Document 11151719
advertisement

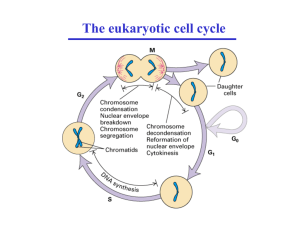
Switches, Oscillators, and the Cell Cycle What to notice so far • There are two ways to design a regulatory cell network: • (1) protein-protein interactions (mutual phosphorylation, etc etc) (time scale: secmin) • (2) gene networks (time scale: hrs day) Gene circuits Protein circuits Protein circuits Kinase M phosphatase Mp Other things to notice • By building up feedback interactions it is possible to obtain new dynamics : • (1) Simple decay to steady state • (2) Switch (bistability) • (3) Oscillator (stable cycles) No feedback x Decay to a single stable steady state No feedback x Decay to a single stable steady state A Positive feedback x Bistability and switch-like behaviour possible A Add negative feedback x y Stable cycles possible Example: Phosphorylation cycle Kinase Inactive M phosphatase Mp Active Decay to a single stable steady state Add positive feedback to kinase Kinase M phosphatase Mp Bistability and switch-like behaviour possible Add further negative feedback Kinase M phosphatase Mp Stable cycles possible Simple mathematical example x A A switch (Generic bistability) The parameter A controls the switch A controls the switch “Switch” (Generic bistability) y The “parameter” y controls the switch The curve dx/dt=0 y y controls the switch y As y varies, we can go around the hysteresis loop y Add negative feedback to the switch x y Now y is dynamic ] x y Switch becomes an oscillator ] Example: This is the Fitzhugh Nagumo model “Switch” (Generic bistability) Bifurcation diagram y “Switch” (Generic bistability) Let us flip it over y “Switch” (Generic bistability) y y The xy phase plane The curve dy/dt=0 The curve dx/dt=0 Oscillator a=0.7, b=0.8, c=3, j=0.35 Get an oscillator Application to the Cell Cycle • Work by John Tyson (Virginia Tech): • The control of the cell division is maintained by an intricate web of signaling pathways, that incorporates many signals to decide when to divide. • The cycle has “checkpoints” at which decisions are made. G1 Slide by John Tyson cell division 1) Alternation of S phase and M phase. 2) Balanced growth and division. M mitosis S DNA replication G2 G1 Slide by John Tyson cell division The cell cycle is the sequence of events whereby a growing cell replicates all its components and divides them more-or-less evenly between two daughter cells ... M mitosis S DNA replication G2 G1 Slide by John Tyson cell division S Cyclin-dependent kinase Cyclin B P M mitosis DNA replication Cdk1 CycB P G2 P Wee1 Cdc20 Cdc14 Wee1 CycB Cdc25 TFBI APC APC-P Cdc14 P CycB Cdc14 CKI Cdc25 CycD CycB CycE TFII Cdh1 TFIA CKI CKI CycA CycD CKI P TFBA CycE CycA Cyc E,A,B CycE CycA TFEA CycB CycD CycA TFEI Checkpoints in phase G1 there is low Cdk and low cyclin buildup of cyclin/Cdk Cdk1 CycB APC is activated, leading to destruction of cyclin and loss of CdK activity. Cyclin is produced and degraded APC is inactivated by phosphorylation Cyclin Active form APC (no phosphate) pi Inactive form APC is inactivated by phosphorylation Cyclin APC This will be modeled by a typical equation that we have already seen. pi Schematic Cyclin Cyclin APC pi Negative feedback Cyclin APC pi APC and Cyclin mutually antagonistic Cyclin APC pi Model Model Model Bistable switch Cyclin Cell Mass Cell mass is the parameter that flips the switch Cyclin Cell Mass P Wee1 Cdc20 Cdc14 Wee1 CycB TFBI APC APC-P Cdc14 P Cdc25 CycB Cdc14 Cdc25 CKI Bistable switch CycD CycB CycE M TFIA CKI CycA CycD 10-1 CKI Cdc2/Cdc13 CKI S/G2 10-2 CycE CycA Cyc E,A,B 10-3 CycE 0 1 [cyclin] mass/DNA TFEA CycA 2 CycB CycD CycA TFII Cdh1 100 [kinase] P TFBA TFEI Activation of APC by Cdc20 (“A”) cYclin A A= Cdc20. It increases sharply during metaphase and activates APC A is turned on by cyclin (sigmoidally) cYclin A Activation of APC by Cdc20 (“A”) cYclin A A= Cdc20. It increases sharply during metaphase and activates APC Three variable model: Now we get a cell cycle.