomes. Genome Res 10, 1351-1358. Bartek, J., Lukas, C., and Lukas, J. (2004). Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 5, 792-804. Bartek, J., and Lukas, J. (2001). Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr Opin Cell Biol 13, 738-747. Bell, S.P. (2002). The origin recognition complex: from simple origins to complex functions. Genes Dev 16, 659-672. Bell, S.P., and Dutta, A. (2002). DNA replication in eukaryotic cells. Annu Rev Biochem 71, 333374. Bell, S.P., and Stillman, B. (1992). ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357, 128-134. Bowers, J.L., Randell, J.C., Chen, S., and Bell, S.P. (2004). ATP hydrolysis by ORC catalyzes reiterative Mcm2-7 assembly at a defined origin of replication. Mol Cell 16, 967-978. Brinkley, B.R., Zinkowski, R.P., Mollon, W.L., Davis, F.M., Pisegna, M.A., Pershouse, M., and Rao, P.N. (1988). Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature 336, 251-254. Brown, E.J., and Baltimore, D. (2000). ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev 14, 397-402. Bunz, F., Dutriaux, A., Lengauer, C., Waldman, T., Zhou, S., Brown, J.P., Sedivy, J.M., Kinzler, K.W., and Vogelstein, B. (1998). Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282, 1497-1501. Burma, S., Chen, B.P., Murphy, M., Kurimasa, A., and Chen, D.J. (2001). ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276, 42462-42467. Canman, C.E., Lim, D.S., Cimprich, K.A., Taya, Y., Tamai, K., Sakaguchi, K., Appella, E., Kastan, M.B., and Siliciano, J.D. (1998). Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281, 1677-1679. Casper, A.M., Nghiem, P., Art, M.F., and Glover, T.W. (2002). ATR regulates fragile site stability. Cell 111, 779-789. Cha, R.S., and Kleckner, N. (2002). ATR homolog Mec promotes fork progression, thus averting breaks in replication slow zones. Science 297, 602-606. Chen, S., and Bell, S.P. (2011). CDK prevents Mcm2-7 helicase loading by inhibiting Cdtl interaction with Orc6. Genes Dev 25, 363-372. Chen, S., de Vries, M.A., and Bell, S.P. (2007). Orc6 is required for dynamic recruitment of Cdtl during repeated Mcm2-7 loading. Genes Dev 21, 2897-2907. Chesnokov, I., Remus, D., and Botchan, M. (2001). Functional analysis of mutant and wild-type Drosophila origin recognition complex. Proc Natl Acad Sci U S A 98, 11997-12002. Cortez, D., Guntuku, S., Qin, J., and Elledge, S.J. (2001). ATR and ATRIP: partners in checkpoint signaling. Science 294, 1713-1716. Costa, A., lives, I., Tamberg, N., Petojevic, T., Nogales, E., Botchan, M.R., and Berger, J.M. (2011). The structural basis for MCM2-7 helicase activation by GINS and Cdc45. Nat Struct Mol Biol 18, 471-477. Dahmann, C., Diffley, J.F., and Nasmyth, K.A. (1995). S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol 5, 1257-1269. Dart, D.A., Adams, K.E., Akerman, I., and Lakin, N.D. (2004). Recruitment of the cell cycle checkpoint kinase ATR to chromatin during S-phase. J Biol Chem 279, 16433-16440. de Klein, A., Muijtjens, M., van Os, R., Verhoeven, Y., Smit, B., Carr, A.M., Lehmann, A.R., and Hoeijmakers, J.H. (2000). Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol 10, 479-482. DePamphilis, M.L. (2005). Cell cycle dependent regulation of the origin recognition complex. Cell Cycle 4, 70-79. Diffley, J.F. (2004). Regulation of early events in chromosome replication. Curr Biol 14, R778786. Diffley, J.F., Cocker, J.H., Dowell, S.J., and Rowley, A. (1994). Two steps in the assembly of complexes at yeast replication origins in vivo. Cell 78, 303-316. DiTullio, R.A., Jr., Mochan, T.A., Venere, M., Bartkova, J., Sehested, M., Bartek, J., and Halazonetis, T.D. (2002). 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol 4, 998-1002. Donzelli, M., and Draetta, G.F. (2003). Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep 4, 671-677. Driever, W., Solnica-Krezel, L., Schier, A.F., Neuhauss, S.C., Malicki, J., Stemple, D.L., Stainier, D.Y., Zwartkruis, F., Abdelilah, S., Rangini, Z., et al. (1996). A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37-46. Duncker, B.P., Pasero, P., Braguglia, D., Heun, P., Weinreich, M., and Gasser, S.M. (1999). Cyclin B-cdkl kinase stimulates ORC- and Cdc6-independent steps of semiconservative plasmid replication in yeast nuclear extracts. Mol Cell Biol 19, 1226-1241. Elson, A., Wang, Y., Daugherty, C.J., Morton, C.C., Zhou, F., Campos-Torres, J., and Leder, P. (1996). Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci U S A 93, 13084-13089. Erzberger, J.P., and Berger, J.M. (2006). Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct 35, 93-114. Evans, T., Rosenthal, E.T., Youngblom, J., Distel, D., and Hunt, T. (1983). Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389-396. Evrin, C., Clarke, P., Zech, J., Lurz, R., Sun, J., Uhle, S., Li, H., Stillman, B., and Speck, C. (2009). A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proc Natl Acad Sci U S A 106, 20240-20245. Flynn, R.L., and Zou, L. ATR: a master conductor of cellular responses to DNA replication stress. Trends Biochem Sci 36, 133-140. Forsburg, S.L., and Nurse, P. (1991). Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol 7, 227-256. Fujita, M. (2006). Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells. Cell Div 1, 22. Gambus, A., Jones, R.C., Sanchez-Diaz, A., Kanemaki, M., van Deursen, F., Edmondson, R.D., and Labib, K. (2006). GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol 8, 358-366. Garcia, V., Furuya, K., and Carr, A.M. (2005). Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 4, 1227-1239. Ge, X.Q., Jackson, D.A., and Blow, J.J. (2007). Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev 21, 3331-3341. Giordano-Coltart, J., Ying, C.Y., Gautier, J., and Hurwitz, J. (2005). Studies of the properties of human origin recognition complex and its Walker A motif mutants. Proc Natl Acad Sci U S A 102, 69-74. Glover, J.N., Williams, R.S., and Lee, M.S. (2004). Interactions between BRCT repeats and phosphoproteins: tangled up in two. Trends Biochem Sci 29, 579-585. Grunwald, D.J., and Eisen, J.S. (2002). Headwaters of the zebrafish -- emergence of a new model vertebrate. Nat Rev Genet 3, 717-724. Guo, Z., Kumagai, A., Wang, S.X., and Dunphy, W.G. (2000). Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UVdamaged DNA in Xenopus egg extracts. Genes Dev 14, 2745-2756. Haffter, P., Granato, M., Brand, M., Mullins, M.C., Hammerschmidt, M., Kane, D.A., Odenthal, J., van Eeden, F.J., Jiang, Y.J., Heisenberg, C.P., et al. (1996). The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123,1-36. Harper, J.W., and Elledge, S.J. (2007). The DNA damage response: ten years after. Mol Cell 28, 739-745. Hekmat-Nejad, M., You, Z., Yee, M.C., Newport, J.W., and Cimprich, K.A. (2000). Xenopus ATR is a replication-dependent chromatin-binding protein required for the DNA replication checkpoint. Curr Biol 10, 1565-1573. Hermeking, H., Lengauer, C., Polyak, K., He, T.C., Zhang, L., Thiagalingam, S., Kinzler, K.W., and Vogelstein, B. (1997). 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1, 3-11. Higa, L.A., Mihaylov, I.S., Banks, D.P., Zheng, J., and Zhang, H. (2003). Radiation-mediated proteolysis of CDT1 by CUL4-ROC1 and CSN complexes constitutes a new checkpoint. Nat Cell Biol 5, 1008-1015. Ho, C.C., Siu, W.Y., Lau, A., Chan, W.M., Arooz, T., and Poon, R.Y. (2006). Stalled replication induces p53 accumulation through distinct mechanisms from DNA damage checkpoint pathways. Cancer Res 66, 2233-2241. Hu, J., McCall, C.M., Ohta, T., and Xiong, Y. (2004). Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol 6,1003-1009. Hua, X.H., and Newport, J. (1998). Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J Cell Biol 140, 271281. Ibarra, A., Schwob, E., and Mendez, J. (2008). Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Natl Acad Sci U S A 105, 89568961. Ilves, I., Petojevic, T., Pesavento, J.J., and Botchan, M.R. (2010). Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Mol Cell 37, 247-258. Im, J.S., Ki, S.H., Farina, A., Jung, D.S., Hurwitz, J., and Lee, J.K. (2009). Assembly of the Cdc45-Mcm2-7-GINS complex in human cells requires the Ctf4/And-1, RecQL4, and Mcm1O proteins. Proc Natl Acad Sci U S A 106, 15628-15632. Jackson, S.P., and Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature 461, 1071-1078. Jares, P., and Blow, J.J. (2000). Xenopus cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev 14, 1528-1540. Jeffrey, P.D., Russo, A.A., Polyak, K., Gibbs, E., Hurwitz, J., Massague, J., and Pavletich, N.P. (1995). Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376, 313-320. Kamimura, Y., Masumoto, H., Sugino, A., and Araki, H. (1998). SId2, which interacts with Dpbl 1 in Saccharomyces cerevisiae, is required for chromosomal DNA replication. Mol Cell Biol 18, 6102-6109. Kamimura, Y., Tak, Y.S., Sugino, A., and Araki, H. (2001). SId3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J 20, 2097-2107. Kastan, M.B., and Lim, D.S. (2000). The many substrates and functions of ATM. Nat Rev Mol Cell Biol 1, 179-186. Kim, Y.C., Gerlitz, G., Furusawa, T., Catez, F., Nussenzweig, A., Oh, K.S., Kraemer, K.H., Shiloh, Y., and Bustin, M. (2009). Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat Cell Biol 11, 92-96. Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B., and Schilling, T.F. (1995). Stages of embryonic development of the zebrafish. Dev Dyn 203, 253-310. Klemm, R.D., Austin, R.J., and Bell, S.P. (1997). Coordinate binding of ATP and origin DNA regulates the ATPase activity of the origin recognition complex. Cell 88, 493-502. Kozlov, S.V., Graham, M.E., Peng, C., Chen, P., Robinson, P.J., and Lavin, M.F. (2006). Involvement of novel autophosphorylation sites in ATM activation. EMBO J 25, 3504-3514. Kramer, A., Mailand, N., Lukas, C., Syljuasen, R.G., Wilkinson, C.J., Nigg, E.A., Bartek, J., and Lukas, J. (2004). Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdkl kinase. Nat Cell Biol 6, 884-891. Kruhlak, M.J., Celeste, A., Dellaire, G., Fernandez-Capetillo, 0., Muller, W.G., McNally, J.G., Bazett-Jones, D.P., and Nussenzweig, A. (2006). Changes in chromatin structure and mobility in living cells at sites of DNA double-strand breaks. J Cell Biol 172, 823-834. Kumagai, A., Lee, J., Yoo, H.Y., and Dunphy, W.G. (2006). TopBP1 activates the ATR-ATRIP complex. Cell 124, 943-955. Labib, K. (2010). How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes Dev 24, 1208-1219. Lee, J., and Dunphy, W.G. (2010). Rad17 plays a central role in establishment of the interaction between TopBP1 and the Rad9-Husl-Radl complex at stalled replication forks. Mol Biol Cell 21, 926-935. Lee, J., Kumagai, A., and Dunphy, W.G. (2007). The Rad9-Husl-Radl checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem 282, 28036-28044. Lee, J.H., and Paull, T.T. (2004). Direct activation of the ATM protein kinase by the Mrel1/Rad50/Nbsl complex. Science 304, 93-96. Lew, D.J., and Kornbluth, S. (1996). Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol 8, 795-804. Lew, D.J., and S, I.R. (1992). A proliferation of cyclins. Trends Cell Biol 2, 77-81. Lisby, M., Barlow, J.H., Burgess, R.C., and Rothstein, R. (2004). Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118, 699-713. Liu, Q., Guntuku, S., Cui, X.S., Matsuoka, S., Cortez, D., Tamai, K., Luo, G., Carattini-Rivera, S., DeMayo, F., Bradley, A., et al. (2000). Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14, 1448-1459. Lou, Z., Minter-Dykhouse, K., Franco, S., Gostissa, M., Rivera, M.A., Celeste, A., Manis, J.P., van Deursen, J., Nussenzweig, A., Paull, T.T., et al. (2006). MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell 21, 187-200. Lukas, C., Bartek, J., and Lukas, J. (2005). Imaging of protein movement induced by chromosomal breakage: tiny 'local' lesions pose great 'global' challenges. Chromosoma 114, 146154. Lukas, C., Falck, J., Bartkova, J., Bartek, J., and Lukas, J. (2003). Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol 5, 255260. Lukas, J., Lukas, C., and Bartek, J. (2004). Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst) 3, 997-1007. Makiniemi, M., Hillukkala, T., Tuusa, J., Reini, K., Vaara, M., Huang, D., Pospiech, H., Majuri, I., Westerling, T., Makela, T.P., et al. (2001). BRCT domain-containing protein TopBP1 functions in DNA replication and damage response. J Biol Chem 276, 30399-30406. Malumbres, M., and Barbacid, M. (2009). Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9, 153-166. Marahrens, Y., and Stillman, B. (1992). A yeast chromosomal origin of DNA replication defined by multiple functional elements. Science 255, 817-823. Masai, H., and Arai, K. (2002). Cdc7 kinase complex: a key regulator in the initiation of DNA replication. J Cell Physiol 190, 287-296. Masai, H., Matsui, E., You, Z., Ishimi, Y., Tamai, K., and Arai, K. (2000). Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a criticial threonine residue of Cdc7 bY Cdks. J Biol Chem 275, 29042-29052. Masai, H., Matsumoto, S., You, Z., Yoshizawa-Sugata, N., and Oda, M. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem 79, 89-130. Masai, H., Taniyama, C., Ogino, K., Matsui, E., Kakusho, N., Matsumoto, S., Kim, J.M., Ishii, A., Tanaka, T., Kobayashi, T., et al. (2006). Phosphorylation of MCM4 by Cdc7 kinase facilitates its interaction with Cdc45 on the chromatin. J Biol Chem 281, 39249-39261. Masumoto, H., Muramatsu, S., Kamimura, Y., and Araki, H. (2002). S-Cdk-dependent phosphorylation of SId2 essential for chromosomal DNA replication in budding yeast. Nature 415, 651-655. Matsuno, K., Kumano, M., Kubota, Y., Hashimoto, Y., and Takisawa, H. (2006). The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol Cell Biol 26, 4843-4852. Matsuoka, S., Ballif, B.A., Smogorzewska, A., McDonald, E.R., 3rd, Hurov, K.E., Luo, J., Bakalarski, C.E., Zhao, Z., Solimini, N., Lerenthal, Y., et al. (2007). ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 11601166. Matsuoka, S., Huang, M., and Elledge, S.J. (1998). Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893-1897. Matsuoka, S., Rotman, G., Ogawa, A., Shiloh, Y., Tamai, K., and Elledge, S.J. (2000). Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A 97, 10389-10394. McGarry, T.J., and Kirschner, M.W. (1998). Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043-1053. Mimura, S., Seki, T., Tanaka, S., and Diffley, J.F. (2004). Phosphorylation-dependent binding of mitotic cyclins to Cdc6 contributes to DNA replication control. Nature 431, 1118-1123. Moll, T., Tebb, G., Surana, U., Robitsch, H., and Nasmyth, K. (1991). The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SW15. Cell 66, 743-758. Montagnoli, A., Valsasina, B., Brotherton, D., Troiani, S., Rainoldi, S., Tenca, P., Molinari, A., and Santocanale, C. (2006). Identification of Mcm2 phosphorylation sites by S-phase-regulating kinases. J Biol Chem 281, 10281-10290. Moyer, S.E., Lewis, P.W., and Botchan, M.R. (2006). Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A 103, 10236-10241. Muramatsu, S., Hirai, K., Tak, Y.S., Kamimura, Y., and Araki, H. (2010). CDK-dependent complex formation between replication proteins Dpbll, Sid2, Pol (epsilon}, and GINS in budding yeast. Genes Dev 24, 602-612. Nakayama, K.I., and Nakayama, K. (2006). Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6, 369-381. Namiki, Y., and Zou, L. (2006). ATRIP associates with replication protein A-coated ssDNA through multiple interactions. Proc Natl Acad Sci U S A 103, 580-585. Nguyen, V.Q., Co, C., Irie, K., and Li, J.J. (2000). Clb/Cdc28 kinases promote nuclear export of the replication initiator proteins Mcm2-7. Curr Biol 10, 195-205. Nguyen, V.Q., Co, C., and Li, J.J. (2001). Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411, 1068-1073. Nurse, P. (2000). A long twentieth century of the cell cycle and beyond. Cell 100, 71-78. Pacek, M., Tutter, A.V., Kubota, Y., Takisawa, H., and Walter, J.C. (2006). Localization of MCM27, Cdc45, and GINS to the site of DNA unwinding during eukaryotic DNA replication. Mol Cell 21, 581-587. Pellegrini, M., Celeste, A., Difilippantonio, S., Guo, R., Wang, W., Feigenbaum, L., and Nussenzweig, A. (2006). Autophosphorylation at serine 1987 is dispensable for murine Atm activation in vivo. Nature 443, 222-225. Perkins, G., and Diffley, J.F. (1998). Nucleotide-dependent prereplicative complex assembly by Cdc6p, a homolog of eukaryotic and prokaryotic clamp-loaders. Mol Cell 2, 23-32. Randell, J.C., Bowers, J.L., Rodriguez, H.K., and Bell, S.P. (2006). Sequential ATP hydrolysis by Cdc6 and ORC directs loading of the Mcm2-7 helicase. Mol Cell 21, 29-39. Rao, P.N., and Johnson, R.T. (1970). Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature 225, 159-164. Remus, D., Beuron, F., Tolun, G., Griffith, J.D., Morris, E.P., and Diffley, J.F. (2009). Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell 139, 719-730. Rogakou, E.P., Boon, C., Redon, C., and Bonner, W.M. (1999). Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146, 905-916. Rowles, A., Tada, S., and Blow, J.J. (1999). Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J Cell Sci 112 ( Pt 12), 2011-2018. Sanchez, Y., Wong, C., Thoma, R.S., Richman, R., Wu, Z., Piwnica-Worms, H., and Elledge, S.J. (1997). Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497-1501. Sangrithi, M.N., Bernal, J.A., Madine, M., Philpott, A., Lee, J., Dunphy, W.G., and Venkitaraman, A.R. (2005). Initiation of DNA replication requires the RECQL4 protein mutated in RothmundThomson syndrome. Cell 121, 887-898. Satyanarayana, A., and Kaldis, P. (2009). Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28, 2925-2939. Schlegel, R., and Pardee, A.B. (1986). Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science 232, 1264-1266. Sclafani, R.A., Tecklenburg, M., and Pierce, A. (2002). The mcm5-bobl bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdkl-dependent steps in Saccharomyces cerevisiae. Genetics 161, 47-57. Sherr, C.J. (1995). Mammalian G1 cyclins and cell cycle progression. Proc Assoc Am Physicians 107, 181-186. Sherr, C.J., and Roberts, J.M. (1995). Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9, 1149-1163. Sheu, Y.J., and Stillman, B. (2010). The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 463, 113-117. Shiloh, Y. (2003). ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3, 155-168. Sorensen, C.S., Syljuasen, R.G., Falck, J., Schroeder, T., Ronnstrand, L., Khanna, K.K., Zhou, B.B., Bartek, J., and Lukas, J. (2003). Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell 3, 247-258. Sorensen, C.S., Syljuasen, R.G., Lukas, J., and Bartek, J. (2004). ATR, Claspin and the Rad9Rad1-Hus1 complex regulate Chk1 and Cdc25A in the absence of DNA damage. Cell Cycle 3, 941-945. Soutoglou, E., and Misteli, T. (2008). Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320, 1507-1510. St Onge, R.P., Besley, B.D., Pelley, J.L., and Davey, S. (2003). A role for the phosphorylation of hRad9 in checkpoint signaling. J Biol Chem 278, 26620-26628. Stucki, M., Clapperton, J.A., Mohammad, D., Yaffe, M.B., Smerdon, S.J., and Jackson, S.P. (2005). MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123, 1213-1226. Sun, H., Treco, D., Schultes, N.P., and Szostak, J.W. (1989). Double-strand breaks at an initiation site for meiotic gene conversion. Nature 338, 87-90. Syljuasen, R.G., Sorensen, C.S., Hansen, L.T., Fugger, K., Lundin, C., Johansson, F., Helleday, T., Sehested, M., Lukas, J., and Bartek, J. (2005). Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol 25, 3553-3562. Tak, Y.S., Tanaka, Y., Endo, S., Kamimura, Y., and Araki, H. (2006). A CDK-catalysed regulatory phosphorylation for formation of the DNA replication complex Sld2-Dpb11. EMBO J 25, 19871996. Takai, H., Tominaga, K., Motoyama, N., Minamishima, Y.A., Nagahama, H., Tsukiyama, T., Ikeda, K., Nakayama, K., Nakanishi, M., and Nakayama, K. (2000). Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev 14, 1439-1447. Tanaka, S., and Diffley, J.F. (2002). Interdependent nuclear accumulation of budding yeast Cdtl and Mcm2-7 during G1 phase. Nat Cell Biol 4, 198-207. Tanaka, S., Umemori, T., Hirai, K., Muramatsu, S., Kamimura, Y., and Araki, H. (2007). CDKdependent phosphorylation of Sid2 and Sid3 initiates DNA replication in budding yeast. Nature 445, 328-332. Trimarchi, J.M., and Lees, J.A. (2002). Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3, 11-20. Tsai, L.H., Lees, E., Faha, B., Harlow, E., and Riabowol, K. (1993). The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene 8, 1593-1602. Tsakraklides, V., and Bell, S.P. (2010). Dynamics of pre-replicative complex assembly. J Biol Chem 285, 9437-9443. Wahl, G.M., and Carr, A.M. (2001). The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol 3, E277-286. Wang, B., Matsuoka, S., Carpenter, P.B., and Elledge, S.J. (2002). 53BP1, a mediator of the DNA damage checkpoint. Science 298, 1435-1438. Ward, J.F. (1988). DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol 35, 95-125. Wasch, R., and Engelbert, D. (2005). Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene 24, 1-10. Weinreich, M., Liang, C., and Stillman, B. (1999). The Cdc6p nucleotide-binding motif is required for loading mcm proteins onto chromatin. Proc Natl Acad Sci U S A 96, 441-446. Wilker, E., and Yaffe, M.B. (2004). 14-3-3 Proteins--a focus on cancer and human disease. J Mol Cell Cardiol 37, 633-642. Wilmes, G.M., Archambault, V., Austin, R.J., Jacobson, M.D., Bell, S.P., and Cross, F.R. (2004). Interaction of the S-phase cyclin CIb5 with an "RXL" docking sequence in the initiator protein Orc6 provides an origin-localized replication control switch. Genes Dev 18, 981-991. Wold, M.S., and Kelly, T. (1988). Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A 85, 2523-2527. Xu, B., Kim, S.T., Lim, D.S., and Kastan, M.B. (2002). Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 22, 1049-1059. Xu, X., Rochette, P.J., Feyissa, E.A., Su, T.V., and Liu, Y. (2009). MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J 28, 3005-3014. Yardimci, H., Loveland, A.B., Habuchi, S., van Oijen, A.M., and Walter, J.C. (2010). Uncoupling of sister replisomes during eukaryotic DNA replication. Mol Cell 40, 834-840. You, Z., Chahwan, C., Bailis, J., Hunter, T., and Russell, P. (2005). ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbsl. Mol Cell Biol 25, 53635379. Zegerman, P., and Diffley, J.F. (2007). Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445, 281-285. Zhao, H., and Piwnica-Worms, H. (2001). ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol 21, 4129-4139. Zhao, H., Watkins, J.L., and Piwnica-Worms, H. (2002). Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A 99, 14795-14800. Zou, L., Cortez, D., and Elledge, S.J. (2002). Regulation of ATR substrate selection by Rad17dependent loading of Rad9 complexes onto chromatin. Genes Dev 16, 198-208. Zou, L., and Elledge, S.J. (2003). Sensing DNA damage through ATRIP recognition of RPAssDNA complexes. Science 300, 1542-1548. Zou, L., Liu, D., and Elledge, S.J. (2003). Replication protein A-mediated recruitment and activation of Rad17 complexes. Proc Natl Acad Sci U S A 100, 13827-13832. Zou, L., and Stillman, B. (2000). Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7pDbf4p kinase. Mol Cell Biol 20, 3086-3096. CHAPTER II: A zebrafish screen for novel DNA damage response genes identifies TICRR Nelly M. Cruz, Cristopher L. Sansam and Jacqueline A. Lees Experimental contributions: C.L.S. designed the screen and conducted the experiments in Figure 2, 6, 7 and 8. N.C. conducted the large scale zebrafish screen. Summary Eukaryotes have evolved numerous checkpoint pathways to protect genomic integrity during normal cell division and in response to DNA damage. Mutations in DNA damage checkpoint components often lead to genetic disorders and cancer in humans. We have screened a collection of zebrafish insertional mutants to identify new components of these checkpoints. Specifically, we assayed activation of the IR-induced G2/M checkpoint by examining the mitotic index of zebrafish embryos after IR treatment. We have identified 12 zebrafish mutant lines that showed persistence of mitotic cells after IR treatment. 5 of these also had aberrant mitotic index without IR treatment, suggesting that the disrupted genes in these zebrafish lines play a role in cell cycle progression. One of the mutant zebrafish that had a defect in checkpoint activation carries an insertion in a previously uncharacterized gene that we have named ticrr. The ticrr zebrafish gene encodes a 220 kDa protein with no identifiable domains that could suggest its biochemical function. We have found orthologs of the Ticrr protein in other species, from primitive metazoans to humans. Characterization of Ticrr will further our understanding of the process of cell cycle regulation and conservation of genome integrity in response to DNA damage. Introduction Upon sensing DNA damage, eukaryotic cells activate a number of signal transduction pathways collectively known as the DNA damage response. Activation of these pathways leads to cell cycle arrest, DNA repair and, when the damage is irreparable, initiation of apoptosis. Three molecularly distinct DNA damage checkpoints induce cell cycle arrest at the G1/S and G2/M boundaries, or a delay in S phase progression, in the presence of DNA damage (Lukas et al., 2004). Many DNA damage sensors and signal transducers are shared by all three checkpoints. ATR and ATM are two protein kinases of the phosphatidylinositol-3 (PI-3) kinase-like family that mediate the initiation of checkpoints in response to DNA damage. ATM responds to double strand breaks (Lee and Paull, 2004), whereas ATR responds more broadly to DNA damage and replication interference (Shiotani and Zou, 2009). ATR and ATM kinases phosphorylate a number of targets that propagate and amplify the signal, including the effector kinases Chk1 and Chk2. Checkpoints trigger cell cycle arrest by regulating CDK activity. Specific CDKcyclin combinations regulate cell cycle progression at different stages of the cell cycle, and they are inhibited after DNA damage by various mechanisms. A key mechanism is the inhibition of the Cdc25 phophatases (CDKs activators) (Donzelli and Draetta, 2003). Additionally, checkpoints activate DNA repair proteins and cause stabilization of the transcription factor p53, which upregulates the transcription of pro-apoptotic and cell cycle inhibiting genes such as BAX and the CDK inhibitor p21 (Giono and Manfredi, 2006). The importance of the DNA damage response in human pathology is well established. Defects in the DNA damage checkpoints lead to accumulation of mutations and chromosomal aberrations, which in turn increase the probability of developing genetic diseases. In fact, mutations in various genes encoding DNA damage checkpoint proteins are associated with a variety of human disorders and these are often characterized by an increased predisposition to cancer (Jackson and Bartek, 2009). Thus, considerable attention has been given to the dissection of components of the DNA damage response and their function. Despite great advances that have been made in recent years, it has become clear that the DNA damage response is a complex network of interacting signaling pathways with many key players, some of which have not been identified (Harper and Elledge, 2007). The zebrafish is as an excellent model in which to conduct genetic screens for vertebrate cell cycle and checkpoint regulators. This is primarily due to its small size and fecundity but also because maternally contributed mRNAs allow embryos to survive to developmental stages at which defects in cellessential genes can be assayed. Through a pilot genetic screen, our lab has validated our ability to identify novel cell cycle regulators using zebrafish (Sansam et al., 2006). In this prior study, mitotic index was assayed through whole mount staining of zebrafish embryos for phosphorylated (Ser 10) histone H3 (pH3). We established that the number of pH3positive cells decreases rapidly when zebrafish embryos are exposed to 15 Gy of ionizing radiation, showing that the G2/M checkpoint is intact in these embryos. We used this assay to perform a large-scale screen of the Hopkins' collection for genes required for the IR-induced G2/M checkpoint. In addition, the pH3 assay facilitated the detection of mutants that have a defect in mitotic progression. Here, we summarize the screen results and highlight candidates of interest for both phenotypes. Of particular interest are the hi1573 and hi3202A mutants, which had robust accumulation of pH3 positive cells after IR treatment. Each of these lines carries a mutation in an uncharacterized gene that we have named ticrr (the origin of this name will be discussed in Chapter 3). We show that the insertions are linked to the observed phenotype and the accumulation of 54 pH3 positive cells is caused by a checkpoint defect and not a mitotic defect. This study further confirms the potential of zebrafish for identification of novel checkpoints and cell cycle regulators. Results A genetic screen for zebrafish mutants with a defective IR response We have designed and validated a screen for the discovery of novel DNA damage response genes in zebrafish (Sansam et al., 2006). Using an antibody against serine-10 of phosphorylated Histone H3, a marker of cells in mitosis (Crosio et al., 2002), we established that zebrafish embyos activate a G2 checkpoint in response to ionizing radiation (IR). Whole mount staining of wild-type zebrafish embryos at 32 hours post-fertilization (hpf) with anti-pH3 revealed that approximately 3% of the cells are in mitosis at this developmental stage (Sansam et al., 2006). Within one hour of exposure to ionizing radiation (IR), the anti-pH3 positive cells are largely absent in wild-type embryos, indicating that there is a robust DNA damage-induced cell cycle arrest in zebrafish embryonic cells (Figure 1A). Based on cell cycle kinetics experiments, we established that G2 lasts more than one hour at this developmental stage (Sansam et al., 2006). To asses the transit of cells from S phase into G2/M, embryos were pulsed with BrdU and chased for varying times after the BrdU pulse, then fixed and stained for both BrdU and pH3. The rate at which BrdU-positive cells entered mitosis reflects the duration of S and G2. Using this assay, it was determined that the average duration of G2 in wild-type embryos is between 1 and 2 h (Sansam et al., 2006). Therefore, the decrease in mitotic index one hour after IR exposure represents a G2 checkpoint and not a G1 or S phase checkpoint. In human cells, a rapidly activated checkpoint requires ATM kinase. To determine if the zebrafish G2/M checkpoint is also dependent on ATMIATR, embryos were treated with caffeine, an ATM/ATR inhibitor, before irradiating and staining them with anti-pH3 antibody. The caffeine-treated embryos maintained a similar level of pH3positive cells to the unirradiated controls, indicating that caffeine prevented activation of the checkpoint (Figure 1A) (Sansam et al., 2006). This indicates that ATM/ATR kinases are required for activating this checkpoint, and more importantly, we can identify embryos with an abrogated checkpoint by examining pH3 staining after exposure to IR. b Figure 1. Zebrafish can be used to screen for IR-induced G2 checkpoint response genes. (A) Mitotic cells were labeled in 32hpf embryos using an anti-pH3 antibody in untreated (-IR), IR-treated (+IR) and caffeine pretreated/IR-treated (+IR; +Caffeine) wild-type zebrafish embryos. (B) Analysis of pH3 showed a low mitotic index in wild-type embryos but not dtl mutant clutchmates I h after IR exposure, which is indicative of a G2 checkpoint defect. Adapted from Sansam, C.L, 2006. The Hopkins laboratory generated a zebrafish collection of approximately 500 heterozygote lines carrying insertional mutations in over 300 different genes required for embryonic development (Amsterdam et al., 2004).The sensitivity of the pH3 assay was verified by staining known checkpoint and cell cycle mutants in the Hopkins collection, and a pilot screen was performed on several mutants, including some with insertions in novel genes. We were able to identify mutants that have an increase number of mitotic cells after irradiation, compared to the non-irradiated embryos. We observed the mitosis after irradiation (MAI) phenotype in fish carrying mutations in SMC1 and MCM7, proteins known to be required in DNA damage checkpoints in mammalian cells, demonstrating that the screen can be used to find mutants that fail to arrest after DNA damage. In addition, zebrafish carrying an insertional mutation in the gene denticleless (dtl) showed a defect in the DNA damage checkpoint (Figure 1B). In a prior study, our lab established both the biological properties and mechanism of action of the human ortholog of the Dtl protein. This analysis showed that Dtl is an essential component of the early G2/M checkpoint and is required for normal cell cycle control, primarily to prevent re-replication (Sansam et al., 2006). We have now applied this screen to a large collection of zebrafish mutants that carry stable viral insertions within 336 different genes. These lines are fully viable as heterozygotes but the homozygous mutants display developmental defects 24-96 hours post-fertilization (hpf) that are typically lethal (Amsterdam et al., 2004). For our cell cycle screen, we intercrossed the heterozygous mutants, treated 50 or more of the resulting embryos at 32 hpf with ionizing radiation (IR), and assayed pH3 staining 1 hour later (Figure 2A). This timepoint in development was chosen for the screen for two reasons. First, the number of pH3 positive cells in wild-type embryos is optimal during this time, allowing easy detection of an increase or decrease in staining. Secondly, maternal transcripts should be largely absent by this time, allowing the emergence of phenotypes that otherwise could have been masked. Lines were considered to have altered mitotic index if approximately one quarter of the embryos showed altered pH3 staining relative to the rest of the clutch. For those lines that displayed an aberrant number of pH3 positive cells, the staining was repeated at least two more independent times in order to confirm the phenotype. PCR genotyping of the embryos was used to confirm that the phenotype was linked to the mutant insert. A Normal Response Mitosis Phenglyoe MAI Phenotyoe ±18 -IR j32 hpf 15 Gy IR -IR -IR 15 hour anti-pH3 stain Figure 2. A zebrafish screen for novel DNA damage response and cell cycle regulators. (A) Scheme of checkpoint screen. Heterozygous carriers were intercrossed to yield clutches of embryos that were expected to consist of 25% homozygous mutants. The clutches were irradiated or left untreated and fixed one hour later, followed by staining with a pH3 antibody. (B) Possible outcomes of the checkpoint assay. The pH3 staining of mutant zebrafish embryos can uncover both a mitosis phenotype and a mitosis after irradiation (MAI) phenotype. Using this approach, we identified 12 mutant lines that showed a high mitotic index compared to their wild-type and heterozygous clutchmates after IR exposure. These mutants can be classified into two classes (Figure 2B). The first class of mutants also displayed a relatively high mitotic index without irradiation, which could be indicative of a delay or arrest in mitosis and suggests that the affected gene is involved in mitotic progression. Alternatively, it could indicate a higher rate of proliferation. In the second . ... .... . ... class of mutants, the non-irradiated embryos did not display a difference in their mitotic index. These are considered to be checkpoint activation failure mutants. The screen results are summarized in Table 1. Table 1. Insertional mutants from the Hopkins zebrafish collection that showed an increase in pH3 staining after IR treatment. Zebrafih Gene line Gene mutated function Casein kinase pH3 staining pH3 staining Increase Increase factor Increase Increase untreated IR-treated hi1002 1, alpha 1 Signal transduction hi1487 Site-1 protease Transcription Regulator hi2578 nopl 0 Ribosome biogenesis Increase Increase hi2820 clasp1 Regulator of microtubule Increase dynamics Increase hi3512B bc2 Unknown Increase Increase hi923 v-ATPase SDF/54 kD Vacuolar proton pump No difference Increase hi933 pbx4 Transcription factor No difference Increase hi1573 hi3202A 5730590G19like Unknown No difference Increase hi1858A CWC25-like Unknown No difference Increase hi2688 paics de novo purine synthesis No difference Increase hi3526B gart de novo purine synthesis No difference Increase hi3685 hi3685 pG 1 uRNA subunit G Transcription No difference Increase Insertionalmutations in zebrafish that affect normal mitotic progression Many mutants in the collection have non-specific developmental phenotypes, such as extensive cell death, small eyes and head, and deformed bodies (Amsterdam, There are two classes of genes that could cause those phenotpyes when 2003). mutated: housekeeping genes and genes involved in cell proliferation. Given that at the time of the screen approximately 20% of the mutated genes were novel or poorly described, we reasoned that novel cell cycle genes could also be identified in our screen, specifically genes that are required for normal mitotic progression. Indeed, in our pilot screen we observed that we could identify mutants for known mitotic proteins, such as polo-like kinase and the kinesin-related motor protein Eg5, because they not only had a high mitotic index after IR treatment, but also in the absence of exogenous DNA damage. By also scoring the mitotic index in unirradiated embryos, we identified several zebrafish lines in which the homozygous mutants had higher pH3 staining compared to wild-type and heterozygous clutchmates (Figure 3, data not shown). hi2820 mutants, which carry an insertion in the gene encoding the evolutionary conserved protein Clasp1, fall into this category. Even though the zebrafish Clasp1 protein has not been characterized, studies with its human (CLASP1), Xenopus (Xorbit/CLASP), Drosophila (MAST/Orbit), and C. elegans (CLS-2/ R106.7) homologs revealed that they all play key roles during mitotic progression (Galjart, 2005). Thus, it is not surprising that disruption of the zebrafish Clasp1 protein causes a defect in mitotic progression. The human Clasp1 protein has a stabilizing function at the kinetochore, which is essential for the bipolar alignment of chromosomes on the mitotic spindle (Maffini et al., 2009; Maiato et al., 2003; Pereira et al., 2006). hi1002 homozygous mutants also had a aberrantly higher number of pH3 positive cells (data not shown). These zebrafish are mutant for Casein kinase 1 isoform alpha (CKla), a multifunctional Ser/Thr kinase. Depletion of the yeast homolog, HRR25, causes defects in a number of processes including chromosomal segregation (DeMaggio et al., 1992). Mammalian CKla localizes to mitotic spindles, suggesting that its role in chromosomal segregation may be conserved in higher eukaryotes (Brockman et al., 1992; Nousiainen et al., 2006). Moreover, one of the mammalian CKIa interacting proteins is the regulator of chromosome condensation 1 (RCC1), which plays a role in nuclear transport and mitotic spindle formation (Dubois et al., 2002). CKla has also been found to act as a negative regulator of the Wnt and Hedgehog signaling pathways (Price, 2006). Thus, the accumulation of pH3 positive cells in the ckla zebrafish mutants could be caused by a block in mitosis as a result of a defect in chromosome segregation, or by aberrant proliferation due to misregulation of the Wnt and Hedgehog pathways. Additional experiments are necessary to distinguish between these two possibilities. Lastly, hi3512B (mutant for Bc2), hi1487 (mutant for site-1 protease, Figure 3) and hi2578 (mutant for Nop1O) also displayed a mitotic phenotype. A role in mitosis has not been described for these proteins to this day. Interestingly, two independent studies found that human BC2 localizes to the midbody of dividing cells (Morita et al., 2007) and to the mitotic spindle (Nousiainen et al., 2006), which is highly suggestive of a role for BC2 during mitosis. (1)1 CL >t +IR Figure 3. hi1487 homozygous mutants show an aberrant accumulation of pH3 positive cells in the absence of exogenous DNA damage. Mitotic cells were labeled in 32hpf embryos using an anti-pH3 antibody in untreated (-IR), IRtreated (+IR) hil487 wild-type and mutant clutchmates. Untreated mutant embryos show an increase in pH3 positive cells, indicative of a defect in mitosis prgression. Disruption of de novo purine nucleotide synthesis in zebrafish abrogates IR-induced cell cycle arrest Several mutants had a higher pH3 staining only after IR treatment, suggesting these mutants fail to undergo an IR-induced G2 arrest (Table 1). A striking observation is that two of these mutants, hi3526B and hi2688, carry insertions in genes that encode for enzymes involved in de novo purine synthesis. Specifically, hi3526B carries a mutation in the gart gene, while hi2688 carries a mutation in the paics gene. These genes encode two multifunctional enzymes that catalyze several steps of inosine monophosphate (IMP) synthesis. The synthesis of purine nucleotides is important for many processes: nucleotides are constituents of the genetic material, cofactors in enzymatic reactions, and the major energy source that drives most cellular reactions. Ribonucleotide depletion and/ or slowing replication forks activates a p53 mediated checkpoint response independently of direct DNA damage, which leads to G1 and G2 arrest (Linke et al., 1996). Interestingly, this response seems to be abrogated by inhibition of de novo purine synthesis pathway. Bronder and Moran showed that inhibition of GART activity in human cell lines leads to nuclear accumulation of p53; however, the transcription of the downstream targets p21, mdm2, and bax, was impaired (Bronder and Moran, 2003). Consequently, cells progress through G1 and G2, and accumulate in S phase due to decreased ribonucleotide pools that impair DNA synthesis. This effect is specific for inhibition of de novo purine synthesis, since inhibition of de novo pyrimidine synthesis does not impair the G1 and G2 arrest (Bronder and Moran, 2003). Our checkpoint assay specifically surveys the early G2 checkpoint, which is thought to be p53 independent (Taylor and Stark, 2001). It is unknown if inhibition of de novo purine synthesis will also have an effect on p53-independent checkpoint activation in response to exogenous DNA damage. However, it is intriguing that two lines in our collection carrying mutations in genes required for pyrimidine nucleotide synthesis, hi3510 and hi2694, were not scored as having a checkpoint defect in our screen (data not shown). hi3510 is mutated for thymidylate synthase, whereas hi2694 carries a mutation in the trifunctional protein CAD, which contains the three first enzyme activities of de novo pyrimidine synthesis. This suggests that the mitosis after irradiation phenotype observed in hi3526B and hi2688 is specifically caused by disruption of the de novo purine synthesis and not by decreasing nucleotide pools in general. An insertional mutation in a novel gene that abrogates IR-induced cell cycle arrest in the zebrafish It is important to note that most of the DNA damage response candidates identified in this screen had a subtle phenotype; the mutants showed a slight, albeit consistent, increase in the pH3 staining after IRtreatment (Figure 4). The reason for the modest phenotype may be the existence of compensatory mechanisms (see discussion). One of the mutants that scored as having a modest defect was hi3685, mutated for RNA polymerase 11, subunit G. The role of RNA polymerase 11 in transcription has been widely described. Recently, a similar screen for G2-M checkpoint genes in Drosophila also identified several subunits of RNA polymerase 11as being required for proper checkpoint response (Kondo and Perrimon, 2011). The role of this protein in checkpoints could be indirect; perhaps it is required for the transcription of other checkpoint proteins. The mutants that showed only a slight increase in pH3 staining were not further pursued in this study. Figure 4. Mitosis after irradiation phenotype in hi923 mutants. 0. Analysis of pH3 staining showed a slight increase in the mitotic index in IR-treated +IR hi923 homozygous mutant embryos compared to wild-type clutchmates. Notably, hi1573 homozygotes showed a robust increase in mitotic index, compared to their wild-type and heterozygous clutchmates, after IR exposure (Figure 5). In contrast, the mitotic index without radiation in the hi1573 mutants was indistinguishable from that of the wild-type embryos (data not shown). This mutant was not only identified as a potential candidate in the larger-scale screen of the mutant collection, but was also scored as having a mitosis after irradiation phenotype in the initial pilot screen. Because its phenotype was robust, we decided to focus our attention on the further characterization of hi1573 mutant embryos. wild-type clutchmate mutant clutchmates Figure 5. hi1573 and hi3202A zebrafish embryos show a Mitosis After Irradiation (MAI) phenotype. Whole mount immunostaining of pH3 showed that IR treatment induces the appropriate block to mitotic entry in 36 hpf wild-type embryos, while hil573 and hi3202A homozygous mutant clutchmates retained a high mitotic index. We mapped the hi1573 viral insertion just upstream of an uncharacterized open reading frame (5730590G19-like) on chromosome 25 (Figure 6A). A second line in the collection, hi302OA, contains a distinct mutant allele of 5730590G19-like with a viral insertion in the first predicted coding exon (Figure 6A). Notably, hi3202A homozygotes have a normal mitotic index in the absence of irradiation (data not shown) and display the MAI phenotype (Figure 5B) just like the hi1573 mutants. The hi1573 and hi3202A homozygotes also have identical developmental phenotypes: at 36 hpf these lines develop a dark head that is characteristic of widespread apoptosis in the central nervous system (Amsterdam et al., 2004). We confirmed that cells in these mutants are apoptotic, with increased acridine orange and anti-cleaved caspase-3 staining appearing at 26 hpf (data not shown). Typically, the retroviral insertions in the Hopkins mutant collection cause a decrease in mRNA expression that results in recessive, loss-offunction phenotypes. We performed quantitative RT-PCR to measure the expression of 5730590G19-like at 36 hpf in wild-type and mutant embryos and found that both the hi1573 and hi3202A insertions cause a strong reduction in mRNA levels of this gene (Figures 6B-C). Taken together, these data indicate that disruption of the novel gene 5730590G19-like causes embryonic lethality and the mitosis after irradiation phenotype. We have named this gene ticrr for reasons that will be described in the following chapter. Hereafter, I will refer to this gene as ticrr. 66 m m I UUIU Y~Tuuyiyi ,uu~ hi1573 hi202A Exon 1 Exon 2 Wild-type Littermates Mutants ~C 08 -t ta 0.6 .2 0.4 Z0.2 X0 wt mut Figure 6. The checkpoint defect results from disruption of a novel gene, ticrr. (A) Schematic of the zebrafish ticrr gene denoting the position of viral insertions in the hil573 and hi3202A mutant lines. (B-C) Quantification of total mRNA by RT-PCR showed that the levels of ticrr mRNA were greatly reduced in both the (B) hil573 and (C)hi3202A homozygous mutants at 36 hpf. A critical step in regulating entry into mitosis is the activation of Cdc2 kinase by Cdc25 phosphatases. In the presence of DNA damage, Cdc25 phosphatases are inhibited, thereby preventing Cdc2 kinase activation. We sought to determine whether the increased mitotic index in the ticrr mutants after DNA damage was associated with high Cdc2 kinase activity. Thus, we performed an in vitro assay using Cdc2-cyclin B1 complexes immunoprecipitated from lysates of 40 hpf embryos that had been irradiated or left untreated. Embryos were identified as either mutant or wild-type based on the presence or absence of the characteristic ticrr developmental phenotype. IRexposure of wild-type embryos resulted in a 14-fold decrease in the phosphorylation of a synthetic peptide substrate for Cdc2 (Figure 7). This is consistent with the decrease in mitotic index and the activation of the G2/M checkpoint in response to IR. In contrast, the activity of Cyclin B1-Cdc2 remained high after DNA damage in the ticrr mutants (Figure 7), consistent with the persistence of mitotic cells. cu 6c 0.-6 -IR CD CN 1 M*+IR 0.4 - S0.2 CU Wt CdC2 0 mut &Q4 Figure 7. Cdc2 kinase activity remains high in ticrr mutants after IR treatment. Cyclin B1-Cdc2 was immunoprecipitated from 40 hpf unirradiated (-IR) and irradiated (+IR) pools of wild-type (+/+ and +/-) and mutant (-/-) hil573 embryos. The total levels of Cdc2 were determined by western blotting (representative experiment shown). Assessment of cyclin B1-Cdc2 kinase activity (mean ± SD; n=3 biological replicates) showed that the hil573 mutants retain high activity after IR treatment. We reasoned that the mitosis after irradiation phenotype and the high Cdc2 kinase activity could arise in two possible ways: the mutation could abrogate the checkpoint that prevents mitotic entry after DNA damage or it could reflect a failure to efficiently exit mitosis. To distinguish between these two possibilities, we performed a nocodazole trapping experiment using 36 hpf embryos. For this assay, nocodazole was added immediately after exposure to IR and then the embryos were incubated for 2 hours before disaggregating and quantifying the percentage of pH3-positive cells with 4N DNA content by FACS. Since nocodazole causes early to mid-mitotic arrest, an accumulation of mitotic cells following IR and nocodazole treatment would indicate that cells continue to enter mitosis and thus a defect in the G2/M checkpoint. In the absence of irradiation, the level of 4N, pH3-positive cells was increased in the nocodazoletreated, versus the untreated, embryos for both the wild-type (3.4-fold) and ticrr mutants (2-fold; Figure 8). IR treatment greatly reduced the level of mitotic cells in the wild-type embryos in both the absence and presence of nocodazole (Figure 8). This is consistent with the existence of a robust G2/M checkpoint. In contrast, the level of 4N, pH3-positive cells in the ticrr mutants was completely unaffected by IR treatment. Indeed, we still observed the 2-fold increase in mitotic cells that results from nocodazole treatment. Taken together, our data show that the G2/M checkpoint is abrogated in the ticrr mutants and these cells continue to enter mitosis following DNA damage. 4- -IR 3 - +IR Co I1 noc. + - wt - + mut Figure 8. Cells in ticrr homozygous mutants continue to enter mitosis after IR treatment. Unirradiated and irradiated 36 hpf wild-type (+/+ and +/-) and hil573 mutant (-/-) clutchmates were maintained in the absence or presence of nocodazole for 2 hrs. The percentage of pH3positive cells was quantified by FACS (mean ± SD; n = 20,000 cells counted for each of three biological replicates). The zebrafish ticrr gene is predicted to encode a 1,824 residues protein (NM_001003887.1) with an anticipated molecular mass of 202 kDa. Genes encoding proteins with significant homology exist in other vertebrates, including humans, mouse and Xenopus (Table 2). We also found putative Ticrr orthologs in other metazoans including the primitive chordates Branchiostoma floridae and Ciona intestinalis, the echinoderm Strongylocentrotus purpuratus, the arthropod Ixodes scapularis, the nematode Caenorhabditis elegans, the cnidarian Nematostella vectensis, and the placozoan Trichoplax adhaerens. The conservation across these proteins maps to specific stretches, yielding high confidence in its significance, thoug the overall conservation is poor (Table 2). We were unable to detect putative Ticrr orthologs in Drosophila and there was no significant homology to any yeast protein. We have uncovered additional roles for this protein, which will be described in the following chapter. Table 2. Conservation between D. rerio Ticrr and orthologs identified by psiBLAST ClustalW siBLAST Species 0. rerio .sapiens M.musculus G.gallus .laevis .floridae C.intestinalis S. purpuratus .scapularis C.elegans N. vectensis T. adhaerens Accession # Length (amino acids) NP_001003887.1* 1824 NP689472.3 NP 084111.1 XP413862.2 AAH73061.1 XP002215127.1 XP002120860.1 XP792617.1 XP002416131.1 NP491752.1 XP001628252.1 XP_002111061.1 1910 1889 1679 (1937**) 1394 1911 1441 2305 (1768*) 1495 919 1845 1069 HSP Length 1126 1114 1113 1098 1147 1172 654 1111 472 1125 515 Results % % Identity Positives 35 35 34 36 22 19 22 20 21 22 18 52 52 50 54 38 34 37 35 37 37 37 % Gaps Alignment % Identity 8 7 13 6 12 16 16 19 15 14 17 30 28 29 30 16 14 11 16 11 14 14 *Sequence used in Psi-BLAST was truncated at amino acid 1181 before a long stretch of low complexity proline-rich sequence. ** Length of predicted XI and Sp proteins from manually refined gene models. HSP=high scoring segment pairs. Discussion To maintain their genome integrity, cells have a complex network of pathways that together compose the DNA damage response. Loss of components of the DNA damage checkpoints often leads to genetic disorders and cancer in humans. We have used the zebrafish to identify new components of these checkpoints. Along the way, we have also identified genes that are potentially required for mitotic progression. The uncovering of these genes raises two important questions: first, what are the mechanisms by which these mutations lead to higher mitotic index in the zebrafish, and second, are these genes involved in similar processes in other vertebrates? What role do the identifiedgenes play in normal cell cycle progression? We observed that a group of the mutants identified in the screen have an aberrant number of pH3 positive cells in the absence of exogenous DNA damage. Histone H3 is phosphorylated on serine 10 during late G2 and becomes dephosphorylated during anaphase, in mitosis (Crosio et al., 2002). We speculate that the accumulation of pH3 positive cells in these mutants could be a result of the inability of those cells to progress through G2/M and exit mitosis. Entry into and progression through mitosis requires precise coordination and many proteins are involved in the process. Thus, this phenotype could be caused by disruption of many pathways that regulate G2/M transition, chromosome segregation and mitotic exit. The other class of mutants described in this study accumulates pH3 positive cells only after IRtreatment. This phenotype has been shown to be characteristic of disruption of the G2/M checkpoint (Sansam et al., 2006; this study). Many checkpoint genes are also important for normal cell cycle progression. The mutants in the Hopkins collection are embryonic lethal, so it is highly probable that the candidates we have identified also have a role in normal cell cycle progression. Defects in normal cell cycle progression could be uncovered by analyzing cell cycle kinetics in the mutant embryos. Further characterization of the cell cycle phenotypes of the mutants identified will help uncover the specific role of the mutated proteins in mitotic progression and/or cell cycle regulation. What role do the identifiedgenes play in the DNA damage response? Two mutants in the collection with disrupted de novo purine synthesis had a slight increase in the number of pH3 positive cells after IR treatment. It is plausible that the impaired IR-induced checkpoint in these zebrafish mutants is caused solely due to the reduction of dNTP pools and the mutated proteins do not have a bona fide checkpoint role. It has been shown that inhibition of de novo purine synthesis causes rapid depletion of GTP and ATP pools. The fact that disruption of the pyrimidine synthesis pathway does not impair the checkpoint response suggests that this effect may be a consequence of depletion of the ATP pools specifically. ATP is used as a substrate by kinases to phosphorylate proteins, a modification that checkpoints highly rely on for signal transmission (Huen and Chen, 2008). In accordance with this hypothesis, p53 is stabilized in response to de novo purine inhibition but is not phosphorylated or acetylated in a number of residues that are important for its function as a transcriptional activator (Bronder and Moran, 2003). Another process important for proper checkpoint function is the ubiquitylation/proteosomal degradation of certain key players (Bassermann and Pagano; Huen and Chen, 2008), a process dependent on ATP. It remains an open question if cells impaired for purine synthesis are unable to arrest due to an indirect effect of the limited nucleotide pools, or whether these enzymes play a direct role in checkpoint activation that has not been described before. We have uncovered a number of candidates that have an IR-induced G2/M checkpoint defect in zebrafish. The difference in pH3 positive cells for most of the mutants identified was small, but highly reproducible. It is possible that the mutated genes have a minor role or are indirectly involved in the checkpoint. In addition, they could be important players of the G2/M checkpoint but other pathways compensate for their depletion. Indeed, activation and maintenance of the G2 arrest requires targeting of multiple regulatory processes involved in the normal progression of the cell from G2 into M phase. Several checkpoint pathways act in parallel and converge at specific nodes. An example of this is the regulation of the mitotic kinase Cdc2-CyclinB, which is inhibited by several mechanisms in response to DNA damage (Stark and Taylor, 2006). Recently, a similar screen for finding novel G2/M checkpoint components was performed in Drosophila (Kondo and Perrimon). For this genome-wide screen, knockdown of individual genes was achieved using RNA interference technology and phosphohistone H3 staining was used to assay mitotic index in cells treated with the anticancer drug doxorubicin, which induces double-stranded breaks. This study found that knockdown of known checkpoint proteins, like ATR, only caused a modest checkpoint defect. Furthermore, the authors were able to show that when ATR knockdown is combined with knockdown of Nbs1, another checkpoint protein, the checkpoint defect is stronger. This is an example of the synergy that exists for checkpoint activation. Thus, the defects in checkpoint activation observed in some zebrafish mutants in my screen could be weak due to compensation. Nevertheless, our screen has been successful in identifying two mutants in the same gene with a robust increase in mitotic index after IRtreatment, suggesting that this protein is a major player in checkpoint activation. These mutations disrupt a novel gene that we have named ticrr. We have confirmed that the aberrant accumulation of pH3 cells in the ticrr mutants is caused by a failure to activate the G2/M checkpoint in response to IRtreatment. In addition to the G2/M checkpoint, DNA damage response checkpoints have been identified at the G1/S transition and during S phase of the cell cycle. After DNA damage, these checkpoints block progression through the cell cycle until the damage is repaired. However, they are mechanistically different and, while some components are shared amongst the three checkpoints, others are checkpoint-specific. Thus, the identified genes could be specifically required for the early G2/M checkpoint or they could have a more general role. In addition, specific types of damage can induce certain pathways of the DNA damage response. It may be informative to examine how these mutant embryos respond to other types of damage, including damage caused by UV radiation and chemotherapeutic agents like camptothecin and doxorubicin, which target DNA topoisomerase I and II, respectively. A more extensive analysis of the mutant zebrafish will be crucial for understanding the role of these proteins in DNA damage checkpoints and cell cycle regulation. Are the identified genes required for normal cell cycle progression and checkpointsin other vertebrates? An advantage of performing screens for DNA damage response genes in zebrafish is that it is relatively straightforward to identify candidate vertebrate orthologs based on protein sequence comparison. This screen has identified a novel gene, ticrr, as a checkpoint regulator. We have identified orthologs of this gene in a number of metazoans, including humans. A particularly useful system for studying cell cycle and DNA damage regulators is human cells, where a wide array of tools is available. The following chapter will describe how we have taken advantage of these tools to gain insight into the molecular basis for Ticrr's role in cell cycle control. Further studies with the orthologs on Ticrr will establish the conservation, and therefore importance, of Ticrr's biological functions. 74 In summary, we have shown the power of the zebrafish to uncover genes required for cell cycle progression and checkpoints. Future characterization of the proteins identified in this screen will lead to a better understanding of the pathways that underlie the DNA damage response and will significantly advance our ability to unravel the complex processes maintaining the integrity of the genome. Materials and methods Zebrafish maintenance Zebrafish were maintained as previously described (Amsterdam et al., 2004). For the screen, heterozygous insertion carriers were intercrossed and at 24 hpf the embryos were manually dechorionated and 1-phenyl-2-thiourea (PTU, 0.003%) added to suppress pigmentation. At 32 hpf, 60 embryos from each clutch were subjected to G2/M checkpoint analysis. G2/M checkpointassays To initiate a DNA damage response, zebrafish embryos were exposed to 15 Gy IR from a 6Co source and analyzed for pH3 staining one hour later as previously described (Sansam et al., 2006). To test whether caffeine could inhibit the zebrafish G2 checkpoint, 32-hpf embryos were placed in 2 mM caffeine and incubated for 30 min at 28.50C before the IR/pH3 assay. For the nocodazole trapping experiment, embryos were exposed to IR, immediately placed in nocodazole (150 ng/mI + 1% DMSO), and incubated at 28.5C for 2 hours before analysis of pH3/DNA content by FACS. Genotyping and expression analysis of zebrafish lines Primers for genotyping were as follows: hil 573 - forward primer (F): AAGCAAGCTACATCTCAAAGCA; hil 573 -reverse primer (R): CGGAAAACCCTGAAGTGTGAT; hi3202A - (F) CAGATCCCCTGGTTATAAGTGTTGC; hi3202A - (R) CAGTTATGGCCTCTCAGA ATGTCGT; reverse primer for provirus: GCTAGCTTGCCAAACCTACAGGT. Ticrr mRNA levels in hil 573 were measured by two-step real time RT-PCR with SYBR Green I Dye (Applied Biosystems) using the primers F: CTGAACAGTTTGCAT GGATGG and R: CTTTCGGCTGTATGTCCTGCT and normalized against zebrafish bactin (F: CATCAGCATGGCTTCTGCTCTGTATGG and R: GACTTGTCAGTGTACA GAGACACCCTG). Ticrr mRNA levels in hi3202A were analyzed by limiting dilution RTPCR using the Ticrr primers above and RPL35 as the reference mRNA (F: GCTGCTTCCAAGCTC TCAAAAATCC and R: TGCCTTGACGGCGAACTTGCGAATG). In Vitro Kinase Assay 50 embryos for each data point were treated with 15 Gy IR, and incubated for 30 minutes at 28.5*C. Embryos were then dechorionated, deyolked and homogenized in 400pl KLB (50mM Tris pH 7.4, 150mM NaCl, 0.1% Triton X-100, 4mM EDTA, 0.1% NP40, 50mM NaF, 0.2mM Na3VO 4, 1OuM Leupeptin, 5ug/ml Aprotonin, 1mM PMSF). The homogenate was adjusted to 1 mg/ml prior to precipitation with anti-Cyclin B1. The beads were washed twice with KLB, and KAB (50mM Tris pH 8.0, 10mM MgC 2 , 1mM EGTA) and incubated for 15 minutes at 300C in 20pl of kinase assay mix [KAB with 40pM cold ATP; 100pM peptide (HATPPKKKRK); (6000Ci/mmol)]. 1mM DTT; 0.5pCi/ul y-32P-ATP Substrate phosphorylation was quantified by filter binding and scintillation counting. FACS Analysis Mutant embryos were identified by either developmental phenotype or PCR genotyping of a fraction of individual, fixed embryos. Cells were disaggregated by triturating embryos in 0.25% Trypsin/1 mM EDTA using a p200 and fixed in 70% ethanol at -200C overnight. Suspensions of cells from 20 wild-type or mutant embryos were pooled and prepared for pH3/propidium iodide FACS analysis as described (Pozarowski and Darzynkiewicz, 2004). FACS analysis was conducted by FACScan (Becton-Dickinson). DNA content was quantified by ModFit LT (Verity Software), and pH3 and BrdU was quantified by FlowJo (Tree Star, Inc.). Acknowledgements I would like to thank Chris Sansam for use of his previously published data, included in Figure 1. I would also like to thank Kate Anderson for maintenance of the mutant lines of fish, and Tim Angelini and Sam Farrington for maintenance of the zebrafish colony. References Amsterdam, A. (2003). Insertional mutagenesis in zebrafish. Dev Dyn 228, 523-534. Amsterdam, A., Nissen, R.M., Sun, Z., Swindell, E.C., Farrington, S., and Hopkins, N. (2004). Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A 101, 12792-12797. Bassermann, F., and Pagano, M. Dissecting the role of ubiquitylation in the DNA damage response checkpoint in G2. Cell Death Differ 17, 78-85. Brockman, J.L., Gross, S.D., Sussman, M.R., and Anderson, R.A. (1992). Cell cycle-dependent localization of casein kinase I to mitotic spindles. Proc Natl Acad Sci U S A 89, 9454-9458. Bronder, J.L., and Moran, R.G. (2003). A defect in the p53 response pathway induced by de novo purine synthesis inhibition. J Biol Chem 278, 48861-48871. Crosio, C., Fimia, G.M., Loury, R., Kimura, M., Okano, Y., Zhou, H., Sen, S., Allis, C.D., and Sassone-Corsi, P. (2002). Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol 22, 874-885. DeMaggio, A.J., Lindberg, R.A., Hunter, T., and Hoekstra, M.F. (1992). The budding yeast HRR25 gene product is a casein kinase I isoform. Proc Natl Acad Sci U S A 89, 7008-7012. Donzelli, M., and Draetta, G.F. (2003). Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep 4, 671-677. Dubois, T., Howell, S., Zemlickova, E., and Aitken, A. (2002). Identification of casein kinase lalpha interacting protein partners. FEBS Lett 517, 167-171. Galjart, N. (2005). CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol 6, 487-498. Giono, L.E., and Manfredi, J.J. (2006). The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol 209, 13-20. Harper, J.W., and Elledge, S.J. (2007). The DNA damage response: ten years after. Mol Cell 28, 739-745. Huen, M.S., and Chen, J. (2008). The DNA damage response pathways: at the crossroad of protein modifications. Cell Res 18, 8-16. Jackson, S.P., and Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature 461, 1071-1078. Kondo, S., and Perrimon, N. (2011). A genome-wide RNAi screen identifies core components of the G-M DNA damage checkpoint. Sci Signal 4, rsl. Lee, J.H., and Paull, T.T. (2004). Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbsl complex. Science 304, 93-96. Linke, S.P., Clarkin, K.C., Di Leonardo, A., Tsou, A., and Wahl, G.M. (1996). A reversible, p53dependent GO/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev 10, 934-947. Lukas, J., Lukas, C., and Bartek, J. (2004). Mammalian cell cycle checkpoints: signalling pathways and their organization in space and time. DNA Repair (Amst) 3, 997-1007. Maffini, S., Maia, A.R., Manning, A.L., Maliga, Z., Pereira, A.L., Junqueira, M., Shevchenko, A., Hyman, A., Yates, J.R., 3rd, Galjart, N., et al. (2009). Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr Biol 19, 15661572. Maiato, H., Fairley, E.A., Rieder, C.L., Swedlow, J.R., Sunkel, C.E., and Earnshaw, W.C. (2003). Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113, 891-904. Morita, E., Sandrin, V., Chung, H.Y., Morham, S.G., Gygi, S.P., Rodesch, C.K., and Sundquist, W.I. (2007). Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26, 4215-4227. Nousiainen, M., Sillje, H.H., Sauer, G., Nigg, E.A., and Korner, R. (2006). Phosphoproteome analysis of the human mitotic spindle. Proc Nat[ Acad Sci U S A 103, 5391-5396. Pereira, A.L., Pereira, A.J., Maia, A.R., Drabek, K., Sayas, C.L., Hergert, P.J., Lince-Faria, M., Matos, I., Duque, C., Stepanova, T., et al. (2006). Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol Biol Cell 17, 45264542. Pozarowski, P., and Darzynkiewicz, Z. (2004). Analysis of cell cycle by flow cytometry. Methods Mol Biol 281, 301-311. Price, M.A. (2006). CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20, 399-410. Sansam, C.L., Shepard, J.L., Lai, K., lanari, A., Danielian, P.S., Amsterdam, A., Hopkins, N., and Lees, J.A. (2006). DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev 20, 3117-3129. Shiotani, B., and Zou, L. (2009). ATR signaling at a glance. J Cell Sci 122, 301-304. Stark, G.R., and Taylor, W.R. (2006). Control of the G2/M transition. Mol Biotechnol 32, 227-248. Taylor, W.R., and Stark, G.R. (2001). Regulation of the G2/M transition by p53. Oncogene 20, 1803-1815. CHAPTER III: TICRR associates with TopBP1 and is required for replication initiation Nelly M. Cruz, Cristopher L. Sansam, Paul S. Danielian, Adam Amsterdam, Melissa L. Lau, Nancy Hopkins and Jacqueline A. Lees The material presented in this chapter was adapted, with permission, from the following publication: *Sansam, C.L., *Cruz, N.M., Danielian, P.S., Amsterdam, A., Lau, M.L., Hopkins, N., and Lees, J.A. (2010). A vertebrate gene, ticrr, is an essential checkpoint and replication regulator. Genes Dev 24, 183-194. *These authors contributed equally to this work. Experimental contributions: C.L.S. conducted the experiment showed in Figures 1, 2 and 3. P.D. aided with the cloning of TopBP1 deletion mutants used in Figure 7. N. Cruz performed the experiments for Figures 4, 5, 7, 8 and 9. Summary Eukaryotes have numerous checkpoint pathways to protect genome fidelity during normal cell division and in response to DNA damage. Through a screen for G2/M checkpoint regulators in zebrafish, we identified ticrr, a previously uncharacterized gene that is required to prevent mitotic entry after treatment with ionizing radiation. ticrrdeficiency is embryonic lethal in the absence of exogenous DNA damage because it is essential for normal cell cycle progression. Specifically, the loss of ticrr impairs DNA replication and disrupts the S-M checkpoint, leading to premature mitotic entry of cells with partially replicated genomes and mitotic catastrophe. Here, we identify the human TICRR ortholog and show that it associates with TopBP1, a known checkpoint protein and a core component of the DNA replication pre-initiation complex (pre-IC). The TICRR-TopBP1 association is stable without chromatin and requires BRCT motifs essential for TopBP1's replication and checkpoint functions. We hypothesized that TICRR acts together with TopBP1 in pre-IC formation, in an analogous manner to the yeast protein Sld3. Consistent with this model, we show that ticrr-deficiency disrupts chromatin binding of pre-IC, but not pre- RC, components. Taken together, our data show that TICRR acts in association with TopBP1 and plays a dual role in pre-IC formation and checkpoint response. Introduction Eukaryotic cells possess numerous mechanisms to ensure genome fidelity. In dividing cells, DNA replication is the primary potential source of errors. To ensure that DNA replication occurs at the appropriate cell cycle stage and only one copy of the genome is synthesized, this process is divided into two temporally distinct steps (Bell and Dutta 2002; Sclafani and Holzen 2007). During G1, the replicative helicase complex, Mcm2-7, is loaded onto origin DNA by the ORC, Cdc6 and Cdtl proteins in a process called pre-replication complex (pre-RC) formation. However, the loaded helicase is inactive, and is only activated upon entry into S phase during pre-initiation complex (preIC) formation. This process requires CDK and DDK (Dbf4-dependent kinase) activity and involves recruitment of additional proteins to the Mcm2-7 complex, including TopBP1, Cdc45 and the GINS complex (Hashimoto and Takisawa 2003; Kubota et al. 2003; Aparicio et al. 2009). Once activated, the helicase and its associated proteins recruit the remaining DNA synthesis machinery leading to the formation of a pair of bidirectional replisomes. In addition to the core replication machinery, multiple checkpoint pathways exist to protect cells from DNA damage arising from replication errors and/or genotoxins (Bartek et al. 2004; Harper and Elledge 2007). Two of these pathways function during DNA replication: the intra-S phase checkpoint stabilizes existing replication forks while inhibiting firing of late origins, and the S/M checkpoint prevents the cell from prematurely entering mitosis before it has fully replicated the genome (Bartek et al. 2004). Abrogation of the latter pathway allows cells to enter mitosis with incompletely replicated chromosomes, leading to chromosome fragmentation, segregation defects and, often, mitotic catastrophe (Canman 2001). In G2, a checkpoint blocks mitotic entry when DNA is damaged. This pathway is rapidly activated when cells are exposed to ultraviolet or ionizing radiation, which primarily causes bulky DNA adducts or double-stranded DNA breaks, respectively. Mutations that prevent cells from appropriately responding to DNA damage cause human developmental disorders, cancer and aging (Harper and Elledge 2007). Importantly, nearly all cancer cells have partially impaired checkpoints and thus checkpoint pathway components have emerged as important targets for anti-cancer drugs. Considerable attention has focused on identifying the proteins that contribute to these checkpoint pathways. This has established the PIKK kinases, ATM and ATR, as playing a central role in DNA damage response (Harrison and Haber 2006). In vertebrates, ATM responds primarily to double-stranded breaks, while ATR is more versatile, responding to a wide range of damage or replication stress (Brown and Baltimore 2003). ATR is a key player in the S/M checkpoint and is also required for the radiation-induced G2/M checkpoint (Nghiem et al. 2001). Once activated, ATR phosphorylates and activates the Chk1 kinase. Importantly, activated Chk1 phosphorylates and inhibits the Cdc25 phosphatases, thereby preventing activation of the Cyclin/CDK kinases and blocking cell cycle progression. Numerous other proteins have been identified as sensors and/or mediators in checkpoint signaling pathways (Harrison and Haber 2006). Human TopBP1 and its orthologs Cut5 (Xenopus laevis), Mus101 (Drosophila melanogaster), Cut5/Rad4 (Saccharomyces pombe) and Dpbl1 (Saccharomyces cerevisiae), are particularly intriguing because they have been shown to be critical not only for checkpoint activation in response to DNA damage, but also DNA replication initiation in unperturbed cells (Garcia et al. 2005). Studies in numerous organisms established TopBP1 as essential for the initiation of DNA replication. Consistent with these observations, TopBP1 associates with Cdc45 and the recruitment of TopBP1, Cdc45 and GINS, which is required for the pre-RC to pre-IC transition, appears to be interdependent (Van Hatten et al. 2002; Kubota et al. 2003; Takayama et al. 2003; Schmidt et al. 2008). SId3, a protein that is essential for pre-IC formation in yeast, also associates with Dpbl 1TopBP1 However, to date Sid3 orthologs have not been identified in higher eukaryotes. Importantly, TopBP1 has also been identified as being essential for both the intra-S and S/M checkpoints in numerous organisms (Garcia et al. 2005). Although it is formally plausible that these checkpoint functions are an indirect consequence of TopBP1's replication function, this is not the case: studies with conditional and separation of function mutants show that TopBP1's checkpoint function can be clearly separated from its action in pre-IC formation (McFarlane et al. 1997; Saka et al. 1997; Hashimoto et al. 2006; Yan et al. 2006). Finally, TopBP1 is also required for activation of the G2/M checkpoint in response to DNA damaging agents (Garcia et al. 2005). The widespread roles of TopBP1 are consistent with the broad spectrum of phenotypes resulting from TopPB1 deficiency. For example, Drosophila mus101TopBP1 mutants display defects in chorion gene amplification, hypersensitivity to DNA damaging agents and mitotic chromosome instability (Yamamoto et al. 2000). The predominant feature of the TopBP1 protein is that it contains multiple BRCT motif repeats (four in yeast and eight in humans) (Garcia et al. 2005). These domains commonly mediate protein-protein interactions, and in certain cases pairs of BRCT domains act as phosphopeptide binding motifs. Structure-function studies have revealed a critical role for the N-terminal BRCT domains in TopBP1 function (Garcia et al. 2005). The BRCT domains in this part of the protein are conserved from yeast to humans. Accordingly, this N-terminal half is both necessary and sufficient for DNA replication (Hashimoto et al. 2006). BRCT motifs I and 11 are required for binding to CDK phosphorylated Sld3 and also for TopBP1's checkpoint function in vertebrates (Lee et al. 2007; Tanaka et al. 2007; Zegerman and Diffley 2007; Yan and Michael 2009). Additional BRCT domains in the carboxy-terminal half of TopBP1 of higher eukaryotes are also involved in the response to DNA damage and replication stress. Specifically, BRCTs VII and VIII are important for ATR-dependent phosphorylation of Chk1 in response to replication stress in Xenopus extracts (Yan et al. 2006; Yan and Michael 2009), and BRCT domain V is required for TopBP1 to form nuclear foci in response to damage or stalled replication forks (Yamane et al. 2002). We have screened a mutant zebrafish collection for identifying novel DNA damage response regulators. The screen led to the identification of a novel gene, ticrr, required to activate the ionizing radiation induced G2/M checkpoint. Upon further characterization, we have identified additional roles of this gene: it is required for Sphase progression and the S/M checkpoint. This spectrum of defects is highly reminiscent of those arising in TopBP1 mutants. Accordingly, we show that TICRR binds to TopBP1 in vivo and is essential for pre-IC formation in a similar manner to TopBP1. In recognition of its broad spectrum of functions, we have named this gene ticrr for TopBP1-interacting, checkpoint and replication regulator. Results Disruption of ticrr impairs S-phase progression and causes premature chromatin condensation DNA damage checkpoint genes are often required for normal cell cycle control. For example, TopBP1 is required for both checkpoint activation in response to DNA damage and for the initiation of DNA replication in undamaged cells (Garcia et al. 2005). Consistent with this precedent, our analysis of the hi1573 and hi3202A zebrafish mutants carrying insertion in the ticrr gene revealed that ticrr is required for cell cycle control in the absence of DNA damage. FACS analysis of DNA content revealed that 40 hpf ticrr mutant embryos had a higher percentage of cells with between 2N and 4N DNA content than the wild-type control, suggesting an accumulation of cells in S-phase MMMP '_ Mir (Figure 1A). To further explore this possibility, we compared the ability of wild-type and ticrr mutant 40 hpf embryos to incorporate the nucleotide analog BrdU (Figure 1B). The wild-type embryos had a high level of cells that had between 2N and 4N DNA content and were BrdU-positive. This is indicative of the high rate of cell proliferation at this early stage of zebrafish development. In contrast, the 40 hpf ticrr mutant embryos incorporated very little BrdU even though a significant fraction of the cells had between 2N and 4N DNA content. This shows that the ticrr-deficient cells are impaired for DNA replication. A B wt mut G2/M 100. S 75 U wt 25 LGII DNA Content (PI) mut BrdU+ D 25 20 T 15 10 DNA Content (PI) Figure 1. Ticrr is required for normal DNA replication. (A) Cell cycle profile from a representative pool of 40 hpf hil573 wild-type (+/+ and +/-) and mutant (-/-) zebrafish embryos and quantification of cells with G1, S or G2/M DNA content (mean ±SD, n=3 biological replicates) showed an increase in S-phase cells in the ticrr mutants. (B) BrdU and PI FACS analysis of cells from pools of BrdU pulse labeled, wild-type and ticrr mutant embryos. Quantification of the BrdU+ population (mean +SD, n=3 biological replicates) showed a dramatic reduction in replicating DNA in ticrr mutants. In addition to this replication defect, the 40 hpf ticrr mutants displayed defects in mitotic progression. First, quantification of the percentage of cells in each phase of mitosis by visual inspection of chromatin morphology of pH3-positive cells showed that 86 the distribution of these populations differed between wild-type and ticrr mutant zebrafish (Figure 2A). Specifically, the ticrr mutants had a much higher percentage of cells that appeared to be in prometaphase, as judged by the presence of condensed chromatin that is not aligned on the metaphase plate. Moreover, they had a reduced percentage of anaphase cells and, within this population, anaphase bridges were prevalent (Figure 2A). To explore this defect further, we analyzed chromosome spreads from colchicinearrested cells (Figure 2B). This revealed a low incidence of cells with highly fragmented, condensed chromosomes in the ticrr mutant, but never wild-type, zebrafish (Figure 2B). This level of chromosomal fragmentation is consistent with cells that have undergone premature chromatin condensation. Given their DNA replication problems, we hypothesized that the ticrr mutants have an impaired ability to activate the S/M checkpoint and thus enter mitosis with partially replicated DNA. To address this possibility, we used FACS to determine the DNA content of pH3-positive cells (Figure 2C). It is well established that histone H3 phosphorylation begins in late G2, peaks during metaphase and then declines through anaphase (Hendzel et al. 1997). Accordingly, in the wild-type embryos, histone H3 phosphorylation is restricted to the cells with 4N DNA content (Figure 2C). In contrast, we find that a high percentage of the pH3-positive cells in the ticrr mutants have less than 4N DNA content. Taken together, the cell cycle defects observed in 40 hpf ticrr mutants show that ticrr is required in the absence of exogenous DNA damage for S-phase progression and also for activation of the S/M checkpoint. We propose that failure of these two processes causes cells to enter mitosis with partially replicated genomes, resulting in the chromosomal abnormalities and loss of anaphase cells observed in the ticrr mutants. Abnormal anaphase 100% abnormal anaphase L anaphase E metaphase * prometaphase * prophase wt mut 4 M<4N 3 *4N r2 0 DNA Content (PI) Figure 2. Ticrr is required for S/M checkpoint function. (A) Quantification of the proportion of pH3 positive cells in various mitotic phases, based on chromatin and spindle morphology, (mean +SD; n=100 cells counted in each of 4 wt and mut embryos) established a defect in mitotic progression and the presence of abnormal anaphase cells. Representative cells with anaphase bridges are shown. (B) Metaphase spreads of cells from colchicine treated embryos showed mitotic cells with fragmented chromosomes. A representative wild-type metaphase spread and examples of abnormal metaphase spreads from mutants are shown. (C) FACS measurement of anti-pH3 and PI staining of cells from wild-type and mutant embryos showed a population of mutant pH3-positive cells with less than 4N DNA content (blue) in ticrrmutants, establishing entry into mitosis before completing DNA replication. If ticrr mutants are defective in DNA replication and the S/M checkpoint, the DNA replication defect should be the primary defect and the mitotic abnormalities a secondary consequence. To test this, we turned to an earlier developmental timepoint when the ticrr defects were just beginning to arise. Since we first detect apoptosis in the hi1573 mutants at 26 hpf, we used 24 hpf embryos to screen for cell cycle defects. At this timepoint, the ticrr mutant embryos are morphologically indistinguishable from the wildtypes. Thus, ticrr mutants were identified by PCR genotyping a small fraction of cells dissociated from individual embryos. The remaining cells from 20 embryos of each genotype were then pooled for cell cycle analysis. FACS showed that there is already an increase in the percentage of cells with between 2N and 4N DNA content in the 24 hpf ticrr mutants compared to the wild-type controls, suggesting that cells are proceeding slowly through S-phase (Figure 3A). Moreover, the ticrr mutants had a lower average level of BrdU incorporation in these S-phase cells than the wild-types (Figure 3B). Thus, the defect in DNA replication clearly precedes the initiation of apoptosis. Notably, analysis of the DNA content of pH3-positive cells showed that, unlike the 40 hpf embryos, pH3-positive cells with sub-4N DNA content are not observed in the 24 hpf ticrr mutants indicating that there is no premature mitotic entry at this timepoint (Figure 3C). These data show that the DNA replication defect is the earliest detectable phenotype and thus is not a consequence of the premature mitotic entry. .................... ....... .... .I. 100 wt Li G2/M MS 75 i50Ef '~0 ~25-U BrdU-] lG1 DNA Content (PI) DNA Content (PI) 30 wt mut mut 4 3 *<4N *4N D15 .io C Wu 6-l CL. 10 TO- DNA Content (PI) Figure 3. A DNA replication defect but not premature mitotic entry occurs in 24 hpf ticrrmutants. (A) Cell cycle profiles from 24 hpf hil573 wild-type (+/+) and mutant (-/-) zebrafish embryos and quantitation of cells with G1, S or G2/M DNA content (mean ±SD, n=3 biological replicates), showed an increase in S-phase cells in mutants. (B) BrdU and PI FACS analysis of cells from pools of BrdU pulse labeled, 24 hpf wild-type and ticrr mutant embryos. Quantification of the BrdU+ population showed no change in the percentage of cells replicating DNA (mean ±SD, n=20,000 cells in each of 3 biological replicates), but the level of BrdU incorporation per cell is significantly decreased in the mutants (mean BrdU signal = Mean[BrdU+ signal]/Mean[BrdU- signal]; Student's t-test p<0.05). (C) FACS measurement of anti-pH3 and PI staining of cells from 24 hpf wild-type and mutant embryos showed no evidence of an S/M defect in the ticrrmutants at this timepoint, as judged by the absence of pH3 positive cells with less than 4N DNA content. TICRR binds TopBP1 The phenotypes of the ticrr mutant embryos are highly reminiscent of those resulting from depletion of TopBP1/Dbpl1/Cut5/Mus101. Specifically, these deficiencies have been shown to disrupt DNA replication, the S/M checkpoint and DNA damage checkpoints (Garcia et al. 2005). This raised the possibility that Ticrr acts in the TopBP1 pathway. In particular, we wondered whether Ticrr might be the vertebrate ortholog of 90 the budding yeast protein, SId3, which interacts with Dpbl 1 and is required for DNA replication initiation (Kamimura et al. 2001; Tanaka et al. 2007; Zegerman and Diffley 2007). To test this hypothesis, we examined whether the Ticrr protein shows any sequence homology with Sid3, or any other protein that might yield insight into its biochemical function. We found putative Ticrr orthologs in other metazoans; the conservation across these proteins maps to specific stretches, yielding high confidence in its significance, but the overall conservation is poor. There was no significant homology with any yeast protein, including SId3. However, given how rapidly the conservation falls off amongst the metazoan Ticrr proteins and also the poor sequence conservation of SId3 across the fungal kingdom, it seemed plausible that Ticrr could be a functional analog of Sld3, and yet bear no significant sequence similarity. Notably, despite their large size, the Ticrr proteins have no known functional motifs that could help to infer their biochemical activity. In the absence of any functional insight from the protein sequence, we investigated the biochemical properties of the Ticrr protein. For these studies, we switched to human cells due to the availability of reagents for known replication and checkpoint regulators. Human TICRR is an uncharacterized gene on chromosome 15 (C15orf42). The NCBI Reference Sequence database contained a predicted full-length TICRR mRNA sequence (NM_152259.3), inferred from partial cDNA and genomic sequences, and the encoded human TICRR protein is predicted to be 211 kDa. We generated a full-length 5,753-bp TICRR open reading frame by amplifying two overlapping cDNA fragments from HeLa cell mRNA. We also used a 30 kDa, carboxyterminal fragment of the TICRR protein to raise multiple polyclonal antisera in mouse and rabbits. These successfully recognized overexpressed TICRR and also an endogenous protein of approximately 250 kDa by both western blotting and immunoprecipitation (Figure 4; data not shown). This band was verified to be TICRR by partial knockdown using TICRR shRNAs (data not shown). Having established that we can detect the endogenous TICRR protein, we assessed its subcellular localization through biochemical fractionation of human cells. TICRR was entirely recovered from nuclear extracts of asynchronously growing HeLaS3 cells and was not present in cytosolic extracts. Moreover, TICRR fractionated with the insoluble nuclear material and was resistant to extraction from nuclei by low salt (150 mM NaCl) and nonionic detergent (1% NP-40) but could be extracted with high salt (0.3 M NaCl) or ionic detergents (0.1% SDS or 0.5% deoxycholate; Figure 4A and data not shown). These results strongly suggested that TICRR associates with chromatin. Dpbl 1 TopBP1 and Sid3 are both chromatin-associated, thus, we further tested whether TICRR was bound to chromatin by treating the insoluble nuclear material with nuclease. For this analysis, we used a component of the origin recognition complex, ORC2, as a positive control. We found that treatment of the chromatin/nuclear matrix material with micrococcal nuclease caused partial release of TICRR and this mirrored the level of release of ORC2 (Figure 4B). These results demonstrate that a large fraction of TICRR is associated with chromatin, as has been previously described for TopBP1 (Garcia et al. 2005). 92 MNase: [Nac - M) 0 0 Figure 4. Human TICRR is a chromatin-associated protein. (A) Nuclei preparations were subjected to sequential salt extractions with the indicated NaCl concentrations. Orc2 is a chromatin-bound protein, while Lamin is structural component of the nucleus. (B) Western blotting of biochemical fractions from HeLa cells showed that the majority of TICRR is present in the chromatin-enriched fraction (chrom) and can be partially released by micrococcal nuclease (Mnase) treatment. Orc2 and alpha-tubulin are chromatin and cytoplasmic markers. WCE = whole cell extract, Si = soluble cytoplasmic fraction, S2 = soluble nuclear fraction. We next asked if the endogenous TICRR and TopBP1 proteins interacted. For this analysis, proteins were extracted from nuclei using ionic detergent (0.1% Sodium Dodecyl Sulfate) and then immunoprecipitated with either pre-immune or anti-TICRR polyclonal rabbit antisera. Subsequent western blotting established that TopBP1 was present in the anti-TICRR, but not in pre-immune, immunoprecipitates (Figure 5A). Moreover, we were able to conduct a reciprocal IP/immunoblotting experiment to show that TICRR co-immunoprecipitated with TopBP1 (Figure 5B). Since both TICRR and TopBP1 are chromatin-associated proteins, it seemed possible that DNA bridged the interaction between these two proteins. However, we were able to show that these two proteins continued to co-IP even if the lysates were pre-treated with ethidium bromide or DNase I (Figure 5A). Thus, we conclude that human TICRR and TopBP1 are associated proteins and this interaction can occur in the absence of DNA. 93 ....... ..... .. +EtBr Input (5%) Ip. EJL +DNase I B, (ly _ CO "D Figure 5. Human TICRR interacts with TopBP1. (A) Immunoblotting showed TopBP1 coimmunoprecipitates with TICRR but not preimmune (PI) antibodies. The association was not affected by treatment with ethidium bromide (EtBr) or DNase I indicating its independence from chromatin binding. (B) Reciprocal IP-western blotting confirmed that TICRR is present in TopBP1, but not IgG, immunoprecipitates. Given the relatively poor sequence homology of TICRR across species, it was important to determine whether the TopBP1 binding ability of human TICRR was conserved in the zebrafish Ticrr protein. Since antibodies were not available for zebrafish Ticrr, we cloned the full length zebrafish Ticrr coding sequence and overexpressed GFP-tagged versions of either zebrafish Ticrr (GFP-zTicrr) or human TICRR (GFP-hTICRR) in human cells along with human TopBP1. Notably, human TopBP1 was recovered with similar efficiency in GFP-zTicrr and GFP-hTICRR immunoprecipitates, even though the GFP-zTicrr protein was poorly expressed (Figure 6). This cross-species binding shows unequivocally that the TICRR-TopBP1 interaction is conserved between human and zebrafish. ....................... IP: a-GFP GFP Input 'Uzebrafish 9 r, Figure 6. Human TopBP1 interacts with TopBPI. IP-western blotting showed that GFP-tagged zebrafish Ticrr associated with human TopBP1 when co-expressed in human cells. The human TopBP1 is a large multifunctional protein whose predominant feature is the presence of eight BRCT domains (Figure 7A). The yeast homologs of TopBP1, Dpbl1 and Cut5, have only four BRCT domains that are highly conserved with BRCTs I, 11,IV and V of the vertebrate TopBP1 proteins (Hashimoto et al. 2006). Prior studies have shown that individual or pairs of BRCT domains mediate interactions with specific proteins, and various known TopBP1 functions have been mapped to specific BRCT domains. The N-terminal half of X. laevis TopBP1, which includes the four highly conserved BRCTs, is both necessary and sufficient for the initiation of DNA replication (Hashimoto et al. 2006). Moreover, BRCT motifs I and 11appear to be particularly important in both replication and checkpoint functions: these are required for Dpbl 1 TopBPI to bind to Sld3 (Tanaka et al. 2007; Zegerman and Diffley 2007) and for interaction with the 9-1-1 checkpoint complex (Furuya et al. 2004; Delacroix et al. 2007; Lee et al. 2007). If the interaction between TopBP1 and TICRR is relevant to their DNA replication functions, then TICRR would be predicted to interact with the N-terminal BRCTs of TopBP1. To address this, we created a panel of human TopBP1 mutants in which specific BRCT domains were either deleted singly or pairwise (AI+ll, All1, AIV+V, AVI and AVII+VlIl) and tested their ability to bind to TICRR in co-transfection assays (Figure 7B). All five of the TopBP1 mutants were expressed at similar levels to the wild-type TopBP1. TICRR co-immunoprecipitated with four of these mutants, AlII, AIV+V, AVI and AVII+VIII, and with wild-type TopBP1. In contrast, no TICRR was recovered in the immunoprecipitate of the Al+ll deletion mutant even though TICRR was present at high levels in these cells. Taken together, our data show that TICRR associates with TopBP1 in vivo and this interaction requires the two N-terminal BRCT domains that have been associated with TopBP1's role in both checkpoint signaling and DNA replication. A 41W 1-6W 0 BRCT domain Input (2%) IP: ot-GFP \X I\ eX\% 4 ) 0A &4~ 4P I> % XtI 0if1 Figure 7. TICRR interacts with the first two BRCT domains in TopBP1. (A) Schematic diagram of TopBP1 protein. (B)Anti-GFP immunoprecipitates and whole cell lysates (input) from cells transfected with GFP-tagged TopBP1 deletion mutants were screened for TICRR and TopBP1 proteins by immunoblotting. We were intrigued to find that TICRR association maps to the region of TopBP1 that is required for Sid3 binding. In yeast, formation of the Sid3- Dpbl known to require CDK 1 TopBPI complex is phosphorylation of Sld3 and this, together with the 96 phosphorylation of SId2, accounts for the CDK-dependence of DNA replication in this organism (Tanaka et al. 2007; Zegerman and Diffley 2007). CDK is also essential for replication in human cells, but there is some doubt as to whether pre-IC components will be the relevant target (DeGregori et al. 1995). Given these questions, we used two complementary approaches to examine the role of CDK phosphorylation in the TICRRTopBP1 interaction. First, we generated a population of HeLa cells that were synchronously re-entering G1 from mitosis, and then cultured them in the absence or presence of the pan-CDK inhibitor roscovitine for 8 hours (Figure 8A). Consistent with the known CDK-dependence of S-phase entry, FACS analysis showed that the untreated cells (-ROSC) were beginning to enter S-phase while the roscovitine treated cells (+ROSC) remained blocked in G1 (Figure 8A). Notably, TopBP1 was recovered at comparable levels in the TICRR immunoprecipitates of both the untreated and treated cells (Figure 8A), suggesting that binding occurs in the presence of CDK-inhibition. In the second approach, we incubated TICRR-TopBP1 immunoprecipitates from asynchronous cell extracts with, or without, lambda phosphatase and then assayed the association of TICRR and TopBP1 by western blotting. Notably, phosphatase treatment significantly increased the mobility of the TICRR protein (Figure 8B), indicating that TICRR is phosphorylated in vivo and that we had successfully removed this modification. Despite this change, there was no detectable difference in the levels of the TICRRTopBP1 complex in phosphatase treated versus untreated cells (Figure 8B). Importantly, this result was not altered by DNAase treatment of the extracts prior to immunoprecipitation (data not shown), indicating that this complex formation occurs in the absence of chromatin binding. Taken together, these synchronization and phosphatase experiments strongly suggest that the interaction between TICRR and TopBP1 can occur in a CDK-independent manner. ....... ............ A +Noc Release 18 hrs Rosc 2 hrs Analyze B 8 hrs Input IP ai-TICRR Phosphatase Phosphatase Inhib AS -Rosc +Rosc - + P1 - + - + a DNA (PI) AS -Rosc +Rosc Input IP: Figure 8. TICRR can bind TopBP1 in a CDK independent manner. (A) HeLa cells were synchronized in mitosis and then allowed to re-enter the cell cycle in the absence (-Rosc) or presence (+Rosc) of roscovitine as depicted. FACS analysis showed that the Rosc. cells were entering S-phase, while the + Rosc. cells remained in G1. IP-western analysis shows that the TICRR-TopBP 1 complex was recovered at similar levels in the -Rosc. and +Rosc. samples. AS=asynchronous. (B) Asynchronous HeLaS3 cell extracts were immunoprecipitated with anti-TICRR antibodies and then either incubated with lambda phosphatase, phosphatase inhibitors or both. The mobility of the TICRR protein was significantly increased in the phosphatase treated sample, versus the controls, but showed no difference in the levels of associated TopBP . Ticrr-deficiencyinhibitspre-IC formation The TopBP1 orthologs in X. Iaevis, budding and fission yeast are known to be essential for the transition of the pre-replication complex (pre-RC) into the pre-initiation complex (pre-IC), an intermediate in the initiation of DNA replication (Garcia et al. 2005). Having established that ticrr is essential for normal DNA replication in zebrafish, and that TICRR and TopBP1 associate in the absence of DNA, we asked whether ticrr is similarly required to form the pre-IC. To address this question, we employed the wild-type and ticrr mutant zebrafish. For this analysis, we used embryos at 40 hpf, the developmental timepoint at which the ticrr deficient cells have a profound replication defect, as judged by S-phase accumulation and strongly reduced BrdU incorporation. Pools of wild-type and mutant embryos were dissociated and used to generate extracts from whole cell or chromatin-enriched fractions. These cells were then assayed by western blotting for chromatin association of the Mcm2-7 complex (using a pan-MCM monoclonal antibody) and the GINS complex (using an antibody against Psfl) that are core components of the pre-RC and pre-IC, respectively (Figure 9). There was no difference in the chromatinassociation of the Mcm2-7 complex in wild-type versus ticrr mutant embryos. In contrast, Psfl showed a significant level of chromatin association in the wild-type embryos but was nearly absent (although still detectable on long exposure) from the chromatinenriched faction in the ticrr mutant, even though the Psfl protein was present at normal levels in the whole cell extracts. Taken together our data show that, in concert with TopBP1, Ticrr is required for the transition from pre-RC to pre-IC explaining its essential role during DNA replication. Total Chromatin Figure 9. Ticrr is essential for the chromatin association of the pre-IC component Psfl. 4- Total cell lysates and chromatin-enriched fractions were prepared from 40 hpf wild-type (+/+ and +/-) and ticrr mutant (-/-) zebrafish embryos. Immunoblotting for core pre-RC and pre-IC components, the MCMs and Psfl respectively, showed that these are expressed at normal levels, but only the MCMs are chromatin loaded, in the ticrr mutants. cr 99 Discussion Using a screen for zebrafish mutants that fail to arrest mitotic entry after exposure to IR, we identified two mutant lines, each with a distinct mutation in the ticrr gene. In addition to the G2/M checkpoint defect, these lines have a profound apoptotic phenotype in the absence of exogenous damage that revealed a more general role in cell survival during development. Consistent with this observation we found that ticrr mutants at 40 hpf failed to incorporate BrdU, demonstrating that loss of ticrr also causes a defect in DNA replication. Instead of arresting in S-phase, many cells in the ticrr mutants proceed into mitosis and display an array of chromosomal abnormalities, including fragmented chromosomes and anaphase bridges, that likely account for the loss of anaphase cells through mitotic catastrophe. Thus, ticrr is required to prevent mitotic entry both following exogenous DNA damage and also when DNA replication is impaired. These phenotypes are highly reminiscent of those arising when TopBP1 and its orthologs are inactivated. Accordingly, we found that the human and zebrafish TICRR proteins both interact with TopBP1 in human cells and ticrr is required for transformation of the pre-RC to the pre-IC in zebrafish. Taken together, these data suggest that TICRR functions with TopBP1 in DNA replication and both the S/M and G2/M checkpoints. TICRR in DNA Replication The replication machinery and its mechanisms of regulation have been studied in numerous organisms. These processes are generally conserved among eukaryotes but a unifying model remains elusive because the order of assembly of pre-IC proteins seems to differ between X. laevis and yeast, and known key components such as Sld3 have not been identified in higher eukaryotes. The initiation of DNA replication is best 100 understood in S. cerevisiae. Here, Dpbl 1 TopBP1, SId2, SId3, GINS, and Cdc45 are all required to transform the pre-RC into the pre-IC. Sld3 and Dpbl 1 TopBP1 form a complex that is induced by the CDK phosphorylation of Sld3 and requires the two N-terminal BRCT domains of Dpbl1 (Tanaka et al. 2007; Zegerman and Diffley 2007). Although the formation of the Dpb11-Sld3 complex is critical for replication initiation in S. cerevisiae, an analogous event has not been defined in higher eukaryotes. Much of our understanding of vertebrate DNA replication comes from studies in X. laevis (Bell and Dutta 2002; Sclafani and Holzen 2007). In this organism Cut5TOPBP1 is also required for the recruitment of DNA polymerases onto chromatin (Hashimoto and Takisawa 2003; Kubota et al. 2003). Thus, the general function of Dpbl 1 TopBP1 in pre-IC formation seems to be conserved in vertebrate TopBP1. Our data show that TICRR displays some of the core properties of the Sld3 protein. First, Ticrr is essential for DNA replication in unperturbed cells and it is specifically required for formation of pre-ICs, but not pre-RCs. Second, TICRR associates with TopBP1 through the N-terminal BRCT motifs I and 11that are conserved with the SId3-interacting BRCTs of Dpb 11TopBP1 However, our data do not address whether the interaction between TICRR and TopBP1 is direct, and other observations are less consistent with the idea that TICRR is a true ortholog of Sld3. First, we note that there is no detectable sequence similarity between these proteins. This is not a particularly conclusive finding, since the Sld3 and TICRR proteins are both poorly conserved even within their own kingdoms, but it does raise questions about both the relationship and mechanism(s) of action of these proteins. The second, and more striking, finding is the apparent discrepancy in the role of CDK phosphorylation in Sld3 versus TICRR regulation. Specifically, CDK phosphorylation of SId3 is required for it to bind to Dpbl 1TopBP1 and this, together with the phosphorylation of Sld2, accounts for the CDK-dependence of DNA replication in yeast (Tanaka et al. 2007; Zegerman and Diffley 2007). In contrast, we find that the TICRR-TopBP1 interaction is 101 completely unaffected by culturing in the presence of the pan-CDK inhibitor roscovitine or treatment with phosphatase. We note that there are potential limitations to these approaches: the roscovitine-induced block to S-phase entry (figure 5F) could reflect a reduction, but not full loss, of CDK activity; and/or the phosphatase may be unable to access the phosphorylated residue(s) mediating the TICRR-TopBP1 interaction, even though it effectively targets other phosphorylation sites (figure 5G). Despite these caveats, our data are most consistent with the notion that TICRR associates with TopBP1 in a CDK-independent manner. On first consideration, this finding seems to imply that TICRR is not human Sld3. However, there is evidence to suggest that the underlying basis for the CDK-dependence of DNA replication differs in human versus yeast cells. First, DeGregori and coworkers have shown that E2F1 expression completely bypasses the CDK-dependence of S-phase in human cells (DeGregori et al. 1995). This argues that there is no absolute requirement for CDK phosphorylation of either pre-RC or pre-IC components in this organism. Consistent with this conclusion, CDK phosphorylation is required for formation of the yeast Sld2-Dpbl1 complex but not the vertebrate counterpart RECQL4-TopBP1 (Matsuno et al. 2006; Tanaka et al. 2007; Zegerman and Diffley 2007). Clearly, additional studies are required to explore how similar or different Sld3 and TICRR are to one another in the context of both DNA replication and, as described below, checkpoint response. TICRR in the SIM and G2/M Checkpoints The S/M checkpoint plays a vital role in ensuring complete replication prior to mitotic entry. Our data show that Ticrr is essential for the integrity of this checkpoint in vivo. Thus, Ticrr joins a short list of proteins that play a dual role in both replication regulation and S/M checkpoint response. The existing dual replication/checkpoint proteins can be divided into two different subclasses, based on their role in the S/M 102 checkpoint. The Mcm2-7 complex, Cdtl and Cdc45 are representative members of the first subclass. The analysis of yeast conditional mutants shows that the loss of MCMs, Cdtl or Cdc45 prior to the initiation of replication allows cells with unreplicated DNA to enter mitosis, but the loss of these proteins in replicating cells does not impair the S/M checkpoint (Tercero et al. 2000; Labib et al. 2001). These findings suggest that these pre-RC and pre-IC components are not directly involved in the S/M checkpoint. Instead, they are required to create the replication structures which signal that S-phase is ongoing and not yet complete. Studies using X. laevis extracts confirm that replication structures are a pre-requisite for S-phase checkpoint signaling in vertebrates and further suggest that the necessary feature is the RNA primer generated by DNA polymerase a (Michael et al. 2000). TopBP1 is an example of the second class of dual replication/checkpoint proteins. Studies with conditional and deletion mutants show that TopBP1's checkpoint function can be separated from its role in pre-IC formation (McFarlane et al. 1997; Saka et al. 1997; Hashimoto et al. 2006; Yan et al. 2006). McFarlene and colleagues identified a temperature sensitive mutant of Cut5 in S. pombe that fails to arrest when treated with hydroxyurea at the semi-permissive temperature, but has normal replication capabilities (McFarlane et al. 1997). Two independent studies in Xenopus egg extracts also showed that the replication and checkpoint functions of TopBP1 could be separated (Hashimoto et al. 2006; Yan et al. 2006). Addition of an oligonucleotide duplex to Xenopus egg extracts bypasses the requirement of replication for checkpoint activation in response to stalled replication forks (Yan et al. 2006). Using this system, it was shown that the Nterminal half of the TopBP1 protein rescues the replication defect caused by depletion of TopBP1 in egg extracts, but is incapable of activating the checkpoint in response to stalled replication forks, suggesting that the checkpoint defect is not a consequence of loss of a complete replication complex and TopBP1 is directly involved in checkpoint 103 signaling. In fact, TopBP1 directly stimulates Chk1 phosphorylation by ATR in an in vitro kinase assay and the C-terminus half of TopbP1 is required for this function. Biochemical data shows that TopBP1 is recruited to the site of replication stress or DNA damage (Furuya et al. 2004; Delacroix et al. 2007; Lee et al. 2007) and once recruited promotes ATR activation (Kumagai et al. 2006). Together, these data strongly suggests that TopBP1 is a bona fide checkpoint protein. Given the precedent of the existing dual replication/checkpoint proteins, it remains an open question whether Ticrr is directly involved in the S/M checkpoint or whether this function simply reflects its role as an essential replication regulator. Based on the following observations, we favor the former hypothesis. First, our data show that TICRR interacts with TopBP1, raising the possibility that it cooperates in TopBP1dependent processes beyond DNA replication, such as checkpoint signaling. Second, our FACS data show that ticrr-deficient cells enter mitosis with partially replicated DNA. This clearly differs from the yeast MCM, Cdtl and Cdc45 mutants, which do not prematurely enter mitosis once S-phase has begun (Tercero et al. 2000; Labib et al. 2001). Third, we originally identified ticrr through a screen for G2/M checkpoint regulators. This showed that ticrr-deficient cells fail to arrest in mitosis in response to treatment with ionizing radiation. Importantly, our FACS analysis shows that these mitotic cells have 4N DNA content. Thus, we believe that this MAI phenotype reflects a bona fide defect in the G2/M checkpoint and is not simply an indirect consequence of the replication and/or S/M checkpoint defects. Consistent with this view, there is no evidence in the literature that the G2/M checkpoint is dependent on appropriate replication initiation. Moreover, included in our zebrafish screen were other known replication gene mutants that did not display the MAI phenotype. Finally, we again note that TopBP1 has a well-documented role in the radiation-induced G2/M checkpoint (Garcia et al. 2005). Given all of these observations, we speculate that TICRR is a previously unknown 104 partner for TopBP1 in its myriad roles as a core regulator of the DNA replication, S/M checkpoint and the G2/M checkpoint machinery. Materials and methods Zebrafish maintenance, collection and genotyping.Zebrafish were maintained as previously described (Amsterdam et al. 2004). Primers used for genotyping were as follows: hil 573 - forward primer (F): AAGCAAGCTACATCTCAAAGCA; hi1573 -reverse primer (R): CGGAAAACCCTGAAGTGTGAT; hi3202A - (F) CAGATCCCCTGGTTATAAGTGTTGC; hi3202A (R) CAGTTATGGCCTCTCAGA ATGTCGT; reverse primer for provirus: GCTAGCTTGCCAAACCTACAGGT. Plasmid construction Human TICRR A fragment of the 5' end of the human ticrr cDNA was generated by RT-PCR (F: CACCATGCTGCTGCTGGACAC; R: GGCCGGGTGAGGAGGAGTCTTTTA) from HeLa cell mRNA and cloned into pENTR/D-TOPO to generate NC1. A short 5' sequence was added to this fragment by ligating two annealed oligos (GGCCGCATGGCATGCTGTCA CAAAGTAATGCTGCTGCTGGACACCGC and GGTGTCCAGCAGCAGCATTACTTTG TGACAGCATGCCATGC) into NC1 to generate NC2. The 3' end of the ticrr cDNA was amplified by RT-PCR (F: GGATCCACTTTGGATTCGGAGGTCCTG, CTGGCTGACTCCTTGATCT) R: TTGGCGCGC and ligated into NC2 to generate NC3. The full-length cDNA cloned into pENTR/D-TOPO (NC3) was then transferred to an expression vector (pDSLPCX-FTM-XB) using the LR Clonase (Invitrogen) Reaction 1l. 105 Zebrafish Ticrr The zebrafish ticrr cDNA was generated by RT-PCR from mRNA of a single adult zebrafish (F: ATGGCCTCTCAGAATGTCG; R: CCGAACGTTAAAATCCATGC). The cDNA cloned in pENTR/D-TOPO was then transferred to an expression vector (pDESTEGFP-C1) using the LR Clonase (Invitrogen) Reaction 11. Human TopBP1 The human TopBP1 cDNA was PCR amplified from pCR-XL-TOPO-TOPBP1 (Open Biosystems; BC126209.1; F: CACCATGTCCAGAAATGACAAAGAACC; R: TTAGTGTACTCTAGGTCGTTTGA) and transferred into pENTR/D-TOPO (Invitrogen) and then pDEST-EGFP-C1 using the LR Clonase (Invitrogen) Reaction 11.In frame deletions within the cDNA encoding TopBP1 were generated by PCR. Final products were verified by DNA sequencing. The TopBP1 BRCT domain deletion mutants carry the following in frame deletions within the 1522 a.a. ORF: A98-306 (BRCTs 1+11), A354452 (111), A547-760 (IV+V), A922-1011 (VI), A1267-1489 (VIl-VII). Except for A922-1 011, the deleted amino acids were replaced by two glycines. FACS analysis For BrdU labeling in zebrafish, dechorionated embryos were incubated with 10mM BrdU/1 5% DMSO on ice for 15 minutes, washed and incubated at 28.5*C for 15 minutes. Mutant embryos were identified by either developmental phenotype or PCR genotyping of a fraction of individual, fixed embryos. Cells were disaggregated by triturating embryos in 0.25% Trypsin/lmM EDTA using a p200 and fixed in 70% ethanol at -20*C overnight. Suspensions of cells from 20 wild-type or mutant embryos were pooled and prepared for pH3/propidium iodide or BrdU/propidium iodide FACS analysis as described (Pozarowski and Darzynkiewicz 2004). FACS analysis was conducted by 106 FACScan (Becton-Dickinson). DNA content was quantified by ModFit LT (Verity Software), and pH3 and BrdU was quantified by FlowJo (Tree Star, Inc.). Human cell culture and chromatin fractionation HeLa, HeLa-S3, and 293FS cells were grown in DMEM with 10% FBS. For the synchronization experiments, HeLa cells were cultured in presence of 100 ng/ml nocodazole (Calbiochem) for 18 hours. Synchronized mitotic cells were recovered by shake-off, replated in DMEM containing 10% FBS for 2 hours and then incubated for a further 8 hrs in the presence or absence of 20 pM roscovitine (Calbiochem). The isolation of soluble fraction (S1), soluble nuclear fraction (S2), and chromatin-enriched fractions were conducted as described (Mendez and Stillman 2000). To solubilize the chromatin-bound proteins, nuclei were treated with 50U of Micrococcal nuclease (Worthington) for 2 minutes at 37*C. Immunoprecipitation Hela-S3 cells were lysed in modified RIPA buffer (50 mM Tris-HCL [pH 8.0], 150 mM NaCL, 1 mM EDTA, 1% NP-40, 0.1% SDS, 0.1 mM PMSF, protease inhibitor cocktail (Roche) 1 mM NaF, 10 mM p-glycerophosphate (b-GP), 200 uM Na3VO 4) for 30 minutes in ice. The soluble supernatant was used for immunoprecipitations. When indicated, lysates were treated with 2000U/ml DNase I (Roche; 30 min at 25 0C) or 20 pg/ml ethidium bromide (30 min on ice) prior to TICRR IP. For phosphatase treatment, precipitates were resuspended in k phosphatase buffer (NEB) with or without 400U k phosphatase (NEB) and phosphatase inhibitors (50 mM NaF and 10 mM Na3VO 4), and incubated at 300C for 30 minutes. The immunoprecipitates were washed four times with RIPA and resuspended in 2X Laemmli Buffer. 107 Zebrafish chromatin preparation Deyolked zebrafish embryos were triturated in 0.25% Trypsin/1 mM EDTA using a p200. Disaggregated cells were filtered through a 35pm nylon mesh and washed once with PBS. The chromatin-enriched fraction was prepared essentially as described (Aparicio et al. 2009). Briefly, cells were lysed in 10 mM HEPES pH 7.9, 0.2 M KOAc, 0.1% Triton X100, 0.34 M sucrose, 10% glycerol, 1 mM 1,4-DTT, protease and phosphatase inhibitors. The chromatin-associated fraction was recovered by spinning at 18,000g for 10 minutes and washed twice in lysis buffer before suspension in Laemmli Buffer. Antibodies A 6-His-tagged N-terminal fragment of human TICRR (NP_689472.3 amino acids 10941348) was expressed in bacteria, purified over Ni2+ NTA-agarose resin (Qiagen), and used to immunize BALB/c mice or New Zealand White rabbits (Pocono Rabbit Farm). Other antibodies were: phosho-H3 (sc-8656-R, Santa Cruz), mouse anti-goldfish CyclinB1 (B112; Katsu et al. 1993), PSTAIRE-Cdc2 (sc-53, Santa Cruz), tubulin (T9026, Sigma), ORC2 (sc-13238, Santa Cruz), LaminA/C (2032, Cell Signaling), TopBP1 (NB100-217, Novus Biologicals and sc-32923, Santa Cruz), rabbit polyclonal anti-human Psf1 (Aparicio et al. 2009), mouse monoclonal anti-human Pan-MCM (Austin et al. 1999), normal mouse IgG (sc-2025, Santa Cruz), and GFP (11814460001, Roche). Acknowledgements Antibodies for this study were provided by Dr. Juan Mendez, Spanish National Cancer Research Center (anti-Psfl) and Dr. Stephen Bell, HHMI and MIT (Pan-MCM). We also thank Stephen Bell, Sebastian Hoersch and members of the Hopkins and Lees labs for helpful discussions during this study and the preparation of this manuscript. This work was supported by Ruth L. Kirschstein NRSAs to C.S. and N.C. J.A.L. is a Daniel K. 108 Ludwig Scholar. References Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. 2004. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A 101(35): 1279212797. Aparicio T, Guillou E, Coloma J, Montoya G, Mendez J. 2009. The human GINS complex associates with Cdc45 and MCM and is essential for DNA replication. Nucleic Acids Res 37(7): 2087-2095. Austin RJ, Orr-Weaver TL, Bell SP. 1999. Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev 13(20): 2639-2649. Bartek J, Lukas C, Lukas J. 2004. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol 5(10): 792-804. Bell SP, Dutta A. 2002. DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333-374. Brown EJ, Baltimore D. 2003. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev 17(5): 615-628. Canman CE. 2001. Replication checkpoint: preventing mitotic catastrophe. Curr Biol 11(4): R121124. DeGregori J, Leone G, Ohtani K, Miron A, Nevins JR. 1995. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev 9(23): 28732887. Delacroix S, Wagner JM, Kobayashi M, Yamamoto K, Karnitz LM. 2007. The Rad9-Husl-Radl (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev 21(12): 1472-1477. Furuya K, Poitelea M, Guo L, Caspari T, Carr AM. 2004. Chk1 activation requires Rad9 S/TQ-site phosphorylation to promote association with C-terminal BRCT domains of Rad4TOPBP1. Genes Dev 18(10): 1154-1164. Garcia V, Furuya K, Carr AM. 2005. Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 4(11): 1227-1239. Harper JW, Elledge SJ. 2007. The DNA damage response: ten years after. Mol Cell 28(5): 739745. Harrison JC, Haber JE. 2006. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet 40: 209-235. Hashimoto Y, Takisawa H. 2003. Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J 22(10): 2526-2535. Hashimoto Y, Tsujimura T, Sugino A, Takisawa H. 2006. The phosphorylated C-terminal domain of Xenopus Cut5 directly mediates ATR-dependent activation of Chk1. Genes Cells 11(9): 9931007. Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. 1997. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric 109 heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106(6): 348-360. Kamimura Y, Tak YS, Sugino A, Araki H. 2001. Sld3, which interacts with Cdc45 (Sid4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. EMBO J 20(8): 2097-2107. Katsu Y, Yamashita M, Kajiura H, Nagahama Y. 1993. Behavior of the components of maturationpromoting factor, cdc2 kinase and cyclin B, during oocyte maturation of goldfish. Dev Biol 160(1): 99-107. Kubota Y, Takase Y, Komori Y, Hashimoto Y, Arata T, Kamimura Y, Araki H, Takisawa H. 2003. A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication. Genes Dev 17(9): 1141-1152. Kumagai A, Lee J, Yoo HY, Dunphy WG. 2006. TopBP1 activates the ATR-ATRIP complex. Cell 124(5): 943-955. Labib K, Kearsey SE, Diffley JF. 2001. MCM2-7 proteins are essential components of prereplicative complexes that accumulate cooperatively in the nucleus during G1-phase and are required to establish, but not maintain, the S-phase checkpoint. Mol Biol Cell 12(11): 3658-3667. Lee J, Kumagai A, Dunphy WG. 2007. The Rad9-Husl-Radl checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem 282(38): 28036-28044. Matsuno K, Kumano M, Kubota Y, Hashimoto Y, Takisawa H. 2006. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol Cell Biol 26(13): 4843-4852. McFarlane RJ, Carr AM, Price C. 1997. Characterisation of the Schizosaccharomyces pombe rad4/cut5 mutant phenotypes: dissection of DNA replication and G2 checkpoint control function. Mol Gen Genet 255(3): 332-340. Mendez J, Stillman B. 2000. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol 20(22): 8602-8612. Michael WM, Ott R, Fanning E, Newport J. 2000. Activation of the DNA replication checkpoint through RNA synthesis by primase. Science 289(5487): 2133-2137. Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. 2001. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Nat/ Acad Sci U S A 98(16): 9092-9097. Pozarowski P, Darzynkiewicz Z. 2004. Analysis of cell cycle by flow cytometry. Methods Mol Biol 281: 301-311. Saka Y, Esashi F, Matsusaka T, Mochida S, Yanagida M. 1997. Damage and replication checkpoint control in fission yeast is ensured by interactions of Crb2, a protein with BRCT motif, with Cut5 and Chk1. Genes Dev 11(24): 3387-3400. Sansam CL, Shepard JL, Lai K, lanari A, Danielian PS, Amsterdam A, Hopkins N, Lees JA. 2006. DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev 20(22): 3117-3129. 110 Schmidt U, Wollmann Y, Franke C, Grosse F, Saluz HP, Hanel F. 2008. Characterization of the interaction between the human DNA topoisomerase Ilbeta-binding protein 1 (TopBP1) and the cell division cycle 45 (Cdc45) protein. Biochem J 409(1): 169-177. Sclafani RA, Holzen TM. 2007. Cell cycle regulation of DNA replication. Annu Rev Genet 41: 237280. Takayama Y, Kamimura Y, Okawa M, Muramatsu S, Sugino A, Araki H. 2003. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev 17(9): 1153-1165. Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. 2007. CDK-dependent phosphorylation of Sld2 and Sid3 initiates DNA replication in budding yeast. Nature 445(7125): 328-332. Tercero JA, Labib K, Diffley JF. 2000. DNA synthesis at individual replication forks requires the essential initiation factor Cdc45p. Embo J 19(9): 2082-2093. Van Hatten RA, Tutter AV, Holway AH, Khederian AM, Walter JC, Michael WM. 2002. The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J Cell Biol 159(4): 541-547. Yamamoto RR, Axton JM, Yamamoto Y, Saunders RD, Glover DM, Henderson DS. 2000. The Drosophila muslOl gene, which links DNA repair, replication and condensation of heterochromatin in mitosis, encodes a protein with seven BRCA1 C-terminus domains. Genetics 156(2): 711-721. Yamane K, Wu X, Chen J. 2002. A DNA damage-regulated BRCT-containing protein, TopBP1, is required for cell survival. Mol Cell Biol 22(2): 555-566. Yan S, Lindsay HD, Michael WM. 2006. Direct requirement for Xmus101 in ATR-mediated phosphorylation of Claspin bound Chk1 during checkpoint signaling. J Cell Biol 173(2): 181-186. Yan S, Michael WM. 2009. TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J Cell Biol 184(6): 793-804. Zegerman P, Diffley JF. 2007. Phosphorylation of Sid2 and Sid3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445(7125): 281-285. 111 CHAPTER IV: Discussion 112 Key conclusions We have screened a collection of zebrafish insertional mutants for genes required for G2/M checkpoint activation in response to IR treatment. The work described in this thesis focuses on the characterization of a novel gene discovered in this screen, ticrr. Our work has demonstrated that Ticrr is required for replication initiation, specifically for the conversion of the pre-RC into pre-IC. In addition to a G2 checkpoint defect in response to DNA damage, ticrr mutants have a defect in the S/M checkpoint that monitors DNA replication during normal cell cycle progression (Sansam et al., 2010). These results indicate that ticrr is a novel vertebrate cell cycle regulator, with essential roles in replication initiation and the surveillance mechanisms protecting the cell from genomic insults during replication, as well as during exposure to exogenous DNA damaging agents. The human TICRR protein binds TopBP1, a known replication and checkpoint factor. This interaction is conserved in zebrafish, suggesting that it is functionally significant. Interestingly, the interaction is mediated through the first two BRCT domains of TopBP1, previously shown to be essential for its replication and checkpoint function. We conclude that TICRR is a TopBP1 partner that probably complements its role in cell cycle regulation. Currently the mechanism of action of TICRR and how it collaborates with TopBP1 is unknown, but merits further investigation. A screen in zebrafish identifies Ticrr We have screened 336 insertional mutant lines to identify novel cell cycle regulators. Five mutant lines had a mitotic phenotype, while nine mutant lines had a checkpoint activation failure in response to IR treatment. These candidates represent 4% of the total lines screened. Although we have proven the sensitivity of the screening 113 assay, it is possible that we have missed some candidates. One of the most important considerations is the timing for screening. We selected 32 hpf as our timepoint for the screen based on the level of proliferation occurring at this developmental stage. However, It is possible that some mutants that showed no change in pH3 staining could have phenotypes at a later developmental stage. On the other hand, some mutants that were not screened because they are highly necrotic at 32 hpf could be screened at earlier stages. In addition, it has been calculated that the zebrafish collection used for the screen represent 25% of all essential genes in this organism (Amsterdam et al., 2004). It is probable that there are other novel genes encoding checkpoint proteins that were not represented in our collection. Nevertheless, this screen was successful in identifying ticrr, a novel gene essential for activation of the IR-induced G2/M checkpoint. This gene is of particular interest for its additional roles during normal cell cycle progression. Ticrr is conserved among metazoans and we anticipate that it will play a role in checkpoints and cell cycle progression in other organisms, including humans. Ticrr is required for DNA replication and functions in a similar manner to Sld3 ticrr zebrafish have a defect in replication, demonstrated by the abnormal accumulation of S-phase cells in the mutant embryos and a significant decrease in BrdU incorporation. In addition, we showed that Ticrr is required for chromatin association of Psfl (part of GINS, a pre-IC component), but not for the association of the MCM2-7 proteins (a pre-RC component). This data suggests that Ticrr plays an important role in the conversion of the pre-RC into the pre-IC. This process is best understood in yeast, and involves the recruitment of a number of proteins to origins in a particular order. In metazoans, studying the mechanism of replication initiation has been more challenging 114 because origins are not well defined and a consensus sequence has not been identified. Additionally, identification of orthologs of the yeast replication factors Sid2 and SId3 has proven very difficult. RecQL4 has emerged as a candidate for the Sld2 ortholog in vertebrates. The N-terminal part of RecQL4 shows limited sequence conservation to Sld2 and this protein is essential for replication initiation in Xenopus egg extracts and human cells (Im et al., 2009; Sangrithi et al., 2005; Xu et al., 2009). Sld3 homology is poor among yeast species so it is not surprising that a homolog in higher eukaryotes could not be identified based on sequence similarity. Nevertheless, the fact that all the other known replication proteins and the fundamental aspects of replication are conserved suggests that an ortholog, or at least a functional analog, exists in higher eukaryotes. Intriguingly, Ticrr shares several characteristics with the yeast Sld3 protein. Similarly to Sid3, Ticrr plays an important role in the conversion of the pre-RC complex to pre-IC. Furthermore, human TICRR interacts with TopBP1, the human ortholog of Dpbl 1, through TopBP1 BRCT domains I and 11. This is reminiscent of the yeast Sld3, which interacts with Dpbl1 through the same BRCT domains, which are highly conserved from yeast to humans (Tanaka et al., 2007; Zegerman and Diffley, 2007). Despite the functional similarities between TICRR and SId3, we were unable to find significant sequence similarity. However, Sanchez-Pulido et al. recently showed that TICRR does in fact contain a domain that shares significant sequence homology with Sld3 (Sanchez-Pulido et al., 2010). Using a conserved region in metazoan Ticrr as the query, they found divergent plant homologs. Then they generated a profile of metazoan and plant Ticrr orthologs and used the HHpred method to compare it with a profile from the Sld3 protein family and found highly significant similarity. The authors concluded that this level of significance indicates that these proteins descend form a common ancestor prior to the divergence of the main eukaryotic lineages. Interestingly, the domain in SId3 115 that interacts with the TopBP1 yeast homolog Dpbl1 does not lie within this conserved domain (Tanaka et al., 2007; Zegerman and Diffley, 2007), nor does the region in TICRR that interacts with TopBP1 (my results, discussed in the Appendix). However, temperature sensitive mutants in yeast Sld3 that abrogate replication lie within this conserved domain suggesting that it is functionally significant and it may bind to a different factor (Sanchez-Pulido et al., 2010). Further studies are needed to elucidate what is the function of this region. Kumagai et al. identified the Xenopus ortholog of Ticrr, named Treslin, in a screen for TopBP1 interacting proteins (Kumagai et al., 2010). Consistent with our findings, they showed that depletion of Xenopus Ticrr/Treslin protein and the human counterpart causes a defect in replication and the pre-IC component Cdc45 is not recruited to chromatin. They also showed that Xenopus Ticrr/Treslin binds Cut5/TopBP1 through the first two BRCT domains in Cut5/TopBP1. This indicates that the function of Ticrr is highly conserved in vertebrates. We haven't directly tested whether the TopBP1 interaction with TICRR is required for TICRR's function in replication initiation. However, this seems very likely since a direct role for TopBP1 orthologs in the initiation of DNA replication has been described (Garcia et al., 2005). The Xenopus and human TopBP1 proteins are required for the recruitment of pre-IC components to chromatin, but not for the formation of the pre-RC (Hashimoto and Takisawa, 2003; Jeon et al., 2007; Matsuno et al., 2006; Van Hatten et al., 2002). Furthermore, the replication defect caused by depletion of TopBP1 in Xenopus egg extracts can be rescued by the N-terminal half of the protein containing BRCT domains I through V (Hashimoto et al., 2006; Yan et al., 2006). This implies that TopBP1 replication functions are all contained in the first half of the protein, which includes the BRCT motifs required for TICRR binding. TICRR mutants that fail to bind TopBP1 will be particularly useful for testing whether the TICRR-TopBP1 interaction is functionally significant, by assaying whether they can rescue the replication 116 defect caused by TICRR depletion. The role of CDK activity in regulating TICRR function in DNA replication It has been known for a long time that CDK activity is essential for replication initiation. Although in yeast SId2 and Sld3 are the minimum CDK targets for replication initiation (Tanaka et al., 2007; Zegerman and Diffley, 2007), in metazoans the CDK targets that are functionally significant for replication initiation remain elusive. The possibility that TICRR may be the functional analog of Sld3 raised a critical question: does CDK regulates TICRR function in an analogous manner, in particular, is TopBP1 binding to TICRR CDK dependent? We identified multiple putative CDK phosphorylation sites in the TICRR sequence; however, our experiments did not show that CDK activity is important for the interaction, even though we confirmed that TICRR is phosphorylated in vivo. Contrary to our findings, Kumagai et. al. reported that Xenopus Ticrr/Treslin binds TopBP1 in a phosphorylation and CDK2 dependent manner (Kumagai et al., 2010). Although the discrepancies between these results remain unclear, there are several possible explanations. First, it is possible that the precise regulation of TICRR function might have diverged in different organisms. There are notable differences in pre-IC regulation even between S. pombe and S. cerevisiae. For instance, Sld3 recruitment to origins is not Cdc45 dependent in fission yeast, whereas SId3 in budding yeast forms a complex with Cdc45 before associating to chromatin and their association is mutually dependent (Yabuuchi et al., 2006). It has not been addressed if the SId3 interaction with Cut5 (TopBP1 homolog) in fission yeast is dependent on CDK activity, but 3 putative phosphorylation sites were mutated in a study and disruption of at least those specific sites didn't affect chromosome replication (Nakijima et al., 2002). It is important to note that this result doesn't rule out that other phosphorylation sites in the 117 protein are required for replication. Alternatively, the difference could be accounted for by the fact that I performed experiments using cancer cell lines. Perhaps there is still a requirement for phosphorylation in normal human cells, but it is bypassed in tumor cells, where the regulation could be different. Lastly, there are caveats to the experiments that I performed that could explain why I didn't observed a disruption of the complex under the conditions used in my experiments. The treatment of cells with roscovitine may not have fully inhibited CDK activity; and/or the phosphatase may be unable to access the phosphorylated residue(s) mediating the TICRR-TopBP1 interaction, even though it effectively targets other phosphorylation sites. Other proteins with functional similarity to TICRR In the last year, two other proteins were identified that have similar characteristics to TICRR. GEMC1 is a novel vertebrate protein identified in Xenopus (Balestrini et al., 2010). Like TICRR, it interacts with TopBP1 and depletion from Xenopus egg extracts or from mammalian cells causes a defect in replication. Cdc45 and the GINS component Sld5 are not recruited to chromatin in GEMC1 depleted cells, indicating that this protein is also required for pre-IC formation. Similarly to Sld2 and Sld3, GEMC1 is phosphorylated by Cdk2 and a phosphomimetic form of GEMC1 stimulates TopBP1 binding, Cdc45 recruitment and DNA replication, indicating that these phosporylations are functionally significant. Nevertheless, the phosphomimetic mutant could not bypass the requirement for CDK2 in replication, suggesting that there are other targets required for initiation of DNA replication (Balestrini et al., 2010). DUE-B is yet another protein recently found to be required for replication initiation that interacts with TopBP1. Initially identified for its ability to bind DNA regions of predicted helical instability (DNA unwinding elements) (Casper et al., 2005), it is also required for Cdc45 loading to chromatin. In contrast to SId3, phosphorylation is not essential for DUE-B 118 interaction with TopBP1 (Chowdhury et al., 2010). An interesting question to investigate in the future is the relationship between TICRR and these other novel replication factors. Some issues that can be addressed are the order in which these proteins are recruited to chromatin during replication initiation, what proteins are required for their recruitment and whether they can bind to each other to form a functional complex or complexes required for replication initiation. It is possible that GEMC1 or DUE-B, or both of them, could be performing the role of Sld2 in vertebrates. The proposed ortholog of Sld2 in higher eukaryotes, RecQL4, is also required for replication initiation, but studies in Xenopus and human cells suggest that it doesn't share the same molecular function as its counterpart in yeast. Further studies are needed in order to understand better the role of these factors, including TICRR, during the initiation steps of DNA replication and how they might work together. We can anticipate that the process of replication initiation in vertebrates is more complex than that of yeast and although some aspects will be very similar, there will be notable differences. Possible roles of TICRR in the maintenance of genome stability The replication machinery works in conjunction with DNA damage checkpoint proteins to protect cells from possible genomic insults occurring during DNA replication. The replication proteins MCM7, RPA, DNA polymerase a, and TopBP1 have been shown to play a role in ATR-mediated checkpoint signaling. Mutations in replication proteins may indirectly affect the intra-S phase checkpoint because replication structures are required for checkpoint activation. On the other hand, certain proteins, such as TopBP1, have direct roles in mediating checkpoint activation. This dual role of TopBP1 has been demonstrated by defining separate domains encoding the replication and 119 checkpoint functions (Hashimoto et al., 2006; Yan et al., 2006). A recent screen performed in Drosophila identified several pre-IC components to be required for G2/M checkpoint signaling, including TopBP1, Cdc45 and RecQL4 (Kondo and Perrimon, 2011), suggesting that these proteins also have a role in checkpoints outside of Sphase. Ticrr fits into the category of proteins with a dual role in replication and checkpoints. We observed that zebrafish ticrr mutants have a number of phenotypes associated with genomic instability. Firstly, cells in the ticrr mutants continue to enter mitosis after IR treatment. Secondly, untreated cells that do not complete DNA synthesis due to a defect in replication fail to arrest and instead enter mitosis prematurely. Thirdly, abnormal mitotic figures and chromosomal fragmentation were detected in ticrr mutants. These phenotypes suggest that Ticrr plays an important role in checkpoint activation in response to DNA damage and during normal replication. It is possible, however, that abnormal mitosis and metaphase arrests in ticrr mutants lead to the persistence of mitotic cells after DNA damage regardless of the G2/M checkpoint. However, our results argue against this possibility, as the mitotic index of untreated ticrrzebrafish embryos did not greatly differ from wild-type zebrafish embryos at the timepoint chosen for the screen. We also confirmed that cells in ticrr embryos continue to enter mitosis after IR exposure using nocodazole, a drug that blocks cells in mitosis, which caused further accumulation of mitotic cells in the mutants. Therefore, the sustained presence of mitotic cells after DNA damage is unlikely to be a secondary consequence of DNA replication or S/M checkpoint defects, but caused by a bona fide G2/M checkpoint defect. We conclude that maternal stores contributions results in a hypomorphic phenotype in ticrr mutants, in which cell cycle progression is only moderately affected but the G2/M checkpoint is severely impaired during early stages of development. Based on the link that other replications proteins have with the ATR pathway, 120 including Ticrr's binding partner TopBP1, it seems plausible that TICRR may be involved in the ATR signaling pathway. Ticrr could have a role in the activation of ATR or recognition of downstream substrates. Although TopBP1 directly activates ATR in vitro in the absence of DNA, the recruitment of TopBP1 to chromatin is required for ATR activation in vivo (Kumagai et al., 2006; Lee and Dunphy, 2010). Several studies suggest that the 9-1-1 complex is involved in TopBP1's recruitment to chromatin, however, these studies do not rule out the possibility that additional proteins may be required for this process. Ticrr interacts with the same BRCT domains in TopBP1 that are required for Rad9 binding. It will be informative to know whether a complex containing both TICRR and Rad9 exist or the binding of these proteins to TopBP1 is mutually exclusive. In addition, TopBP1 has been associated with mediating the bridge between ATM and ATR signaling pathways in response to DSBs. ATM phosphorylation of a critical residue in the ATR-activation domain of TopBP1 is required for ATM-dependent activation of ATR in response to DNA damage (Yoo et al., 2007). Furthermore, human and Xenopus TopBP1 associate with the MRN component Nbsl (Morishima et al., 2007; Yoo et al., 2009) which mediates the recruitment of ATM to TopBP1 in response to DSBs and this has been suggested as a mechanism for the activation of ATR in response to DSBs (Yoo et al., 2009). Interestingly, the Nbsl interaction also occurs through the first two BRCT domains in TopBP1. Since Ticrr was found to be required for G2/M checkpoint activation in response to IR, which induces DSBs, an interesting possibility is that Ticrr may have a role mediating the activation of ATR through its interaction with TopBP1. Kumagai et al. observed that depletion of Ticrr/Treslin in human cells causes an increase in H2AX phosphorylation, indicative of DNA damage, which is consistent with 121 our findings that chromosomal fragmentation occurs in the ticrr zebrafish mutants (Kumagai et al., 2010). However, Chk1 fails to become phosphorylated in the activating residues S317 and S345 even after treatment with the drug aphidicolin, which induces stalled replication forks (Kumagai et al., 2010). These results suggest that, similarly to zebrafish Ticrr, the human ortholog is involved in checkpoint signaling. However, this study did not address the phosphorylation status of Chk2 in Ticrr depleted cells; therefore it is unclear whether this defect is specific for Chk1 and the molecular basis for this defect. In any case, this data suggest that the function of Ticrr in checkpoints is conserved in vertebrates. S. cerevisiae SId3 has been recently identified as a target of the intra-S phase checkpoint. In response to stalled replication forks, the Rad53 kinase (homolog of Chk2, downstream of Mecl/ATR) inhibits origin firing by phosphorylating SId3, preventing it from binding to Dpbl1 (Lopez-Mosqueda et al., 2010; Zegerman and Diffley, 2010). The yeast SId3 proteins have not been described to regulate other checkpoints. While it is not known whether Ticrr is targeted by checkpoints in a similar manner, I suspect that Ticrr will have a more general role in checkpoint signaling. The vertebrate TICRR proteins are considerably larger than the SId3 proteins, suggesting that they may have acquired additional interaction partner and additional functions through evolution. Identification of other Ticrr interactors will further amplify our understanding of the functions of this protein. References Amsterdam, A., Nissen, R.M., Sun, Z., Swindell, E.C., Farrington, S., and Hopkins, N. (2004). Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A 101, 12792-12797. Balestrini, A., Cosentino, C., Errico, A., Garner, E., and Costanzo, V. (2010). GEMC1 is a TopBP1-interacting protein required for chromosomal DNA replication. Nat Cell Biol 12, 484-491. Casper, J.M., Kemp, M.G., Ghosh, M., Randall, G.M., Vaillant, A., and Leffak, M. (2005). The c122 myc DNA-unwinding element-binding protein modulates the assembly of DNA replication complexes in vitro. J Biol Chem 280, 13071-13083. Chowdhury, A., Liu, G., Kemp, M., Chen, X., Katrangi, N., Myers, S., Ghosh, M., Yao, J., Gao, Y., Bubulya, P., et al. (2010). The DNA unwinding element binding protein DUE-B interacts with Cdc45 in preinitiation complex formation. Mol Cell Biol 30, 1495-1507. Garcia, V., Furuya, K., and Carr, A.M. (2005). Identification and functional analysis of TopBP1 and its homologs. DNA Repair (Amst) 4, 1227-1239. Hashimoto, Y., and Takisawa, H. (2003). Xenopus Cut5 is essential for a CDK-dependent process in the initiation of DNA replication. EMBO J 22, 2526-2535. Hashimoto, Y., Tsujimura, T., Sugino, A., and Takisawa, H. (2006). The phosphorylated Cterminal domain of Xenopus Cut5 directly mediates ATR-dependent activation of Chk1. Genes Cells 11, 993-1007. Im, J.S., Ki, S.H., Farina, A., Jung, D.S., Hurwitz, J., and Lee, J.K. (2009). Assembly of the Cdc45-Mcm2-7-GINS complex in human cells requires the Ctf4/And-1, RecQL4, and Mcm1O proteins. Proc Natl Acad Sci U S A 106, 15628-15632. Jeon, Y., Lee, K.Y., Ko, M.J., Lee, Y.S., Kang, S., and Hwang, D.S. (2007). Human TopBP1 participates in cyclin E/CDK2 activation and preinitiation complex assembly during G1/S transition. J Biol Chem 282, 14882-14890. Kondo, S., and Perrimon, N. (2011). A genome-wide RNAi screen identifies core components of the G-M DNA damage checkpoint. Sci Signal 4, rsl. Kumagai, A., Lee, J., Yoo, H.Y., and Dunphy, W.G. (2006). TopBP1 activates the ATR-ATRIP complex. Cell 124, 943-955. Kumagai, A., Shevchenko, A., and Dunphy, W.G. (2010). Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell 140, 349-359. Lee, J., and Dunphy, W.G. (2010). Rad17 plays a central role in establishment of the interaction between TopBP1 and the Rad9-Hus1-Radl complex at stalled replication forks. Mol Biol Cell 21, 926-935. Lopez-Mosqueda, J., Maas, N.L., Jonsson, Z.O., Defazio-Eli, L.G., Wohlschlegel, J., and Toczyski, D.P. (2010). Damage-induced phosphorylation of Sld3 is important to block late origin firing. Nature 467, 479-483. Matsuno, K., Kumano, M., Kubota, Y., Hashimoto, Y., and Takisawa, H. (2006). The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol Cell Biol 26, 4843-4852. Morishima, K., Sakamoto, S., Kobayashi, J., Izumi, H., Suda, T., Matsumoto, Y., Tauchi, H., ide, H., Komatsu, K., and Matsuura, S. (2007). TopBP1 associates with NBS1 and is involved in homologous recombination repair. Biochem Biophys Res Commun 362, 872-879. Nakajima, R., and Masukata, H. (2002). SpSId3 is required for loading and maintenance of SpCdc45 on chromatin in DNA replication in fission yeast. Mol Biol Cell 13, 1462-1472. Sanchez-Pulido, L., Diffley, J.F., and Ponting, C.P. (2010). Homology explains the functional similarities of Treslin/Ticrr and Sld3. Curr Biol 20, R509-510. 123 Sangrithi, M.N., Bernal, J.A., Madine, M., Philpott, A., Lee, J., Dunphy, W.G., and Venkitaraman, A.R. (2005). Initiation of DNA replication requires the RECQL4 protein mutated in RothmundThomson syndrome. Cell 121, 887-898. Sansam, C.L., Cruz, N.M., Danielian, P.S., Amsterdam, A., Lau, M.L., Hopkins, N., and Lees, J.A. (2010). A vertebrate gene, ticrr, is an essential checkpoint and replication regulator. Genes Dev 24, 183-194. Tanaka, S., Umemori, T., Hirai, K., Muramatsu, S., Kamimura, Y., and Araki, H. (2007). CDKdependent phosphorylation of SId2 and Sid3 initiates DNA replication in budding yeast. Nature 445, 328-332. Van Hatten, R.A., Tutter, A.V., Holway, A.H., Khederian, A.M., Walter, J.C., and Michael, W.M. (2002). The Xenopus Xmus101 protein is required for the recruitment of Cdc45 to origins of DNA replication. J Cell Biol 159, 541-547. Xu, X., Rochette, P.J., Feyissa, E.A., Su, T.V., and Liu, Y. (2009). MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J 28, 3005-3014. Yabuuchi, H., Yamada, Y., Uchida, T., Sunathvanichkul, T., Nakagawa, T., and Masukata, H. (2006). Ordered assembly of Sld3, GINS and Cdc45 is distinctly regulated by DDK and CDK for activation of replication origins. EMBO J 25, 4663-4674. Yan, S., Lindsay, H.D., and Michael, W.M. (2006). Direct requirement for Xmus101 in ATRmediated phosphorylation of Claspin bound Chk1 during checkpoint signaling. J Cell Biol 173, 181-186. Yoo, H.Y., Kumagai, A., Shevchenko, A., and Dunphy, W.G. (2007). Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J Biol Chem 282, 17501-17506. Yoo, H.Y., Kumagai, A., Shevchenko, A., and Dunphy, W.G. (2009). The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM. Mol Biol Cell 20, 2351-2360. Zegerman, P., and Diffley, J.F. (2007). Phosphorylation of Sld2 and SId3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445, 281-285. Zegerman, P., and Diffley, J.F. (2010). Checkpoint-dependent inhibition of DNA replication initiation by Sid3 and Dbf4 phosphorylation. Nature 467, 474-478. 124 APPENDIX A: Characterizing the functional significance of the TICRR-TopBP1 interaction Nelly M. Cruz and Jacqueline A. Lees 125 We identified TopBP1 as a binding partner of the zebrafish and human TICRR proteins. TopBP1 is a multifunctional protein with key roles in replication and ATRmediated checkpoint signaling. Little is known about the biological significance of TopBP1's interaction with TICRR. This appendix contains data that further characterizes the TICRR-TopBP1 interaction and investigates mechanisms by which TICRR regulates replication initiation in human cells. Results and discussion The TopBP1 interaction domain lies within the C-terminal half of TICRR Having determined the region of TopBP1 that associates with TICRR, we sought to define the region of TICRR participating in this interaction. Human TICRR is a large 220 KDa protein that based on sequence analyses has no identifiable functional domains. We prepared GFP tagged fragments of TICRR containing either the N-terminal half, (residues 1-969), or the C-terminal half (residues 970-1910) of the protein and cotransfected these fragments with full-length TopBP1 into 293T cells. By immunoprecipitating the tagged TICRR protein with anti-GFP antibody, we observed that TopBP1 binds to the C-terminal but not the N-terminal fragment of TICRR (Figure 1). We then tested several smaller C-terminal fragments of TICRR to define more precisely the minimum requirements for binding to TopBP1. We observed that deletion of residues 1565 to 1910 of TICRR, which excises the very end of the C-terminus, did not affect the binding of TopBP1 (Figure 1). However, any deletion that removed residues 976-1565 dramatically reduced the interaction with TopBP1 (Figure 1 and data not shown). Importantly, a fragment consisting of residues 976-1261 binds to TopBP1, indicating that this region is sufficient for the interaction (Figure 1). 126 976 WT 1261 1565 1910 IP: a-GFP TopP1 indng + -M 2 3 +-n -: 6 + - Figure 1. Residues 976-1261 of TICRR protein are required and sufficient for TopBPI binding. (A) Schematic diagram of TICRR truncations and summary of the abilities of the indicated forms to interact with TopBP1. (B) Anti-GFP immunoprecipitates from cells transfected with GFPtagged TICRR truncation mutants were screened for TICRR and TopBP1 proteins by immunoblotting. Two putative CDK phosphorylationsites are highly conserved in TICRR There are many consensus CDK sites within the TICRR sequence. Interestingly, the vast majority of these sites are located within the C-terminus of the protein. Furthermore, we identified a proline-rich region in which many of these sites are clustered. This region contains 54 serine or threonine residues followed by proline, the minimal CDK consensus site; 13 of these also contained an arginine or lysine two positions downstream of the proline, which is the full consensus site for CDK phosphorylation. Interestingly, this region spans the TopBP1 interaction domain identified by our binding assays. Sanchez-Pulido and colleagues found significant homology between the TICRR 127 and Sld3 sequences (Sanchez-Pulido et al., 2010). CDK phsophorylation of Sld3 at two sites, Thr 600 and Ser 622, is required for Dpbl 1 TopBP1 binding to SId3 and subsequent pre-IC formation. As described in Chapter 3, we examined whether phosphorylation is required for the TICRR-TopBP1 complex formation by treating cells with the CDK inhibitor roscovitine as well as phosphatase treatment of cellular lysates, but did not detect a disruption in the interaction. We note that there are limitations to this experimental approach (discussed in Chapter 3). Therefore, we have decided to use a more direct approach in order to test if phosphorylation plays a role in TICRR's replication function. By searching the TICRR protein sequences for highly conserved CDK consensus motifs, we identified a consensus sequence containing serine 1001 (in human TICRR) that is present in all metazoan species examined, including the primitive chordates Branchiostoma floridae and Ciona intestinalis (Figure 2). We aligned this sequence with the CDK sites in S. cerevisiae Sld3 required for replication and found that it is highly similar to the consensus sequence of Sld3's Serine 622 site (Figure 2). We identified a second consensus sequence highly conserved across TICRR metazoan species 32 residues upstream of Ser 001 (Threonine 969). Although the conservation is low, there is similarity between this consensus sequence and the sequence encompassing Sld3's Thr 600 (Figure 2). To test whether these conserved sites play a role in TopBP1 interaction, we replaced them with alanine residues, which cannot be phosphorylated. Co- immunoprecipitation experiments showed that there was no significant difference in the binding capabilities of TICRR when either site was changed to alanine (Figure 3). In contrast, the double mutant had reduced binding to TopBP1. It is important to note that the interaction was not completely disrupted by these mutations (Figure 3). It is possible that other phosphorylation sites or sequences are contributing to the interaction and loss of phosphorylation at these two sites is not sufficient for complete disruption of the 128 complex. Alternatively, these amino acids may have an important function in replication initiation that is independent of TopBP1. As previously discussed, the mechanistic details of replication initiation differ among species. We are currently developing a robust system for performing rescue experiments to examine whether these phosphorylation sites play a role in TICRR's function. Homo sapiens Mus musculus Gallus gallus Xenopus laevis Gasterosteus aculeatus Takifugu rubripes Danio rerio Branchiostoma floridae Capitella sp Nematostella vectensis Strongylocentrotus purpuratus xodes scapularis Lottia gigantea Ciona intestinalis Schistosoma mansoni Saccharomyces cerevisiae Homo sapiens Mus musculus Gallus gallus Xenopus laevis Gasterosteus aculeatus Takifugu rubripes Danio rerio Branchiostoma floridae Capitella sp Nematostella vectensis Strongylocentrotus purpuratus Ixodes scapularis Lottia gigantea Ciona intestinalis Schistosoma mansoni Saccharomyces cerevisiae K74A' P' 74vAET:3 2RCZAAI:A-~ V2 LVL K \ "r:qN~ K :K2- IvEEZ PEK 'iRDIDLR. IvEEC'vK ADADL:G c-AD- DL: a - 7LK IEE v CDDO -KDDEv L Figure 2. Sequence alignment of conserved CDK consensus motifs in the TICRR protein. Sequence alignements of putative CDK motifs for Thr 969 (A) and Ser 1001 (B). Sld3 sequences including the CDK sites Ser600 (A) and Thr622 (B) were included in the alignment for comparison. 129 IP: a-GFP N Figure 3. Mutation of Thr 969 and Ser 1001 in the TICRR protein causes a reduction in TopBP1 binding Thr 969 and Ser 1001 were changed to alanine redues in the GFP-tagged TICRR protein. AntiGFP immunoprecipitates from cells transfected with GFP-tagged TICRR truncation mutants were screened for TICRR and TopBP1 proteins by immunoblotting. To test the roles of TICRR in replication, we used a siRNA that was described by Kumagai and colleagues (Kumagai et al., 2010) to knockdown the TICRR protein in human cells. Using these cells we could introduce mutant forms of TICRR and assay which domains of TICRR are required for replication. This siRNA causes robust depletion of the TICRR protein 48 hours after transfection (Figure 4A). We confirmed the replication defect previously observed by Kumagai et al. that is consistent with the replication defect observed in the zebrafish ticrr mutants. Specifically, we compared the ability of U2OS cells transfected with siRNA against TICRR (siTICRR) or a control siRNA (siControl) to incorporate the nucleotide analog bromodeoxyuridine (BrdU). Cells transfected with siTICRR incorporated relatively little BrdU, in comparison with control cells even though a significant fraction of the cells contained between 2N and 4N DNA content (Figure 4B). This demonstrates that TICRR-deficient cells are impaired for DNA replication. 130 +TICRR TICRR TopBP1 +siResTICRR - siControl siTICRR 21.1% 5.91% - . - --. DNA content (PI) Figure 4. Loss of TICRR in human cells causes a replication defect. (A) U2OS cells were transfected either with a control siRNA or TICRR-specific siRNA, along with wild-type TICRR or a form of TICRR that is resistant to knockdown. There is robust decrease of the wild-type TICRR protein but not of siRNA resistant TICRR (sires-TICRR) 48 hours after transfection. TopBP1 protein levels are shown as a loading control. (B) BrdU and PI FACS analysis of BrdU pulsed labeled siControl and siTICRR cells. Quantification of the BrdU+ population showed a dramatic reduction in replicating DNA in siTICRR cells. We used FACS to determine the DNA content of phosho-histone H3 positive cells during normal cell cycle progression in TICRR depleted cells (Figure 5). As expected, histone H3 phosphorylation is restricted to cells with 4N DNA content in control cells (Figure 5). In contrast, we find that a high percentage of the pH3-positive cells in the TICRR deficient cells have less than 4N DNA content, indicative of a S/M checkpoint defect. This phenotype is equivalent to the defect observed in cells isolated from ticrr mutant zebrafish embryos, further confirming that TICRR's roles are conserved among vertebrates. 131 DNA content (PI) Figure 5. Ticrr is required for S/M checkpoint function in human cells. FACS measurement of anti-pH3 and PI staining of siControl and siTICRR cells showed a population of mutant pH3-positive cells with less than 4N DNA content in TICRR deficient cells, indicative of entry into mitosis before completing DNA replication. TICRR-depleted cells underwent morphological changes including enlargement and flattening of the cells on culture dishes (Figure 6). These changes started to become noticeable 48 hours after transfection of the TICRR siRNA. The size of the nuclei of TICRR-deficient cells increased by as much as 3-4-fold relative to the control cells. These morphological changes are the typical phenotypes of senescent cells (Hwang et al., 2009). Consistent with these morphological changes, TICRR-deficient cells stained positive in a senescence associated P-galactosidase assay (Figure 6). Importantly, the control cells did not undergo any morphological changes and did not stain in the pgalactosidase assay (Figure 6). Most likely, this senescence phenotype is a consequence of DNA damage accumulating in TICRR-depleted cells due to defects in replication and activation of the S/M checkpoint. 132 siControl siTICRR Figure 6. TICRR knockdown induces cellular senescence in U2OS cells U2OS human cell lines were transfected with control siRNA or TICRR-specific siRNA, every 4 days for a period of 12 days. Cells were stained with p-galactosidase stain and viewed under a phase contrast microscope. In summary, TICRR deficiency causes replication and S/M checkpoint defects, as well as cellular senescence in U2OS cells. These phenotypes can be assayed for rescue upon the expression of different mutant forms of TICRR, including phosphomutants and truncations that are defective for TopBP1 binding. These assays will enable the identification of functional domains within the TICRR protein. A TICRR mutant that localizes to PCNA and RPA foci Biochemical experiments have shown that TICRR is a chromatin-associated protein. We noticed that the GFP-TICRR fusion protein consisting of residues 1565-1910 1910), the C-terminus (TICRR' 56 5of the protein, localizes to foci within the nucleus while wild-type GFP-TICRR localization is diffuse throughout the nucleus (Figure 7). A large 19 ' 0 had foci and the size percentage of cells expressing GFP-TICRR' 565and number of these varied between cells. Nuclear foci are characteristic of sites of ongoing replication, as well as the sites of stalled replication forks and DNA damage. We sought to determine what these foci might be by performing co-localization experiments using 133 indirect immunofluorescence to locate proteins known to localize to nuclear foci. We stained for phosphorylated H2AX (6H2AX), a marker of double strand breaks and the replication proteins RPA and PCNA. In the absence of DNA damage, we did not detect increased H2AX in cells transfected with GFP-TICRR156 1910 , indicating that there is no increase in DNA damage in these cells (data not shown). When treated with yIR, GFP positive cells accumulated 6H2AX foci in a comparable manner to GFP negative cells (Figure 8). However, GFP-TICRR'5 51910 foci did not colocalize with ylR-induced 6H2AX foci. In addition, IR treatment did not induce any obvious changes in the foci pattern of 910 (Figure 8). GFP-TICRR' 565-1 GFP DAPI TICRR: Figure 7. GFP-TICRR 565 '9'0 Merge 1565 1910 I 1 localizes to foci within the nucleus. GFP expression of cells tranfected with full length GFP-TICRR or a truncation containing residues 1565-1910. A schematic representation of the TICRR protein showing the location of the truncated fragment in orange is included. 134 GFP-TICRR 1565-1910 8-H2AX Figure 8. GFP-TICRR'*5-'9'* foci do not colocalizes with 8H2AX. U2OS cells transfected with GFP-TICRR1565-19 10 were treated with ionizing radiation, followed by methanol fixation and staining for 6H2AX one hour after treatment. On the other hand, the GFP-TICRR' 5 ,1910 foci co-localized with PCNA and RPA 910 foci in unperturbed cells (Figure 9). This indicates that TICRR' 719~1 is localizing to either sites of ongoing DNA replication or sites of stalled replication forks. The hypothesis that these foci represent stalled replication forks seems unlikely because the GFP-TICRR' 565-1910 positive cells did not accumulate 8H2AX foci in the absence of ylR. H2AX is phosphorylated at sites of stalled replication forks and forms foci that colocalize with RPA after replication inhibition (Balajee and Geard, 2004). We analyzed the cell cycle of cells transfected with GFP-tagged wild-type or 135 TICRR 565 1910 ~ (Figure 11). Cells expressing TICRR 1565-' 10 showed an increased accumulation of cells containing between 2N and 4N DNA content when compared to GFP negative cells within the same population, or with cells expressing wild-type TICRR (Figure 10), indicating that expression of the truncated form of TICRR is causing a defect during replication. A BrdU incorporation assay will be informative to test whether replication is occurring or whether cells are blocked in S-phase. It is possible that replication is occurring at a slower rate, leading to the accumulation of cells in S phase. Alternatively, a regulatory mechanism of origin firing may have been lost, and more cells are undergoing replication. This mutant may be exerting its dominant effect by sequestering an important factor for replication. Given that this fragment of the protein is lacking the region that binds to TopBP1 it is likely that the primary factor being sequestered is not TopBP1. It will be informative to elucidate what are the binding partners of this TICRR fragment and whether these interactions reflect functions of wild-type TICRR during replication. Currently, we do not know if wild-type TICRR localizes to replication foci, whether it moves with replication forks during normal replication or if it re-localizes to replication forks when replication is inhibited. All these questions merit further investigation and the 1910 fusion protein GFP-TICRR 1565could be a useful tool for dissecting the roles of TICRR in replication. 136 GFP-TICRR 1565-1910 RPA GFP-TICRR 1565-1910 PCNA Figure 9. GFP-TICRR' 565 1910 foci colocalizes with RPA and PCNA. U2OS cells transfected with GFP-TICRR 1 .19'. were stained for RPA (A) and PCNA (B). 137 100% G2/M ~~80% Us 70% 2 60% U 50% 0 40% U Gi 30% 20% 10% WT GFP- WT GFP+ Mut GFP- Mut GFP+ DNA content (PI) Figure 10. Expression of GFP-TICRR 565 '9'0 impairs S-phase progression. 65 (A) Cell cycle profiles from cells expressing GFP-tagged wild-type TICRR and GFP-TICRR'15 65 ~ 1910. Quantification of the GI, S or G2/M DNA content of wild type TICRR or GFP-TICRR ~ 19 10 transfected cells reveals an increase in S-phase cells in the population expressing GFPTICRR 565-1910 . Materials and methods Plasmid construction Restricition enzymes were used to cut the TICRR sequnece into fragments that were cloned into pENTR/D-TOPO, and then into pDest-GFP expression vector using the Gateway recombianation cloning technology (Invitrogen). Human cell culture and transfections 293FS and U2OS cells were grow n in DMEM supplemented with 10% FBS. Lipofectamine RNAiMAX from Invitrogen was used, following the manufacturer's instructions, to transfect 25nM siRN As into U2OS cells. DNA transfections were 138 performed using Mirus TranslT-LT1 transfection reagent. FACS analysis For BrdU labeling, U2OS cells were incubated with 10mM BrdU/1 5% DMSO on ice for 30 minutes, washed and incubated at 28.50C for 15 minutes. Cells were fixed in 70% ethanol at -20*C overnight. Samples for pH3/propidium iodide or BrdU/propidium iodide FACS analysis were prepared as described (Pozarowski and Darzynkiewicz 2004). FACS analysis was conducted by FACScan (Becton-Dickinson). DNA content was quantified by ModFit LT (Verity Software), and pH3 and BrdU staining was quantified using FlowJo (Tree Star, Inc.). Immunoprecipitation 293FS cells were lysed in modified RIPA buffer (50 mM Tris-HCL [pH 8.0], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.1% SDS, 0.1 mM PMSF, protease inhibitor cocktail (Roche) 1 mM NaF, 10 mM p-glycerophosphate (b-GP), 200 [M Na3 VO 4 ) for 30 minutes in ice. The soluble supernatant was used for immunoprecipitations with anti-GFP antibody (11814460001, Roche). The immunoprecipitates were washed four times with RIPA and resuspended in 2X Laemmli Buffer. Immunofluorescence U20S cells were plated on coverslips and fixed with cold methanol for 10 minutes at 200C. For PCNA, soluble proteins were pre-extracted prior to methanol fixation as described (DiMicco, RD et al. 2006). Antibodies used were a-PCNA (PC10) from Santa Cruz, a-5H2AX from Upstate (#05-636) and a-RPA (Calbiochem, NA19L). Coverslips were mounted for imaging with Vectashield containing 1 ug/ml DAPI on a glass 139 microcope slide. Slides were examined using a Zeiss Axioplan 11confocal microscope. Antibodies The following antibodies were used in this study: phosho-H3 (sc-8656-R, Santa Cruz), TopBP1 (NB100-217, Novus Biologicals and sc-32923, Santa Cruz), GFP (11814460001, Roche), PCNA (PC10, Santa Cruz), 6H2AX (Upstate #05-636) and RPA (Calbiochem, NA1 9L). Acknowledgements I would like to thank members of the Lees lab for helpful comments and discussions. This work was supported by Ruth L. Kirschstein NRSAs to N.C. J.A.L. is a Daniel K. Ludwig Scholar. References Balajee, A.S., and Geard, C.R. (2004). Replication protein A and gamma-H2AX foci assembly is triggered by cellular response to DNA double-strand breaks. Exp Cell Res 300, 320-334. Hwang, E.S., Yoon, G., and Kang, H.T. (2009). A comparative analysis of the cell biology of senescence and aging. Cell Mol Life Sci 66, 2503-2524. Kumagai, A., Shevchenko, A., and Dunphy, W.G. (2010). Treslin collaborates with TopBP1 in triggering the initiation of DNA replication. Cell 140, 349-359. Pozarowski, P., and Darzynkiewicz, Z. (2004). Analysis of cell cycle by flow cytometry. Methods Mol Biol 281, 301-311. Sanchez-Pulido, L., Diffley, J.F., and Ponting, C.P. (2010). Homology explains the functional similarities of Treslin/Ticrr and Sld3. Curr Biol 20, R509-510. 140 141