co 0) I ~
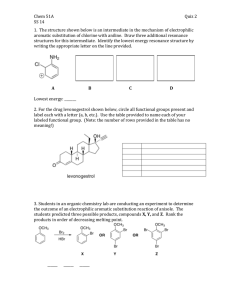
advertisement

4.
Answer the questions in the table below for the compounds Indole and Quinoline (8 points)
!
co
0)
..
Indole
Quinoline
I~
N
\
H
Number of 'It electrons?
Aromatic? Yes or No
What type of orbital is the
nitrogen lone pair in?
{o
~es
p
~
.&
N
(0
YRS
"5f'­
Which of the two compounds is the stronger base and why? 5. Predict the major product in the reaction below and provide a complete mechanism that
accounts for its formation. The mechanism must show movement of electrons with curved
arrows, any non-zero formal charges, and all intermediates. Identify the electrophilic
species in the reaction and show, using curved arrows, how AICh facilitates its formation.
(6 points)
:M 1\
•• Q
+
~-c -~ ~-;iA1-CR
(
0-
~_~_~'f_ __
Name:_ _ _ _
7 Mar 2016
Prof. Urban
5C226 Exam 2 Chap 16 17
1. The structures shown below represent the cycloheptatrienyl (tropylium) cation and anion. Use
Huckel's Rule and Molecular Orbital theory to classify each as either aromatic or antiaromatic.
Draw the energy levels for the 1t MO's and populate them with electrons to justify your answer.
(8 points)
o
+
a.
Cerr€.­
"1 P AU '> -=-lTfr\Os
-1t,
-lIv
-i\«
-
4)
A-~tI\kll c
..1k:... i' 'I.- Jx if}
A(liJMk't1 L­
kII,
­
b.
o
1v-
~I
--I \
(
2.
c..f l'\
+1..
( 0
JJ
no"", L,w...
<fr- rr e-
ft:\ 1'r5 )
Which of the following are predicted by Huckel's Rule to be aromatic? Circle all that apply.
(5 points)
+
0
~t1
0
~
~#
Jf
e.­
+
()
()A~L
LVlok- ~~\~ ~.
+
til e­
iT~-
0f00+
())\~ I'~)
A:-{lilM;fi1
L
3. Rank the compounds below in their expected rate in electrophilic aromatic substitution
reaction. (1 = fastest, 4 = slowest) (4
\ .e.\ II).
('I'o~tl(l.\ U ~
k\v(,..X\.rvf1
oY
5'
CH 3
NH2
pointsL
vt. v'" o..( /P vI/<
d
-ti 1'-'\
J..:v"'+~
° fA. c\J..: vI!.1-:v-'\..
\ 0
() \ qj)f.t"P
N0 2
1
)l
/(!,
HN
CH 3
6 6 6 6
\
~
ki--oJ'/
i\Q)J
. rY"
Ifr
GC(.-z,
/'0
~~ Draw the arenium ion intermediate that results from nitration at the para position of
~
trifluoromethylbenzene. (9 points)
vJ' ~
a. Draw the arenium ion's resonance structures.
~ b.
r{vrt
~
V\\..J
p-" It 0- '\'" >2!CI'<'
~ c. ~
\~ Predict and explain the effect of the trifluoromethyl group on rate or reaction relative to
benzene (Le., is the ring activated or deactivated and why?).
r~3
L~
~~-4 ~ ~ ~ (f)~o
3
6
HN03.
a..) I /':
~
HzS04 ~
-
H
~
.
CF~ d < S1~~\ i'),5
~ ~~ 1­
tJt'L.
~ Io.,.~.
"" s I,,,, V
wil\
eW&­
f'I-t.fe..-di' ed"O 10+ d~s ~ bil ;as
i"
~
/ ' - /.Jlj
~
~
e F, ,S cllfU;t;vo.+'- O. \2..h
b)
c. F3
c,F,
ortho/para-directing and why?).
1.)
CF
l
Predict and explain the reaction's regiochemistry (i.e., is the CF3 group meta-directing or
c.
@
c.~"''1-
d-
0/f I'O~r+; 1M"
7. The reaction below is a nucleophilic aromatic substitution.
Q+ NOz
HO
Q
•
NOz
a.
Provide a complete mechanism for the reaction using curved arrows. Show formal
charges and re~?nance structures for any inter~ediates, if appropriate. (6 pOints)
~r-~
y
~
:O\\
.. ----'/
\\
-, rJO'l-
~p.5 '9~ !~H
/:
<./~0
E:;i* -/ \\ =:-J
~
1.-
~
r--
I)
\>
Oil.
••Q
f\~
.r_\.2~ -~
~
rJ:::-D~
I'~ .. G):,r;>.' b. Ot\
4>J
-
F.- () ~
.,J
~fl
N°l..­
<'~ k:l q,.-lJ
\""":>~-
~
6Q
"\
~
..
/IJ==O
+- ~-
I
-/Jot­
FQo\
II
".0
Is the compound below expectedt~tTh~a 'faster or slower rate in this reaction?
e~
,. \\
lZ,'o/@l;'
"
_D·
p 0V dRf
~-fR u;1f bv slNiJ'-. MDt. 1:;~ r
10 ~
tiA ~b 'j;2A.~-M ~ V'~ w~fll1 ;{-- I~ {YI.e-k­
rr;u. 5,
e... -h> ~ F-), /~ c/.l~ vdA#­
Explain. (3 points)
I
I
(}r
aJ..~ Cor~O ~r~ sfcv-efwu!. ~II\ ~
(a-J-
V'rvfY A.
I.wf (IV
.
,5
..fI»-tMJ.~
8. Fill in the blanks. Provide the missing product(s), starting materials, or reagents. Indicate
stereochemistry and/or product mixtures (major vs minor products) as appropriate. (40 points)
...... t6~\A
1) KMn04 • NaOH. 6
•
2) Hp+
O-C01l-\
?
Zn(Hg)
..
HCI
?
o
(Jlc~
Br,
..
FeBr3
?
..
H
0
~~y+~cl
..
~oV
Nv.IJO'l-) ~
0
.. ?
NI
~
yCI
?
N0 2 N0 2
GVC,tJ
6
e;.1,.. ) ~eJ'S
..
~I
9
CI
N=N+ NO z
H,
--Pt-­
?
..
?
CN
6
9. Provide a synthesis of these compounds from the indicated starting materials. More than one
step may be necessary. Your synthesis may produce both enantiomers of any target that is
chiral. (B points)
.;5
from
0
I..\,JIJ/J/\, ko~
"7
OH
b.
&
//
from
CI
0
NO'\..­
~)
l-=::
I
f{ IUO,
:>
l-i 1 50'-( cPp..
\~
>
-/
ptC1 3
10. Are the syntheses below expected to work as planned? Or, is there a flaw in the design? Will it
work? Yes or No. For any that you answer "No", explain the error in the synthetic design.
(6 points)
0
,\~1
b.
>-CI,. Alels
(5 -
-
H2SO4 ­
HN03
/::;
N0 2 H2SO4
H2SO4 S03H
S03H
Hp /::;
&~,
1.0