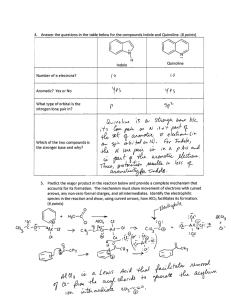
Hydrodenitrogenation of indole and quinoline with a nickelous chloride -... catalyst system
advertisement

Hydrodenitrogenation of indole and quinoline with a nickelous chloride - gaseous hydrochloric acid
catalyst system
by Thomas Joseph Buller
A thesis submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of
MASTER OF SCIENCE in Chemical Engineering
Montana State University
© Copyright by Thomas Joseph Buller (1973)
Abstract:
A supported NiCl2- gaseous HC1 catalyst was used in the hydro-denitrogenation reactions of indole, a
nonbasic compound, and quinoline, a basic compound. The reactions were carried out at a reactor
temperature of 400&deg,C; a reactor pressure of 850 psig; space velocities of 4.0, 1.6, 1.0, and 0.7275
LHSV; a hydrogen rate of 500 SCF/bbl of liquid feed; a chloride to nitrogen content of eight to one;
and using p-xylene as the carrier oil.
Nitrogen compositions were determined by a Kjeldahl analysis and a Mohr test was used to determine
chloride contents. The amine forms of basic and nonbasic nitrogen compounds from indole and
quinoline runs were isolated. These compounds were then identified using gas chromatography and
mass spectrometry.
In this research, nitrogen was found easier to remove from the product oil of quinoline than indole at
4.0 LHSV. However, little difference, was' observed for lower -space velocities.
Different reaction mechanisms were proposed for the denitrogenation of indole and quinoline. It
appeared that hydrochloride intermediates are present in the reaction sequences and that nitrogen leaves
the reaction zone as ammonium chloride. Also, possible limiting steps in the reaction sequences were
proposed. In presenting this thesis in partial fulfillment of the require­
ments for an advanced degree at Montana State University, I agree
that the library shall make it freely available for inspection. I
further agree that permission for extensive copying of this thesis for
scholarly purposes may be granted by my major professor, or, in his
absence, by the Director of Libraries.'
It is understood that any
copying or publication of this thesis for financial gain shall not be
allowed without my written permission.
'i
HYDRODEHTROGEMTIOIf OF IIfDOLE AHD QUIWOLIIfE
WITH A WICKELOUS CHLORIDE - GASEOUS HYDROCHLORIC
ACID CATALYST SYSTEM
"by
Thomas Joseph Buller
A thesis submitted to the Graduate Faculty in partial
fulfillment of the requirements for the degree
of
MASTER OF SCIEWCE
in
Chemical Engineering
\
Apprrared:
Head, Major Department
Chairman, Examing Committee
Graduate“Dean
MOWTAWA STATE UHIVERSITY
Bozeman, Montana
December, 1973
iii
ACKNOWLEDGEMENTS
I would like ter,thank the entire Chemical Engineering staff,
particularly my advisor Dr. F.P. Mc Candless > for their help and
support in this project. 'I would like to thank Mr. J. Tillery andMr. A. Huso for maintaining and repairing the equipment.
Also, I would like to thank the Chemistry departments at hoth
Montana State University and the University of Montana for allowing me
to use their mass spectrometers.
Finally, I would like to thank the Department of Chemical
Engineering and the Petroleum Research Fund for financial support
in this research.
iv
TABLE OF CONTENTS
page
List of T a b l e s ....................
vi
List of F i g u r e s ............................................... vii
A b s t r a c t ...................
I.
II.
III.
ix
Introduction ............................................
I
A.
B a c k g r o u n d ..........................................
I
B.
T h e o r y ......................
3
Research Objectives . . .
.............................
Experimental Apparatus, Procedure, and Analyses.
A.
B.
Materials
8
...
9
• • '...............* ...................
9
1.
Charge S t o c k ............
9
2.
Catalyst and F i l l e r .........................
10
Equipment . . . . . . . . . . . . . . . . . . . .
10
o
C.
D.
IV.
Operating Procedures . . . . . . .
...............
13
1.
Process Conditions ...........................
13
2.
Reactor Charging ........................
14
3.
Reactor Operation
I^
............................
Analytical Methods ............................ .
.
15
Results and D i s c u s s i o n ................................
17
A.
Nitrogen and Chlorine Analyses.....................
17
B.
Identification of C o m p o u n d s ........ .
23
I.
...........
I n t r o d u c t i o n .................... . . . .
23
V
page
2.
Basic Compounds from Quinoline Buna . . . . . . .
23
3.
IfonBasic Compounds from Quinoline Runs
29
ty.
Basic Compounds from IndoleR u n s ................ 33
5-
Ifonbasic Compounds fromIndole R u n s .............. '36
. . . .
6 . Summary ........................................... 38
C.
V.
Reaction M e c h a n i s m s .................................. 39
Summary and Conclusions
VI.
VII.
VIII.
................................... 48
Recommendations for Future W o r k .....................
A p p e n d i x ................................................. 50
Literature Cited
............... ..
. . . ........... 74
49
vi
LIST OF TABLES
page
l6
TABLE I :
Chromatographic Columns.....................
TABLE 2 :
Nitrogen and Chloride Analyses of White Crystal.
. .
17
TABLE 3
:Mass Spectrum
for Trace U of Figure 9
TABLE U
:Mass Spectrum
for Trace 7 and p-Propylaniline . . . . .
TABLE 5
:Mass Spectrum
for Trace U of Figure 1 0 ...........
. 31
TABLE 6
:Mass Spectrum
for Trace 7 of Figure 1 0 ...........
. 33
TABLE 7 : Mass Spectra Data on Traces 3,
............26
28
.and 6 .............33
of Figure' 12
TABLE 8
:Mass Spectrums of
TABLE 9
:Mass Spectrum
Trace 5 and o-Ethylaniline
....
35■
for Trace 7 of Figure 1 2 ............35
TABLE 10 : Mass Spectra Data on Traces S 9 ^ 9 and . 5 .......... 36
of Figure 13
TABLE 11:
Approximate Compositions of Identified Nitrogen.
Compounds
. .
38
vii
LIST OF FIGURES
page
FIGURE I
: Common Heterocyclic Hitrogen C o m p o u n d s ...........
FIGURE 2
: The Electrons in the P Orbitals and
i t
2
............. 7
Clouds of Pyrrole and Pyridine
FIGURE 3 :
Schematic Diagram of Reactor S y s t e m ........ . . . .
11
FIGURE It : Detailed Diagram of the R e a c t o r .................. 11
FIGURE 5
: Effect of Space Time on Hitrogen ..................... 20
Removal from Oil
FIGURE 6
: Curvilinear Regression Line for Quinoline Runs
FIGURE 7
Curvilinear Regression Line for Indole Runs
. . . 21
....
FIGURE 8
: Chromatogram of Mixture of Basic Compounds .........
FIGURE 9
: Chromatogram of Basic Compounds from 1,6 LHSV
22
2b
'• • - 25
- Quinoline Runs
FIGURE 10 :
Chromatogram of Honbasic Compounds from Ir.O . • • . 3 0
LHSV Quinoline Runs
.
FIGURE 11 :
p-Xylene, p-Xylene Dimer, and Ions................. 32
Figure 12
Chromatogram of Basic Compounds from 1.6 LHSV •, * - 34
Indole Runs
FIGURE 13 :
Chromatogram of Honbasic Compounds from 1.6 LHSV
Indole Runs
• 37
viii
page
FIGURE
:
Possible Reaction Mechanism for Quinoline . . . .
hi
Hydrodenitrogenation
FIGURE 15 :
Possible Reaction Mechanism for Indole
Hydrodenitrogenation
........
43
ix
ABSTRACT
A supported NiClg - gaseous HCl catalyst was used in the hydrodenitrogenation reactions of indole, a nonbasic compound, and quinoline,
a basic compound.
The reactions were carried out at a reactor tempera­
ture of 400°C; a reactor pressure of 850 psig; space velocities of 4.0,
1.6, 1.0, and 0.7275 LHSV; a hydrogen rate of 500 SCF/bbl of liquid feed;
a chloride to nitrogen content of eight to one; and using p-xylene as the
carrier oil.
Nitrogen compositions were determined by a Kjeldahl analysis and
a Mohr test was used to determine chloride contents.
The amine forms of
basic and nonbasic nitrogen compounds from indole and quinoline runs were
isolated.
These compounds were then identified using gas chromatography
and mass spectrometry.
In this r e s e a r c h n i t r o g e n was found easier to remove from the product
oil of quinoline than indole at 4.0 LHSV. However, little difference,
was' observed for lower -space velocities.
Different reaction mechanisms were proposed for- the denitrogenation
of indole and quinoline. It appeared that hydrochloride intermediates
are present In the reaction sequences and that nitrogen leaves the
reaction zone as ammonium chloride. Also, possible limiting steps in the
reaction sequences were proposed.
I- INTRODUCTION
A.
BACKGROUND
Indole and quinoline are heterocyclic nitrogen compounds which have
been found in fossil fuel stocks.
Indole, a nonbasic compound, has been
isolated in petroleum crude oil'(28) and in coal tar (3 ).
Quinoline, a
basic compound, has been identified in crude oil (2k, 28), coal tar (3),
and in hydrocracked shale oil (4, 7)•
The structure and other signifi­
cant data on both these compounds as well as on other important nitrogen
bases and nonbases are presented in Figure I.
Nitrogen compounds are' not desirable in fossil fuel stocks.
There
is now: much evidence to suggest that the nitrogen in fuels is as important
as the nitrogen in air in the formation of nitrogen oxides during combus­
tion (.10, 3l).
Nitrogen oxides in the presence of sunlight undergo a
photochemical reaction with hydrocarbons to form smog.
Also, in the
petroleum industry, nitrogen is a poison for many catalysts.
Its effect
is most notably seen in catalytic reforming where nitrogen decreases the
isomerization efficiency of dual-functional catalysts (2, 12, 15).
Today, with the United States experiencing a domestic shortage of
production crude oil, different sources of energy must be found.
One
potential source is the Green River shale oil formation of Colorado, Utah
and Wyoming.
It contains an estimated one trillion barrels of oil.
However, it also contains 2.k percent nitrogen (?)•
Before this oil can
be utilized, the nitrogen content must be reduced considerably.
Hydrodenitrogenation has been found to be the best way of removing
nitrogen from petroleum stocks (12).
In this destructive hydrogenation
basic
c o :-:i’
o u ;:d :
Aniline
79. :o
m.p.
116 C
Quinoline
m.v. 93.13
m. p.
b.p. l8L C
129.16
Indoline
119.17
m.p. -16 C
237 c
NOMBASIC COMPOUNDS
Pyrrole
. 67.09
-2h C
b.p. 131 C
Indole
m.v. 117.15
m.p. 52 C
b.p. 253 C
FIGURE I: Common Heterocyclic Nitrogen Compounds
Carbazole
. 167.21
m.p.
- 3 -
process the most widely used catalysts are cobalt molybdate, nickel
molybdate, and nickel tungsten sulfide.
These catalysts systems
generally denitrogenate nonbasic compounds easier than basic ones (12).
Also, most nitrogen from these treatments ends up as ammonia.
These
systems are also better suited for desulfurization than denitrogenation.
This department, in hope of finding a better denitrogenation
catalyst system, has been, doing work on a niekelous chloride-gaseous
hydrochloric acid catalyst system since 1966 (11,19,20).
This work has
primarily been aimed at determining, the operating variables for maximum
denitrogenation.
However, work has also been done to determine the
relative ease of denitrogenating a number of heterocyclic nitrogen
compounds.
1)
This system has shown some unusual characteristics:
Basic compounds are generally easier to denitrogenate
than nonbasic compounds.
2)
There is a higher denitrogenation than desulfurization
activity.
3)
Hydrochloride intermediates are suspected in the reaction
sequence.
H)
Nitrogen appears to leave the reaction zone as ammonium
chloride.
The work detailed in this report has been done to find a mechanism to
account for these characteristics.
B.
THEORY
In understanding the mechanism proposed in this report, "basicity"
- 4 -
Pyrrole P Electrons
Pyrrole
it
Clouds
Pyridine
it
Clouds
unshared
electrons
Pyridine P Electrons
FIGURE 2: The Electrons in the P Orhitals and u Clouds
of Pyrrole and Pyridine
- 5 is a very important concept.
Generally, heterocyclic compounds are
arbitrarily classified as "basic" or "nonbasic" by their ability to
react with a perchloric acid-acetic acid solution.
Basic compounds
can react with this solution and nonbasic compounds can not.
The reason for this difference can be found by studying the
electronic configurations of the pyrrole and pyridine carbon and
nitrogen atoms.
As can be seen from Figure I, pyrrole and pyridine
are very similar in molecular structure to indole and quinoline
respectively.
In pyrrole, a nonbasic compound, the electronic configurations
the carbon and nitrogen atoms are as follows:
Is
CARBON
(y)
2s
g)_o_g, o
sp
Is
NITROGEN
2p
2
hybridization
2s
2p
-O O 0,0
sp
2
hybridization
- 6 Each carbon and nitrogen atom is bonded to three other atoms by a
bonds using the trigonal-planar, sp
2
hybridization orbitals.
This
leaves one electron in a p orbital for each of the four carbon atoms
and two electrons in a p orbital for the nitrogen atom.
these six p orbitals gives rise to a
of the molecule.
cloud above and below the plane
it
The electrons in the
sharred by all five atoms (3).
Overlap of
it
clouds are delocalized and
This gives a stabilizing effect to
the ring known as an "aromatic sextet."
It should be noted that in
pyrrole there are no unsharred electrons in the outer valence state.
In pyridine, a basic compound, the electronic configuration of
the carbon and nitrogen atoms is as follows:
CARBON
0 O OQO
sp
NITROGEN
hybridization
O ,O O 0,0
sp hybridization
As can be seen only the electronic configuration of the nitrogen atom
has changed.
In pyridine nitrogen in bonded to only two other atoms and
- 7 only one electron in a p orbital is needed to complete the aromatic
sextet.
Therefore, there are two unshared
orbital which can be shared with acids (3).
orbitals and the
tt
electrons in the sp
2
The electrons in the p
clouds of pyrrole and pyridine are shown in
Figure 2.
Since the basic or nonbasic classification is an arbitrary one
and since some heterocyclic nitrogen nonbases can react with strong
acids, a more universal definition of basicity will be used.
report, the Lewis theory of basicity will be used.
Lewis stated that
a base was anything which has an unshared pair of electrons.
and quinoline fit this classification.
For this
Pyridine
Lewis also stated that an
acid was anything which could attach itself to such a pair of electrons.
For instance:
+
HCl
Pyridine
Base
Pyridine
Acid
Hydrochloride
Pyrrole and indole while not forming salts with even strong acids cannot
be considered acids so the classification of "nonbase" will be used for
these compounds.
II.
RESEARCH OBJECTIVES
The primary objective of this research was to identify intermediate
compounds in the hydrodenitrogenation of indole and quinoline which then
could be used to speculate as to possible reaction mechanisms.
Other
objectives were to prove the existence of. ammonium -chloride and hydro­
chloride intermediates.
III.
EXPERIMENTAL APPARATUS, PROCEDURE AND ANALYSIS
MATERIALS
I.
Charge Stock
The two nitrogen compounds used in this work were indole and
quinoline.
They were chosen because they are both found in coal
tar and crude oil and because they are both available in large
quantities, in pure form, and at reasonable prices. The nonbasic
compound indole was obtained in reagent grade from the Aldrich
Chemical Company.
The basic compound quinoline was supplied by
the J. T. Baker Chemical Company in reagent grade.
The carrier oil initially chosen was "Peneteck" a commercial
mineral oil produced by the Pennsylvania Refining Corporation.
However, this oil proved to be unuseable since it interfered with
some nitrogen peaks on the chromatograph.
Therefore, p-xylene, a
compound with a retention time less' than the nitrogen compounds was
selected.
This compound was obtained 99% pure from the Chevron
Chemical Company.
The charge stock also included methylene chloride and hydrogen.
Some of the hydrogen reacted with the methylene chloride to form
gaseous hydrochloric acid under the reactor conditions.
Hydrogen
was supplied in 2000 psig cylinders by H . R. Oxygen Supply of
Billings,
Montana.
The methylene chloride was obtained in
reagent grade from the J. T. Baker Chemical Company.
H
O
pump
flask
scrubbers
FIGURE 3: Schematic Diagram of Reactor System
11
pressure gauge
rupture disk
aluminum block
catalyst support
thermowell
catalyst pellets
I controller
insulation
metal can
heating coils
Variac
glass-wool plug
section for NE,Cl accumulation
support rod
FIGURE I4: Detailed Diagram of the Reactor
- 12
2. . Catalyst and Filler
The catalyst used in this work was prepared hy Fedoruk (ll).
He impregnated HiClg on a 1/8 hy 1/8 inch Harshaw alumina pellet.
He determined the nickel content of the pellets to he 7.72 percent.
By using only this catalyst, the nickel content of the pellets was
eliminated as a variable in this work.
The Norton Company supplied the filler.
It consisted of inert
alumina pellets and was used to support the catalyst pellets in the'
reactor.
B.
EQUIPMENT
A schematic diagram of the equipment is shown in Figure 3.
The
liquid charge stock was pumped into the top of the reactor by a Lapp
diaphragm pump.
cylinder.
Here the liquid combined with hydrogen from a pressurized
The' hydrogen rate was measured on a calibrated. Brooks high-
pressure gas rotameter after the hydrogen had been deoxygenated in an
Englehard deoxo unit and dehumidified in a molecular seive drying unit.
A detailed diagram of the reactor is shown in Figure
A pressure
gauge which measured the reactor inlet pressure was attached to the top
of the reactor.
Also attached was a 2000 psig rupture disc - in the
event of overpressure this disk would depressurize the charge to the
vent system.
The reactor was' a schedule 80, corrosion resistant. Inconel alloy
pipe 52 inches in length and one inch in diameter.
A six inch diameter
aluminum block enclosed the upper 60 percent of the pipe.
Wrapped
- 13 around the block were three heating coils each with a Variac.
The
middle coil was automatically regulated by a Wheelco Capacitrol onoff controller . Extending down the middle of the reactor as far as
the bottom of the aluminum block was a thermowell.
One iron-constantan
thermocouple measuring a temperature in the middle of the reactor
supplied the input signal to the on-off controller.
Three other
thermocouples connected to a Leeds and Worthrup indicating potentiometer
measured the temperature at the t o p , middle and bottom of the reactor.
The product after leaving the reactor passed through a Grove back­
pressure regulator and into a flask.
The liquid was collected in the
flask and the gas first bubbled through a caustic bath to remove the
acidic vapors and then was vented.
C.
OPERATING PROCEDURE
I.
Process Conditions
In order to identify intermediate products from the reactions,
the system was operated at less than optimum nitrogen removal
conditions.
These reduced conditions were:
a)
The reactor temperature was 400°C.
b)
The reactor pressure was 850 psig.
c)
Hydrogen rate was 5000 SCF/BBL of feed.'
d)
The nitrogen content of the feed was 0.66 percent.
e)
The chloride to.nitrogen ratio was eight to one.
f)
Liquid feed pumping rates were 4.0, 1.6, 1.0, and 0.7275
LHSV for-the quinoline runs and 4.0, L. 6 , and 1.0 LHSV
for the indole runs.
2.
Reactor Charging
The 250 ml of filler and 100ml of catalyst used each run were
dried at 400°C for 2k hours.
These were cooled in a desiccator.
In charging the reactor, half of the filler was poured down the
inverted pipe with the thermowell already in place.
Then the
catalyst, followed by the remaining filler, was added.
After each
layer was in place, the side of the pipe was tapped to insure
complete and uniform settling.
Finally, a glass-wool plug and a
stainless steel coll were positioned to support the catalyst and
■filler as the pipe was turned upright.
Then, with the pipe
enclosed in an aluminum block, the heating coils were turned on
and the reactor was brought up to temperature.
3.
Reactor Operation
With all fittings tightly sealed and the reactor at ^OO0G ,
the back-pressure regulator was pressurized to about 825 psig with
hydrogen.
The reactor itself could then be pressurized to 85O psig
inlet pressure-with the selected hydrogen gas flow.
for gas leaks, the feed pump was started.
After checking
By measuring the
volumetric feed rate of the oil, the adjustable stroke on the
piston pump was set to give the desired space velocity.
During
the run, the temperatures in the reactor were closely watched and
the two manual variacs were adjusted to keep the reactor top
and bottom temperatures as close to 400°C as possible.
To insure
- 15 a completely "lined-out" catalyst, 500 ml of feed were reacted
before any product was sampled.
After 200 ml of product oil
were collected, the reactor was "shut down."
This process
consisted of shutting off the hydrogen rate, the feed pump,
and the heating coils..
Also, the reactor system was depressurized.
When the system had cooled, the reactor was emptied and the
entire system was cleaned.
The catalyst and support were
discarded without being reused.
hot water and acetone.
The reactor tube was washed with
With the indole runs material which had
accumulated in the bottom of the reactor and on the support coil
was also saved for further analysis.
D.
ANALYTICAL METHODS
The nitrogen content of the samples was determined by a standard
Kjeldahl method (l8 ),
Two analyses of each sample were run and an
average of the two analyses was recorded.
Kel-paks obtained from
Matheson Scientific Company which contained a weighed amount of K^SO^
and HgO proved to be time saving.
The chloride content of samples was
determined by the Mohr volumetric method (33).
The nitrogen compounds in the product samples were separated into
basic and nonbasic fractions and then concentrated according to a
method outlined by Hartung and others (l4).
These concentrates were
tentatively identified chromatographically.
The unknown concentrates
were injected into the chromatograph and the peaks observed were compared
for retention time with known compounds.
Also, retention data from
- 16 Poulson (24) proved to be quite useful.
The three columns which
proved to be most successful were given in Table I.
TABLE I:
Chromatographic. Columns
Column No.
,
2
I
3
.
Stationary Phase
Carbowax 20M
Carbowax 20M .
Carbowax 2OM
Support
Chromosorb P
Chromosorb P
Chromosorb P
Special Treatment
KOH
KOH
Length
17 ft.
10 ft,
5 ft.
The KOH treatment was applied as outlined by Smith and Radford (26)
to reduce severe tailing of the nitrogen compounds which was encoun­
tered on untreated columns. The treatment was very successful. The
chromatograph used was a Varian Aerograph 200.
The recorder was a
Sargent Model SR.
The primary tool for identifying the nitrogen compounds was
mass spectrometry.
Two mass spectrometers were used in this research.
One was a Varian Model Ch5 which was located in the Chemistry Depart­
ment of Montana State University,
The other was a Variant Model MAT
111 located in the Chemistry Department of the University of Montana.
Both analyzed samples which had passed through one of the columns
described in Table I.
In this way, the samples were separated into
individual compounds before they were analyzed - unless two or more
compounds had identical retention times.
IV.
A.
RESULTS M D DISCUSSION
Nitrogen and Chloride Analyses'
After every quinoline or indole run, a white crystaline solid was
found deposited on the upper section of the support coil and a large amount
of brown solid was found in the bottom of the reactor.
Previous workers
(ll, 19) felt that the white crystal was probably ammonium chloride.
The
data in Table 2 confirms their hypothesis.
TABLE 2:
NITROGEN AND CHLORIDE M L A Y S E S OF WHITE CRYSTAL
% N
% Cl
1st
26.67
66.76
2nd
27.49 '
66.21
3rd
26.95
67.64
Average
27.04
66.87
Standard
Deviation
0.295
0.510
Actual Value
For Ammonium
Chloride
26.18
66.28
The slight deviations of the experimental values from the actual values are
likely due to small bias in the experimental techniques.
Also, ammonium
chloride sublimes at 3^0°C at atmospheric pressure and so would be expected
to .sublime in the upper section of the cooling portion of the reactor.
Previous workers (ll, 19) felt that the brown solid might be hydro­
chloride salts of the basic nitrogen compounds.
Data taken in this work
supports this hypothesis.- For each of the nine indole runs the nitrogen and
- 18 chloride contents of the solid were found.
The nine nitrogen analyses
showed an average content of 12.67% with a standard deviation of 0.365.
The nine chloride analyses showed an average content of 39.46% with a
standard deviation of 1.379.
Nitrogen and chloride analyses were not run
on the solid from the quinoline runs.
It must he noted here that nitrogen
and chloride analyses were calculated on a different basis.
The chloride
samples were dried at IlO0C for one hour before being weighed, but the
nitrogen samples were not dried.
It will be shown later in this report that aniline was identified in
both the quinoline and indole runs.
Aniline in the presence of hydro­
chloric- acid forms aniline hydrochloride (2l).
nitrogen a n d ,27.35% chloride.
values.
This compound has 10.81%
These values are lower than the experimental
However, if some ammonium chloride were present in the brown solid
and if one takes into account the different basis for the nitrogen and
chloride analyses, the experimental values are possible.
In the 4.0 and 1.6 LHSV quinoline runs and the 4.0 LHSV indole runs,
some of the hydrochloride salt, in the form of a very viscous liquid,
flowed into the product flask.
The salt did not usually enter the product
flask until about 500 mis, of feed had been reacted.
The constrictions in
the bottom of the reactor and the back-pressure regulator obviously
restricted the flow of the salt.
Since not all of the salt made it into
the product, flask, it was .decided, to,:exclude the salt in the product flask
from the samples for nitrogen analysis.
This was accomplished by simply
decanting the oil product off the very viscous salt.
However, the data
Percent of Nitrogen Removed
100
Operating Conditions
Pressure: 850 psig
Temperature: ^OO C
Hydrogen Rate: 5000 scf/tbl
Cl/N: 8.0
A indole
Q quinoline
80 H -------------------- 1---------------- ---- 1------------------- 10.0
0.5
1.0
1/LHSV
FIGURE 5: Effect of Space Time on Nitrogen Removal From Oil
1.5
- 20 from the nitrogen analyses of the oil product cannot he looked upon as
kinetic data hut more as solubility data.
standpoint, this data is good.
But from a practial engineering
If this process were utilized in a refinery,
one would only need a holding tank to separate the oil from the hydro­
chloride salt.
'
Figure 5 shows the effect of reactant space time on the nitrogen
removal from the oil.
This figure shows that at low space times (high space
velocities).the nitrogen is more completely removed form the quinoline
product than from the indole product.
the higher space times, little
However, the figure shows that at
difference can he detected.
Figure 5 cannot
he compared with the results of previous workers (ll, 19) since the nitrogen
analyses were run on a different sample.
off the top of a settled oil.
Previous workers drew their sample
However, the samples taken in this research
were from a well mixed oil.
The lines through the data in Figure 5 were constructed hy a curvilinear
regression technique.
Straight lines could have been drawn through the data
hut these lines would have gone above 100% removal for the higher space
times.
The curvilinear regression technique consisted of replotting the
'' *
I + P
data with the same abscissa hut with the ordinate changed to In --------- ,
I - P
where P is the fraction nitrogen removed from the oil. The resulting plots
linearized the data as can be seen from Figures 6 and 7*
A linear regression
technique was used to construct the straight lines through the new data
points.
Then the equations for the straight lines were transformed to the
original variables and plotted in Figure 5*
Besides being curved, these
1+p
I-P
5-0
1.0
“
0.0
0.0
1.0
0.5
1/LHSV
FIGURE 6: Curvilinear Regression Line for Quinoline Runs
1.5
6.0
I/LHSV
FIGURE 7: Curvilinear Regression Line for Indole Runs
j
- 23 -
asymtotically approach 100% nitrogen removal.
The regression calculation
and analysis of variance tables for the data in Figures 6 and J is given
in the appendix.
B.
Identification of Compounds
1.
Introduction
Figure 8 is a chromatogram of a known mixture of basic compounds.
It shows that several compounds can have identical retention times.
Therefore, in this research, mass spectra data was essential to
identify compounds with certainty.
This figure also shows that ^-substituted aniline is more strongly
absorbed on Carbowax 2CM than a ring-substituted aniline.
o-Methyl-
aniline has a longer retention time than N-methylaniline.
Likewise,
p-propy!aniline has a longer retention time than N-n-propylaniline.
The reason for this is that- a primary nitrogen is more strongly
absorbed than a secondary nitrogen.
It should be noted that in subsequent identification of basic
nitrogen compounds the amine forms of the compounds are identified.
However, as was shown earlier, the basic nitrogen compounds leave
tlie reactor in their hydrochloride salt forms.
The amines are formed
by treating the salts with a dilute potassium hydroxide solution.
In this paper,, in order to avoid confusion between chromatogram
"peaks" and mass spectrum "peaks" the former will be referred to as
"traces."
2.
Basic Compounds from Quinoline Runs
Figure 9 is a typical chromatogram of the basic compounds from a
Chromatograph Variables:
Temperature: 222 C
Helium Flow: 30ml/min
Column: 17-ft Carbowax 20M
ro
I
F I GURE 8:
I: Syringe cleaning solution
2: Aniline
N-Methylaniline
N-Ethy1-o-toluidine
3: o-Methylaniline
N-n-Propylaniline
I+: o-Ethylaniline
5: p-Propy!aniline
Quinoline
6: 1,2,3, ^-Tetrahydroquinoline
C h r o m a t o g r a m of M i x t u r e of Basic Compounds
Chromatograph Variables:
Temperature: 220 C
Helium Flow: 30ml/min
Column: 17-ft Carbowax 20M
I: Syringe cleaning solution
2: p-Xylene
3: Aniline
U: H-Allylaniline
5: Unknown
6: Unknown
7: o-Propylaniline
8: Quinoline
9: a Methylaniline
10: 1,2,3,4-T etrahydroquinoline
I
M
vn
I
FIGURE 9:
Chromatogram of Basic Compounds from 1.6 LHSV Quinoline Runs
- 26 -
1.6 LHSV quinoline run.
The aniline, quinoline, and 1,2,3,^-tetra-
hydroquinoline traces were identified by comparing the retention times
and mass spectra data of the traces with those of the pure compounds.
Trace 4 is postulated to be N-allylaniline.
A comparison of
Figures 8 and 9 shows that trace H has a retention time similar to
O-methylaniline and N-n-propylaniline.
However, the mass spectrum
for this compound as listed in Table 3 corresponds to neither 0methylaniline or N-n-propylaniline.
TABLE 3:
M/E
MASS SPECTRUM FOR TRACE b of Figure 9
Relative Intensities
Comments
77
32
78
17
79
20
IOif
13
105
24
106
100
107
62
117
27
118
i4
132
45
133
38
146
7
Contamination
147
7
Contamination
Mass spectra■data for o-methylaniline, N-n-prppylaniline, and several
other compounds is listed in the appendix.
the base peak for the compound is 106.
As can be seen from Table 3,
This base peak is typical of
- 27 substituted anilines (5).
The parent peak of 133 with a large 132
peak is very similar to I ,2,3,H-tetrahydroquinoline.
However, the
retention time is wrong for this compound to be 1 ,2 ,3 ,4-tetrahydroquinoline.
Therefore, a compound must be found with a molecular weight of
1.2.3.4- tetrahydroquinoline and a retention time of N-n-propylaniline.
The obvious answer is a N-substituted aniline with an unsaturated
three-carbon side chain.
One possible candidate is N -allylaniline.
This compound could easily be formed by opening the pyridine ring of
1.2.3.4- tetrahydroquinoline.
1,2,3,4-Tetrahydroquinoline
N-Allylaniline
Traces 5 and 6 were not large enough to be analyzed on the mass
spectrometer but these might be o-methylaniline and o-ethylaniline
based on retention times.
The mass spectrum for trace T is listed in
Table U along with data for p-propy!aniline.
- 28 TABLE k:
MASS SPECTRA DATA FOR TRACE 7 AHD p-PROPYLANILIRE
Trace 7
m/e
p-Propylaniline
Relative Intesities
m/e
Comments
Relative Intesities
55
3
55
4
77
27
77
11
79
14
79
9
106
100
106
100
107
12
107
10
118
9
118
5
135
18
135
18
lk6
15
Contamination
I k rJ
12
in trace 7
Table U shows that trace 7 has more than one compound.
However,
it also shows that the major compound in trace 7 is very similar to
p-propylaniline.
A mass spectra comparison with N-n-propylaniline
would be good, but the retention time, would rule it out.
However, the
retention time for p-propylaniline corresponds to trace 8 and not trace
7.
But o-propylaniline could have a retention time like that of trace
7 and would have a similar mass spectrum.
With the propyl group in
the ortho position to the primary nitrogen there could be a shielding
effect to reduce the absorption of the nitrogen.
Thus o-propylaniline
probably has a shorter retention time than p-propylaniline.
Un­
fortunately, no o-propylaniline was available to test the hypothesis.
Trace 9 could not be isolated sufficiently from trace 8 to get a
separate mass spectrum.
However, the mass spectrum of trace 8 has
- 29 contamination peaks at lk2 and 1^3 corresponding to 15% and 22%,
respectively, of the quinoline base peak, 129.
Peaks 11+2 and 11+3
correspond to a methyIquinoline.
When programming the column temperature on the 5-foot Carbowax
column, traces were visible beyond I ,2 ,3 ,4-tetrahydroquinoline which
were too broad on the large column to be seen.
These traces were
invariably composed of more than one compound.
However, large
mass peaks were observed at 156, 157, 170 and 171.
The first two
numbers correspond to a dimethy!quinoline or an ethylquinoline.
The
last two correspond to a trimethylquinoline, a methylethylquinoline,,
or a propylquinoline.
It is interesting to note that Ryffel (25) identified aniline,
o-methylaniline,oo-ethylaniline, o-propylaniline, quinoline and
1, 2j3,l+-tetrahydroquinoline in the product oil of a destructive
hydrogenation of quinoline over a cobalt-molybdate catalyst.
3..
Honbasic Compounds from Quinoline Runs
A chromatogram from a 4.0 LHSV quinoline run is given in. Figure 10.
A 1.6 LHSV run was used in all the other identifications.
However,
with the quinoline runs the nonbasic compounds were such a m i nor'
fraction of the product that the 4.0 LHSV runs had to be used to obtain
a chromatogram with visible traces.
Traces I .and 2 of Figure 10 are p-xylene and an unknown compound.
Trace 3 has not been identified.
A mass spectrum of trace 3 has a
base peak of l4l with other significant peaks at 115, 139, 142, l43
Chromatograph Variables:
Temperature: 221 C
Helium Flow: 50 ml/min
Column: 10-ft Carhowax 20M
I:
2:
3:
U:
5:
p-Xylene
unknown
unknown
Dimer of p-Xylene
a Methylindole
6: a CgH -indole
I
I
U)
I
*
FIGURE 10:
Chromatogram of Nonhasic Compounds from 1+.0 LHSV Quinoline Runs
- B l ­
and 1 U 5 .
Trace 4 is probably the dimer of p-xylene.
Table 5 is a mass
spectrum for this trace.
TABLE 5:
MASS SPECTRUM FOR TRACE h OF FIGURE 10
m/e
Relative Intensities
89
8
91
7
103
11
10 U
■
10
105
100
106
11
118
27
180
11
195
29
210
31
Figure 11 shows the structure and molecular weight of p-xylene,
the p-xylene dimer, and some fragmentary ions.
The mass to charge
ratio of these ions agrees with those of the mass spectrum for trace 4.
No p-xylene dimer was available to compare for retention time or mass
spectrum, but it is strongly suspected that the trace is the dimer.
Trace 5 seems to be a methylindole.
A mass spectrum of the trace
had a base peak of 130 with 131 as another large peak.
Dimethylindole or ethylindole appears to be the main constituent of
trace 6 .
However, as can be seen from Table 6 , a methylindole and a
C^H^-indole are also present.
CH3X o y c H 3
CH3X o y c H 2-CH2X o y c H 3
p-Xylene
p-Xylene Dimer
210
106
CH3~<roy CH2~ CHQ -^o V
CH3-<5>-
M - CH
195
91
CHy-tCcT)- CH 2“ CH 2 *
119
CH3—<^0^>—CH2 *
105
•< 2 ) - CH2 - CH2- CS> •
180
FIGURE 11:
p-Xylene, p-Xylene Dimer, and Ions
- 33 -
TABLE 6:
MASS SPECTRUM OF TRACE 6. OF FIGURE IO
m/e
k.
Relative Intensities
Comments
103
11
115
11
130
39
Methylindole
131
9
Methylindole
143
19
144
100
CgH^-Indole
145
59
CgH^-Indole
157
i4
C^Ht-T-Indole
158
22
C^Ht-T-Indole
Basic Compounds from Indole Runs
A typical chromatogram of the basic compounds from a 1.6 LHSV indole
run is presented in Figure 12.
The identification of these traces was
done in the same manner as with the basic traces from the quinoline
runs.
TABLE 7:
Trace No.
Mass spectra data on traces 3, 4, and 6 is present in Table %.
MASS SPECTRA ON TRACES 3, 4, and 6 OF FIGURE 12
Compound
Parent
Peak
Base
Peak
Other Major
Peaks
93
93
64, 65, 66
77, 79, 89
3
Aniline
4
o-Methylaniline
■107
106
6
o-Propy!aniline
135
■ 106
107, 120, 121, 129
Trace 5 is largely but not exclusively o-ethylaniline.
The mass spectrum
of this trace along with that of pure o-ethylaniline is presented in
Table 8 .
vt
Chromatograph Variables:
Temperature: 221 C
Helium Flow: 30 ml/min
Column: 17-ft Carhowaoc 20M
u>
I
I: Syringe cleaning
solution
2: p-Xylene
3: Aniline
^ : o-Methylaniline
5: o-Ethylaniline
6: o-Propylaniline
a C_H„-aniline and
a C)H^-aniline
height trace 3
2.65 height trace It
FIGURE 12:
Chromatogram of Basic Compounds from 1.6 LHSV Indole Runs
- 35 -
TABLE 8 : MASS SPECTRUMS OF TRACE 5 AND 0--ETHYLAUILINE
Trace 5
m/e
o-Ethylaniline
Relative Intensities
77
21
78
m/e
Relative Intensities>
77
21
6
78
5
79
21
79
14
91
k
91
4
106
100
106
100
107
67
107
9
k
120
4
121
id
121
39
122
1
122
4
120
■
,■
, It is probable that the contamination is o-methylaniline.
Commen-
Contaminat ion of
Trace 5
This
compound has a parent peak. 107 almost as intense as its base peak
106.
Also, the o-methylaniline and o-ethylaniline traces overlap as
can be seen from Figure 12.
/
The mass spectrum for Trace J is presented in Table 9•
MASS S P E C T R U M FOR TRACE r
J OF FIGURE 12
TABLE 9:
m/e
Relative Intensities
13
91
93
Comments
'
12
106
100
107
36
120
' 6l
121
12
134
23
a C^H^-Aniline
135
38
a CgH^-Aniline
149
16
a C^H^-Aniline
- 36 -
This trace seems to be composed of a C^H^-aniline and a C^H^aniline.
Hartung and coworkers (lU) hydrogenated indole using a supported,
sulfactive catalyst under the relatively mild conditions of 300 psig. ,
315°C, and 4.0 LHSV.
The basic products which they isolated were
H-ethyIcyclohexylamine , n-octylamine, g-cyclohexylethylamine, 3-phenylethylamine, o-ethylanilines indoline, quinoline, dimethylquinoline,
1,2,3,4-tetrahydroquinolihe , and indole (from an indole polymer).
5.
Honbasic Compounds from Indole Runs
A typical chromatogram of the nonbasic compounds from a 1.6 LHSV
indole run is given in Figure 13.
The identification of these traces
was done in exactly the same manner as with the nonbasic traces from
the quinoline runs.
The mass spectra data on traces 3, 4, and 5 is
presented in Table 10.
TABLE 10:
Trace Ho.
MASS SPECTRA DATA OH TRACES 3, 4, and 5
Compound
Parent
Peak
Base
Peak
Other Major
Peaks
3
Dimer of p-Xylene
210
105
106, 118, 165, 195
4
a Methylindole
131
130
101, 102, 103
a CgH^-Indole
145
l44
103, 115, 117, 130
5
'
Chromatograph Variables:
Temperature:
221 C
Helium Flow:
50 ml/min
Column:
10-ft Carhowax 20M
FIGURE 13:
I : p-Xylene
2: Unknown
3: Dimer of p-Xylene
4: a Methylindole
5: a C ^ -indole
Chromatogram of Nonhasic Compounds from 1.6 LHSV Indole Runs
- 38 -
With the 5-foot Carbowax column small traces appeared which were
not visible on the longer columns.
These traces had mass spectra
numbers equivalent to carbazole (167) and to methylcarbazole (l80 and
l8l).
Hartung and coworkers (l4) identified the following nonbasic
compounds from their work: • 3-isopropylindole, indole, 1,3-dimethyl2-ethylindole, 3-propylindole, 1-ethylindole, 2-tert-butylindole,
other indole types, and carbazole types.
Si
Summary of Identification of Nitrogen Compounds
A listing of the identified nitrogen products along with their
approximate compositions, which were calculated using peak heights,
is presented in Table 11.
TABLE 11:
APPROXIMATE COMPOSITIONS OF IDENTIFIED NITROGEN COMPOUNDS
QUINOLINE RUNS
Basic Compounds
I .6 LHSV Runs
Compound
Nonbasic Compounds
4.0 LHSV Runs
Composition
Compound
Composition
Aniline
29.1%
a Methylindole
16.1%
N-Allylaniline
29.6
a CgH [.-Indole
83.9
o-propylaniline
11.7
a C^H^-Indole
Trace
Quinoline
Methylquinoline
11.3
6.1
1,2,3,4-Tetrahydro
quinoline
12.2
CgH ,.-Quinoline
Trace
CgH^-Quinoline
Trace
100.0
•
100.0
-
— 39 —
TABLE 11:
APPROXIMATE COMPOSITIONS OF IDENTIFIED NITROGEN COMPOUNDS (Cont).
INDOLE RUNS
Basic Compounds
1.6 LHSV Runs
Compound
Nonbasic Compounds
4.0 LHSV Runs
Composition
Compound
Aniline
57.4#
a Methylindole
o-Methlaniline
21.'6
a CgH^-Indole
o-Ethylaniline
' 12.4
o -Propylaniline
8.6
a C^H^-Aniline
Trace
a C^Hg-Aniline
Trace
Composition
45.2#
'
54.8
Carbazole
Trace
Methylcarbazole
Trace
100.0
100.0
The one major surprise of this listing is the absence of indole and
indoline from the indole slate.
This fact combined with the fact that
quinoline and 1 ,2 ,3 ,4-tetrahydroquinoline are present in the quinoline
slate suggests that different mechanisms are involved in the hydrodenitrogenation of quinoline and indole and that basicity must play
an important role in this difference.
C.
Reaction Mechanisms
The nickelous chloride-gaseous hydrochloric acid catalyst behaves as
dual functional catalyst.
It contains sites for a hydrogenation function
and for an acid function.
The hydrogenation function is responsible for
hydrogenation and dehydrogenation reactions.
The acid function is
responsible for cracking, isomerization,, and polymerization reactions (2 ).
With this catalyst system, the following sites for hydrogenation and
acidic activities are possible:
-UoHYDROGENATION
ACIDIC
I)
Ni
HCl
2)
H+ (NiCl3 )-
H+ (NiCl3 )-
3)
H+ (NiClgOH)-
H+ (NiClgOH)
These site combinations all involve nickel and hydrochloric acid.
The
reason for this is that McCandless (19, 20) showed that "a minimum amount
of nickel and a minimum partial pressure of HCl are required for good
denitrogenation activity."
With the first of the above site combinations the hydrogenation
/
functions would take place at a nickel site on the catalyst surface.
The
acidic functions could take place either where hydrochloric acid is
absorbed on the catalyst surface or possibly in the gas phase with gaseous
hydrochloric acid.
In either case, the nitrogen compound would have to
migrate or diffuse from the nickel site to the hydrochloric acid site in
order for the denitrogenation reaction to go to completion.
With the last two active sites an entire denitrogenation reaction
could conceivably take place at one location on the catalyst surface.
The H+ (NiClgOH)
liquid feed.
site is possible since there might be some water in the
Unfortunately, this research did not obtain the type of data
necessary to identify the site combination actually present on the surface
of this catalyst.
Using•the product slates in Table 11 possible reaction mechanisms for
the denitrogenation of quinoline and indole were postulated in Figures
and 15, respectively.
These mechanisms are very similar.
The primary
lU
NiCl
HCl Co)
Salt of Quinoline
Quinoline
I ,2-Dihydroquincline
I ,2,3,^-Tetrahydroquinoline
-continued-
FIGURE l U :
Possible Reaction Mechanism for Quinoline
Hydrodenitrogenation
- 1*2 -
-continued-
-N-CH-C=CH1
Salt of
N-Allylaniline
Salt of o-Propylaniline
Propane
Salt of Aniline
+
Benzene
FIGURE ll+:
NHi Cl
+
Ammonium
Chloride
Possible Reaction Mechanism for Quinoline
Hydrodenitrogenation (cont.)
NiCli
Indole
Indoline
NiCl
Salt of Indoline
Salt of
o-Ethylaniline
-continued
FIGURE 15:
Possible Reaction Mechanism for
Indole Hydrodenitrogenation
-W-
Methane
Salt of
o-Methylaniline
Salt of Aniline
Ethane
Salt of Aniline
EH, Cl
Ammonium
Benzene
FIGURE 15:
Chloride
Possible Reaction Mechanism for Indole
Hydrodenitrogenation (cont.)
EiCl
difference is that indole must first be reduced to a basic compound
before it can react with the active site H+ '(NiCl ) .
being a basic compound can react directly.
However, quinoline
One might speculate that it
is this reduction step which causes indole to be more difficult to
denitrogenate at 4.0 LHSV than quinoline.
However, since indole was not
identified in the 4.0 or 1.6 LHSV product oil from the indole runs, it is
likely that indole was reduced quickly and irreversibly.
Therefore, the
reduction step would not be the limiting step in the reaction sequence.
In Figure I4
the products of reactions 1,3,4 and 5 were identified in
their amine forms.
Also, ammonium chloride., one of the products of reaction
6 , was identified.
I ,2-Dihydroquinoline was not identified but this
compound has been found in the product of a mild reduction of quinoline (25).
It is likely that the salt of I ,2-dihydroquinoline is formed in the reduction
of quinoline but that it is immediately reduced to the salt of I ,2 ,3 ,4-tetrahydroquinoline.
In Figure 15, the products of reactions 3, 4, 5 and the ammonium
chloride from reaction 6 were identified.
indoline was identified.
However,
neither indole nor
.
But this fact could be explained if reactions
1,2, and 3 were fast and irreversible.
Then there would be little indole
or indoline in the reaction product to be identified.
The reactions in Figures I4 and 15 are reasonable.
mechanisms could be possible.
However, other
For instance, it is possible that the
o-methylaniline is an end product and not an i n t o
Jiate in the indole
— 46 —
reaction sequence.
This would mean that reaction 5 would not procede.
Most of the aniline would then come from reaction 4.
A sure way to test
this mechanism would be to react the salt of o-methylaniline at the same
reactor conditions as indole.
Then a comparison of the reaction rates
and product slates would indicate if step 5 is in the reaction sequence.
As such runs and analyses were not made in this research, it is impossible
to positively identify the reaction sequences for the denitrogenation of
indole and quinoline,
The presence of methylated quinolines and indoles in the quinoline
product and high molecular weight anilines, carbazoles, and methylated
indoles in the indole product is the result of destructive alkylation.
Hydrocarbons from cracking reactions react with anilines, indoles and
quinolines.
Methylated indoles and quinolines are harder to denitrogenate
than indole and quinoline (12).
This means that complete denitrogenation
of quinoline and indole would be difficult even at long reactor space time.
Since alkylated anilines were identified in the product oil of indole
and not quinoline runs this would suggest that the denitrogenation of indole
is limited by one of the aniline or methylated aniline reactions.
The
salts of these compounds must have a long residence time in the reactor to
allow alkylation to take place.
Using similar logic, it would appear that quinoline must have a long .
residence time in the reactor to allow quinoline to be .alkylated.
This
would suggest that either the initial reaction of quinoline to form the
-U t salt with the active site or the reduction of this salt to the salt of
I, 2-dihyroquinoline is the limiting reaction.
The former case seems to
he more likely.
If in future work even more reduced conditions were selected and the
product slates for these conditions were obtained for a number of space
velocities then one could speculate with more assurance as ,to possible
limiting reactions.
V.
SUMMABY
and
conclusions
The following conclusions can he drawn from this work on the
hydrodenitrogenation of indole and quinoline:
1.
It is easier to remove nitrogen from the product oil of quinoline
than indole at 4.0 LHSV, hut at lower space velocities little
difference is observed.
2.
Nitrogen leaves the reaction zone as ammonium chloride.
3.
Hydrochloride intermediates appear to he present in the reaction
sequence.
4.
The amine forms of basic and nonbasic nitrogen compounds from
indole and quinoline runs can he isolated.
5.
Different reaction mechanisms with different limiting steps must
he proposed for the denitrogenation of indole and quinoline.
6.
Destructive alkylation reactions seem to occur.
7.
Some of the p-xylene appears to dimerize.
VI.
RECOMMENDATIONS FOR FUTURE WORK
Future work should "be aimed at testing the hypotheses proposed in
this report concerning the type and location of the active sites, the
reaction sequences, and the limiting reactions.
The three programs
outlined "below might be useful.
1.
An intensive analysis of reacted catalyst pellets for nitrogen,
nickel, and chloride content might shed some light on the type
and location of the active sites.
2.
Reaction of proposed intermediate compounds a t .the same reactor
conditions as indole and quinoline and subsequent comparison of
reaction rates and product slates would test hypotheses concerning
reaction sequences.
3.
Analysis of rate data and product slates from the denitrogenation
of quinoline and indole at a number of space velocities and at
less severe "reduced" conditions than those of this research would
test hypotheses concerning limiting reactions in the reaction
sequence.
VIII.
APPENDICES
- 51 -
Linear Regression Calculations for Quinoline
Curvilinear Regression Line (Figure 6)
X = 1/LHSV
1.00
1.375
3 .6864
3.6759
4.1846
. 3.2619
3.5075
3.8275
3.8493
3.0941
3. 6OI 8
3.5638
3.7647
.25
.625
3.2591
n = 12
ZX = 9.75
ZY = 43.2766
X = 0.8125
Y = 3.6064
ZX2 = 10.0313
ZY2 = 157.0639
ZXY = 36.44 i 4
(ZX)2 /n a 7.9219
(ZY)2Zn = 156.0720
(ZX)(ZY)Zn = 35.1622
Zx2 = 2.1094
= .9919
L = Zxy/Zx^ = 0 .606^
Y = Y +
b(X-X) = 3.606k + .6064(X - .8125)
Zxy = 1.2792
- 52 -
Analysis of Variance Table for Quinoline
Curvilinear Regression
\
Source of Variation
Degrees of
Freedom
Sum of
Squares
Mean Square
F_
C
Linear Regression
on Space Times
I
.7757
.7757
36.9k
Deviation from
Linear
2
.0U 82
.02^1
1.15
Pooled Within
Space Times
.8
.1680
.0210
Total
11
.9919
F-test for Slope of Line
Hq
F-test for Fit of Data to Line
: Slope is zero (b = 0)
H q : Fit is good
: Slope is not zero
H^ : Fit is not good
a = .05
a = .05
F
F
c
= 36.9k
Rejection region : We reject
H q if [Fc 3 > 5-32
Conclusion : Reject H q
C
- 1.15
Rejection region : We
reject H q if [Fc ] > k.k6
Conclusion : Accept H0
- 53 -
Linear.Regression Calculations for Indole
Curvilinear Regression
ix =
1+P
-P
.625
2?6555
3.2718
4.2950
2.4)498 ■ 3.0412
4.6469
2.5224
3.7161
5.625
x = 0.625
IY =
Y =
3.2535
29.8522
7)
1.00
.25
X = 1/LHSV
Y = ln(
Line (Figure
n=9
3.3169
IY2 = 103.8046
IXY = 20.5440
(ix)2/n = 3.5156
(IY)2Zh = 99.0171
(IX)(IY)Zn = 18.6576
Ix2 = 0.8438
Iy2 = 4.7875
Ixy = 1.8864
• IX2 = 4.3594
b = Ixy/Ix2 = 2.2356
Y = Y +
b (X-X) = 3.3169 + 2.2356(X-.625)
.
- 54 -
Analysis of Variance Table for Indole
Source of Variation
Curvilinear
Regression
Degrees of
Freedom
Sum of
Squares
Mean Square
F
C
Linear Regression
on Space Times
I
4.2172
4.2172
50.93
Deviation from
Linear
I
0.0736
0.0736
0.89
Pooled Within
Space Times
6
0.-4967
0.0828
Total
8
4.7875
F-test for Slope of Line
Hq
: Slope is zero (b = 0)
H^ : Slope is not zero
■ F-test for Fit of Data to Line
H0 :
HA :
Fit is good ■
Fit is not good
a = .05
a = -05
F q = 50.93
FC =
Rejection region : We
Rejection region : We
reject H q if [Fq ] >'5,99
Conclusion : Reject Hn
U
0.89
reject H q if [F ] > 5-99
Conclusion : Accept
- 55 -
Mass Spectrometry
In a mass spectrometer a beam of electrons bombards a molecule.
The molecule is broken into ions.
The ions have a particular ratio
of mass to electronic charge, or m/e ratio.
Most ions have a charge
of one so that means that the m/e ratio is the mass of the ion.
A
’’molecular ion" or "parent ion" is an ion which has not lost any
mass and has lost only one electron:
+
-
M
+
e
------M
-
+
Se
Molecular
Ipn
A table or plot of all m/e ratios found for a particular
molecule and the respective relative intensities is called a "mass
spectrum".
In a plot of the m/e ratios the peak with the largest
intensity" is called the "base peak".
It is given a value of 100
and all other relative intensities are calculated from it.
the base peak occurs at the molecular ion.
Often
A mass -spectrum for a
molecule can be highly characteristic of that molecule (3)•
This
can be seen by looking at the API mass spectrums (6) and the experi­
mental mass spectrums which follow.
Thus the mass spectrometer is
a very powerful tool for identifying unknown compounds.
- 56 -
EXPERIMENTAL MASS
SPECTRA DATA
Para Xylene
o-Methylaniline
Relative
Intensities
m/e
m/e
23
77
78
79
89
91
92
105
20
12
6
100
8
k
106
7
77
78
79
89
106
107
o-Ethylaniline
Relative
Intensities
21
55
77
78
79
93
io 6
107
135
21
77
78
79
91
7
21
10
100
6l
5 .
lU
4
106
100
107
9
4
39
4 .
120
121
122
N-n-Propylanillne
m/e
Relative
Intensities
m/e
Relative
Intensities
10
20
5
13
4
100
10
18
p-Propylaniline
m/e
55
77
79
106
107
Relative
Intensities
4
11
9
100
10
118
5
135
18
- 57 -
EXPERIMENTAL MASS
SPECTRA DATA
Indoline
1,2,3,^-Tetrahydroquinoline
Relative
■
Relative
m/e________ Intensities___________________ m/e_____________ Intensities
89
17
91
5
90
13
103
4
91
39
104
7
117
28
115
3
118
100
117
11
119
59
118
i4
129
3
130
11
132
100
133
OO
134
5
- 58 -
MASS SPKCTHAL DATA
A m orican Pvtroleum Institute R e s e a r c h Project 4 4
P i t t s b u r g h , Pa.
C a r n e g ie In stitu te of T e c h n o l o g y
C o n t r i b u t e d by t h e St md ird Oil Cynfhmy ( Indi i n a ) , w h i t i n g ,
S n r ia l Na. 123?
A n ilin e ( g i s )
Mass-Chargv
Ratio
(»i 'f)
2
12
U
11*
15
16
21*
25
26
27
28
29
30
31
31.5
32
32.5
33.5
36
37
33
39
1.0
U
12
13
13.5
Ui
Ui.5
IiS
Ii6
16.5
Ii7
M .l
18
Ii9
50
In d ia n a
Typrt
Relative Intensities
fui ivnuiiig %ollsgv» of
volts
70 roll*
.01
of
Peak
Ma.v.Charge
Ratio
(W7C)
Si
52
53
SL
55
.29
.20
.69
1.03
.20
.10
.39
2.06
3.IL
8.OL
.69
.78
.29
.10
.10
.20
d
d
d
60
61
62
63
6L
65
66
67
68
73
7L
75
76
77
78
79
66
67
88
89
90
91
92
.L9
•L9
3.92
7.L5
17.8
6.37
6.18
1.76
.78
.10
.59
1.18
.20
.78
8.82
.19
.10
.10
.69
3.92
d
d
d
m
Tyne
of
Peak
P
I
i
~ 9 ih
95
A n ril
Relative Intensities
fo r ionizing v iiliagrs of
70
ro/fs
Mass ChargRatio
Type
( H l7C)
Peak
rolls
for iom zii.g
70
Viihiigriof
rolls
vo lts
L.Sl
5-30
1.86
L.71
.20
.29
1.67
2.25
I*.61
2.9L
16.3
33.L
L.31
.20
.29
.98
.69
1.15
1.18
1.67
.10
.10
.10
.10
.10
.20
.98
10.6
100.
6.67
.20
Sensitivity for base peak
if
p ip e tte
IH IVifIOHf
93
. ADDITIONAL INFORMATION
RETASTABLE ION TRANSITIONS
L7.1 (93*)->(66*)
'SO. IOStI
Relative Intensities
10?
Spniitjvity for n Hutane
I n d i v i s i o n s p e r m icro n
R e l a t i v e I n t e n s i t i e s f o r C y c lo h e x a n e
427
69
3 1 .1
Relative Intensities for n Butane
81* 100
15
27
29
43
58
S e n s it iv it y in d iv is io n s per p ip e tte
81* 333
r=--rearrangement
m =m etaslable* ion
p --parent peak
i= iso to p e peak
5 .8 4
3 8 .8
4 5 .1
10 0 .
1 0 .6
d= doubly-charged ion
(«1 i t f u s o p e a k )
COMPOUND
M ASS SPLCTKOMKTKH
Name:
M odel:
2 1 -1 0 2
A n il in e
K lcitron l-u r m it
9 3 .1 2
III
Molecular
Weight
( Cltcher ):
SvmI-structural form ula
v o lta g e s:
0 ” "!
Purity
Source:
I
9 .0
mirroomprrr.r
toffs Il (W V)
3692 I 28
2
Tem perature of ionization cham ber:
rolls
1503
----- e C
Basis of pressure measurem,
J . T . Baker C h em ica l Compargr
Hiofr Jn rcrnt
.0 0 1 m l l i q u i d p i p e t t e
LABORATORY:
S ta n d a rd O il Company ( I n d i a n a ) , h l i i t i n g , I n d ia n a
Stt i ll No. W
- 59MASS SPECTRAL DATA
A m e r ic a n Petroleum Institute R e s e a r c h Project 4 4
P ittsb u rg h , Pa.
C a r n e g i e I n s t i t u t e of T e c h n o l o g y
i s n t r i t'utr-J Iiy th e S t .n .i ir.i Oi I Company ( l n j i . 1n .1l ,
N -E thyl i n i I i n e
T yoc
(»|/«*)
T eak
I n J ia m
A p r il 3 0 . I 9 Vb
S e r ia l No. liM 3
( 9 V .)
Mass-Chargv
Ratio
Ahi t i n g ,
MAss-Charge
Ratio
Ri-lati\f* Intensities
fm ionifIhg VoIiMKfI of
2
.3 $
12
13
Iii
15
16
.2 0
.2 9
.6 7
4 .0 0
• 15
2$
26
27
28
29
30
31
.2 9
3 .0 4
1 1 .0
11. b
5 .2 9
3 .1 1
.1 6
37
3 7 .$
38
39
to
U
12
13
14
4 4 .5
45
4 5 .5
46
4 6 .5
1 .3 5
.0 9
3 .7 7
1 2 .1
2 .2 2
2 .6 0
2 .6 6
1 .0 6
2 . 5°
.0 7
.1 3
.2 0
.0 5
.1 8
d
d
d
d
Tvne
of
Teak
(nii i*)
volts
""70 volt*
55
56
57
5 7 .5
58
5 8 .5
59
5 9 .2
5 9 .5
60
6 0 .5
61
62
63
64
65
66
67
68
MiLSS-Chargi
R atio
Relative Intensities
for iomiinp voUhkm of
70
volts
r o ll!
d
d
d
d
71
.0 7
73
74
75
76
77
78
79
80
81
.1 8
1 .1 1
1 .0 6
1 .0 9
2 3 .3
5 .O8
6 . 3b
.6 6
.0 5
BS
.0 5
70
volt*
VO/ttf
.4 7
.2 b
.Ob
9 3 .4
94
95
103
104
105
106
107
IOd
2 .6 6
.9 8
100.
7 .7 6
.1 3
117
n s
H 9
120
121
122
123
.S i
1 .6 4
•8b
6 .3 b
3 6 .5
3 .1 0
.0 9
P
i
I
.0 5
48
49
50
51
5 1 .5
52
$2 .5
$3
a
5 3 .5
si
d
1 4 .6
.1 1
5 .4 1
87
88
89
.0 7
• 0$
.2 9
d
1 0 .7
.7 3
.7 3
91
92
93
2 .7 9
2 .2 4
1 .7 5
Sensitivity for base peak
in divi#lO*« pcA-mw»w p i p e t t e
541
Sensitivity for n-But.ine
i n d i v is i o n !is p e r m icro n
764
A D DITIO NA L INFORM ATION
R e l a t i v e I n t e n s i t i e s f o r C y c lo h e x a n e
HEiTASTAELE ION TRANSITIONS
$ 9 .2
93 .U
T; r
Peak
(" C f)
.1 6
.1 8
.1 8
.0 4
.1 6
1 .1 1
.3 6
.2 7
• b9
.2 2
.9 7
.4 9
1 .0 6
3 .0 6
2 .3 1
8 .3 2
3 .0 1
.5 1
.0 9
Relative Intensities
for lonizmtr V«HI»*«» vI
69
8b
(1 0 6 ‘ ) -» ( 7 9 * )
*27
(1 2 1 * )-9 (1 0 6 * )
*1$
3 1 .b
R elative Intensities for n-Butane
100
T5
zr
22#
43
S e n s it iv it y in d iv is io n s per p ip e tte
8b
SYMBOLS:
6627
r = rearrangement
m = m eta sta b le ion
(ditfnso pink )
P = p a re n t peak
i= iso to p e peak
5 .8 9
3 8 .8
4 5 .1
100.
. 10.6
58
d=douLly-chargtxl ion
M A SS SPKCTKOM KTKH
COMPOUND
21-102
Nam e.
N-EthZ ! a n i l i n e
Electron current (
Molecular
Height
1 2 1 .1 6
Molecular
Formula
CgH1 1 N
___
Semi structural Formula
Ion accelerating
v o lta g e s:
»102115
Purity
Source:
C U cr -e r
'):
9 .0
•irn'e)
2
Temperature of ioni'/ation ch a m b er:
miVroamperes
volts
(m f )
3692
——
28
volts
1508
*-C
Basis of pressure m easurem ent:
S y n t h e a ia
mo/r pvrernt
. 0 0 1 ml l i q u i d p i p e t t e
Date of m easurement
LABORATORY:
S ta n d a rd O i l C ccpany ( I n d i a n a ) , W h itin g , I n d ia n a
I1/2S/511
S v i i.ii Ni..
-SoMASS SPCCTI!AL DATA
AmeitCAn P e t r o l e u m I n s ti t u te Res oA ich P i o j o c t 4 4
P ittsburgh, Pa.
CArnogio I n s t i t u t o ol T e c h n o l o g y
C o n t r i b u t e d by t i e A t l a n t i c R e f i n in g Coipany, fir i IaUel ph ia, P e n n s y lv a n i a
Mavs-Chargv
Ratio
T vp1
On 1r)
Ve1-Ik
.M.iss-Chargi
Ratio
Relative liiirnsito s
fm iunUMig Vultel:' $ of
3(37
38
39
60
a
O43
4 3 .5
U
4 4 .5
45
4 5 .5
46.
47
48
4 8 .5
49
4 9 .5
50
51
52
53
54
55
5 5 .5
5 5 .7
56
5 6 .5
57
5 7 .5
58
5 8 .5
59
5 9 .7
60
61
62
6 2 .5
63
64
November 30, 1951
S e r i a l No. G3d
C a r ba zo le (gas)
7C volt'
.1 2
.9 2
1 .9 0
5 .6 3
2 .6 4
2 .7 6
.8 6
4 .0 8
.0 3
1 2 .5
.0 3
.3 0
.0 8
.0 9
.0 2
.1 7
.0 3
.2 5
.0 3
2 .5 5
3 .0 4
1 .4 2
.8 8
.3 9
2 .3 6
.1 5
.3 6
1 .0 8
.4 0
1 .9 7
.3 9
.5 5
.0 3
.0 5
.0 9
.2 6
.8 6
2 .3 8
.0 2
4 .5 0
1 .1 7
d
d
d
d
d
d
d
d
d
d
(W f)
(6
67
6 7 .5
68
< 8 .5
69
6 9 .5
70
7 0 .5
71
7 1 .5
72
73
74
75
76
7 6 .5
77
78
79
80
81
8 1 .5
82
6 2 .5
83
6 3 .5
64
8 4 .5
85
86
87
88
69
90
91
92
93
94
9 4 .5
95
Rvlativv Intensities
of
Vcak
d
d
d
d
d
d
d
d
d
d
d
for ionizing Viiltagi-S of
volts
Mass-Chargt
Ratio
T- c
(m c)
Peak
rJQ VOlt S
.2 3
1 .2 5
.0 *
.9 9
.2 1
2 .5 4
4 .9 6
2 .3 4
4 .3 6
1 .2 5
.0 2
.0 5
.4 3
2 .0 3
2 .2 0
1 .2 5
.0 3
1 .1 4
.3 3
.4 0
.2 0
.6 3
.1 2
.6 2
3 .2 0
2 .2 1
1 6 .3
2 .3 2
.1 1
.8 8
1 .3 4
1 .8 8
1 .4 9
1 .8 6
.5 6
1 .8 3
1 .1 5
.3 8
.1 6
.0 5
.4 5
Relative Intensities
for ionizing voltage* of
volts
96
97
98
99
102
103
10 4
105
106
107
108
109
HO
111
112
113
«7q volts
.3 6
.6 9
.9 9
.6 9
.4 8
.1 5
.1 5
.2 7
.1 1
.1 8
.U
.3 4
.4 0
.6 9
.5 0
2 .1 7
1 .6 7
1 .8 6
.8 1
.3 1
.0 7
.1 3
.0 4
.1 5
.1 1
IU
115
11 6
117
118
11 9
12 0
121
122
123
124
125
126
127
128
129
130
131
132
134
135
136
.U
.1 3
.2 5
.3 4
.4 3
.3 8
.U
.06
.0 7
.0 5
.0 5
.1 1
.1 2
S e n sitiv ity for base peak
■* divxiiOKt per m u ron
44
167
AS
S e e s itiv ity for n-Butane
ADDITIONAL INFORMATION
35.9
R ela tiv e Intensities for n-Butane
15
27
29
4.3
58
Vapor T e n p er a tu r e - 560°F
M agnet C u rren t - . 8 8 am peres
SYMBOLS:
r - rvarraogement
m -=InctastnbIc ion
( ililfiise pt-ak)
p parent peak
is-iso to p e peak
3 8 .3
45.1
100.0
10.1
d —doubly-charged i
M ASS S I*KCTKOM KT KR
COMVOUNU
Mtklvl: CEC 2 1 -1 0 1 ( M o d ifie d )
C a r b a z o le
MoloviilAr
Mulrnilar
Weight
Klevtron viirrenl (c a tc h e r
S» mi-structural Kvrmula
Ion accelerating
voltages:
Konnula
C12NoH
1 6 7 ,2 0
£Tc h i 4 la -ic IiM i4 Ii
Vurtty
.Source:
F a s t e n Kodak Compiny
):
(m r )
57
micron Mfirr/x
I oil.*
(w > )
volt-
1880
530
TvmiKraturf of ionizai">n rh.im lifr:
Ilasis of pressure n it.
hinlc fn rrrnt
LAHO kATOItY:
Tbe A t l a n t i c H e f ln ln g C o e;a n y - I h l l b d e l p h l a , P e n n s y lv a n ia
nt: p v t K ea a u rcn en t
200
S f r i I ' N i. M m
C irh i z o l c ( m r I
M astO ia rfi
_
Wf I
137
131
139
149
141
Ty ix*
of
IVak
Uvlaiivv InlviiMtivs
tin !"Iiiiiiiir v»i!lai;i i of
volts
rolls
.7 4
1 .3 9
9.80
6 .6 9
1 .3 1
I'.Z-
.11
143
144
141
."I
146
.0 7
.0 4
.0 4
147
146
149
150
151
152
153
154
155
156
157
156
159
160
161
]'2
163
164
165
1(6
1(8--169
170
171
172
173
174
175
176
177
178
179
IPO
131
1*2
183
.C7
.0 9
.2 0
.2 6
.10
.0 5
.10
.0 3
.06
.02
.0 5
.0 3
.06
.0 7
.21
2.02
2.46
1 4 .2
100__
n:h
.03
.03
.01
.02
.01
.02
.12
.0 9
.7 8
.5 7
.2 7
.2 4
.09
.08
in
.11
W
166
1P7
188
139
.02
.01
100
191
192
193
194
195
196
197
.0 6
.0 2
.11
.02
.1 4
.21
.22
.3 6
.20
.12
.04
SI.ixsOiargi
Ualio
(m'r)
Tynr
Prak
U vlatnr lu ll nsilir*
.".IaxtCliarf I
fo r lo n u in j* w u lU f r s of
(mU)
Type
of
IVak
U v U tiv v
ll.tv iiM liv a
for KiiiiIing ta ils f r a of
—
62 —
MASS SPECTRAL DATA
A m c i i c d n P c t i o l e u m I n s ti t u te R c s o d r c h P r o j e c t 4 4
P i t t s b u r g h . Pa.
C a r n e g ie In stitu te of T e c h n o l o g y
C o n tr ib u te d by th e At Ijn t ic Re f in i n g Conpin y , Phi I jJ e I p h ia , P e n n s y lv a n ia
I n d o le ( g a s )
S e r ia l No. 6 ? 3
fo r
Vvak
( m 'e )
7 0 v o lts
Y o lta
SE
37
3 7 .5
38
3 8 .5
39
3 9 .2 5
ZO
41
42
4 2 .5
43
4 3 .5
44
4 Z .5
Mass-Vhaige
Ratio
Kclntive Intensities
iemiing VOllhg. I Of
Tyoc
.3 1
3 .1 6
.0 2
4 .9 8
.0 1
9 .1 1
.0 1
1 .8 0
1 .1 7
.1 7
.0 9
1 .1 2
.2 2
2 .5 7
d
d
d
d
4 5 .5
46
4 8 .5
d
.0 0 3
4 9 .5
50
5 0 .5
51
5 1 .5
52
53
54
55
5 5 .5
56
5 6 .5
57
5 7 .5
58
5 8 .5
59
5 9 .5
d
.0 1
.9 1
.0 6
L .L b
d
.0 2
4 .4 1
.0 0 3
2 .6 5
.3 3
.1 9
.1 2
.0 8
.1 6
.2 2
.2 2
1 .3 7
1 .1 3
1 2 .2
1 .0 9
.0 5
.3 4
3 .2 4
6 .6 7
d
d
d
d
d
61
62
d
d
71
72
73
74
75
76
77
78
79
80
81
82
8 2 .5
83
8 3 .5
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
ICO
102
d
d
(»• ' e )
63
64
6 4 .5
65
6 5 .5
66
67
68
ADDITIONAL
of
Peak
d
d
I
I
Mass-Charge
IIntio
November 3 0 , 1951
Mass-Charge
Relative Intensities
fur ioniimg voltage* of
v o lts
R atio
Peak
(« ie )
103
10 4
105
106
107
103
109
HO
111
11 2
113
114
115
116
11 7
118
119
120
121
122
123
124
125
126
127
128
129
130
131
132
*70
T 3 .5
5 .0 1
.0 3
1 .5 9
.0 4
.3 3
.2 3
.0 4
.0 9
.0 4
.2 7
1 .1 0
.9 9
.8 4
.5 5
.6 4
.0 6
.0 1
.0 3
.0 2
.0 1
.0 5
.0 1
.2 2
.7 9
1 .3 5
1 .6 9
2 .9 6
2 4 .1
4 0 .0
3 .6 1
.2 2
.0 2
.0 1
.0 3
.0 2
.0 4
Relative Intensities
for ionmng VOltagrr or
Ty „
v o lts
71.0)0"""
.0 2
. .0 2
.0 1
.0 1
.0 1
.0 3
.0 4
.0 9
.0 5
.0 6
.4 4
.8 8
8 .1 5
100.
8 .8 7
.3 3
.0 1
.0 1
.0 0 3
.0 1
.0 0 3
.0 1
.0 1
.0 1
.0 2
.0 2
.6 6
.4 1
.0 4
P
Sensitivity for base peak
103
.0 2
.0 8
t * d iv u w n u p # r W iirre*
117
16
Sensitivity for n-Butane
43
3 9 .4
R elative Intensities for n-Butane
Vapor T em perature - 560»?
M agnet C u rr e n t - .8 8 a n p eree
SYMBOLS:
r=-rearrangem ent
m —mctRstdble ion
p = p a ren t peak
i= iso to p e peak
COMPOUND
Molecular
Formula
1 0 0 .0
1 0 .4
M ASS SPECTRO M ETER
'
Semi structural Formula
CfBTN
Purity
Eastman Kodak Company
43
58
d=doubly-charged ton
HCICHIj CCICh' iT n H
1 1 7 .1 4
Source:
3 7 .4
(itiflusewnk)
N ,m e: I n d o le
Molecular
Weight
IS
27
CEC 2 1 -1 0 1 (Modi f i e d )
Electron current, (c a t c h e r
) :
(m /r )
Ion accelerating
57
v o lta g es:
Temperature of ionization cham ber:
B . , i , o f prfM ur, m ,.,u r c m o n t:
m
9
microamperes
volts
(m e)
200
1880
volts
530
•c
He e s u r e o e n t
tnolr prrermt
Date of measurement
LABORATORY:
The A t l a n t i c R e f in in g Cotryvmy - P h ila d e lp h l e , P e n n a y lv h n la
2/26/51
— 63 —
MASS SPECTRAL DATA
Am ericon Pefioleum Im tiiule R e ieo rch Project 44
C ollege Stofion, Texoi
TeKOi A & M University
( .MiiinLntf-il by ihr Vni-iri Oil Cum|ianv of ( .ilifomia, Mn a. California
he I el l%r
Of
to
Charfr
|n l
el,-v I
0 .3387eaprrri
lOfimn#
l0 i e l
24
25
26
27
28
29
30
31
32
32.5
33
34
0.05
0.22
2.41
5.14
36
37
37.5
38
38.5
39
39.5
40
40.5
41
42
43
43.25
43.5
43.75
44
44.5
45
45.25
45.5
46
0.10
1.77
0.03
3.70
0.05
7.95
0.02
I t 16
0.02
1.17
1.46
0.18
0.05
0.03
0.05
0.07
0.06
0.01
0.01
0.02
0.01
For
48
11 inp woltepri
welli
0.33fl7aef,rri
For Ion !lira i oIIIjrrs of
for ifwiiinx loltigre of
of
70 »e |i ,
70 m in
0.01
»al 11
0.01
0.72
0.16
6.44
0.19
10.74
0.54
5.37
0.25
1.28
0.48
0.07
0.03
0.05
0.05
0.17
0.15
0.09
0.03
0.11
1.30
3.83
0.05
7.61
0.50
3.65
6.29
11.03
7.54
0.99
0.04
0.08
Al Wapmet ir fir Id ef
of
0.3387a.pe»
0 .0 6
48.5
49
49.5
50
50.5
51
51.5
52
52.5
53
54
55
56
56.5
57
57.5
58
58.5
59
60
61
62
62.5
63
63.5
64
64.5
65
65.5
66
66.5
67
0. 16
0.07
0.03
0.01
0.02
0.01
0.02
ion
70
w el l,
P e la t Iwr ln le n n I w
Al Ojrurtlr tirld of
Of
0. 3!87aaprf ri
,ea r.
2 .8 6
InienMly
I w
Al earivrl if Iir lit ef
Of
v o l n r - a of
11
I we IIi I e ii > i
P rln l
l>
Al eiegertit fir I I of
IeT
Octobe r 31. 1963
S e r ia l No. 1907 '
2-M ethyl in d o le
0.01
0.03
0.39
2.51
3.13
3.34
14.92
2.62
0.28
0.11
0.05
0.15
0.09
0.08
0.30
0.67
0.97
0.80
•4.42
3.44
0.67
0.07
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
0 .2 2
0 .0 2
109
111
112
113
114
115
116
117
118
119
120
0 .0 1
0 .0 2
0 .0 3
0 .0 2
0 .1 0
0 .2 1
0 .3 4
0.21
0 .1 3
0 .2 6
0 .0 4
0 .0 1
123
124
0 .0 3
0 .0 1
no
126
127
128
129
130
131 p
132
133
134
0.02
0.07
0.36
97
98
99
105
106
•
0 .1 0
0 .3 2
2 .9 2
3 .6 3
1 0 0 .0 0
7 8 .5 2
7 .6 1
0 .4 4
0 .0 2
0 .2 9
0 .2 7
100
101
1.63
4.87
10.26
2.98
102
103
104
COMPOINM
Name: 2 -Methyl in d o le
Molecular
Molecular
Weight
Formula
131.17
n-HEXADECANF.
n-BUTANE
Approximate Approximate Approximate
Boiling Point Freezing Point
Ilrnsttyz iI
C9H9-N
'c
•c
Magnetic Field:
Magnetic Field:
Total Ionization:
Total Ionization:
4iv/eieron/*irr»«ej>«rr
Semi-structural Foimula:
2 3 .011
4i»/IeeWeZaicreeeprr «
Sensitivity .1
CAc
d>.
WiwZleetrfa Zairraeaprrr
Sensitivity nt
43:
4 i » / a i r r o n /a i r r e e e p r r #
57:
r f i w / m ir r o n Z e ir r e m p r r r
rfi w Z l e a t r f a / a i r r e a e p r r t
d i * / I a a W a Z e irre e e p rrr
I/ll
0 .4 6 5 eeprrrr
15.6
______
l / l l ______________
A tla litt /n ir e n ly
I* Ifni I Ir
57
Source: U. S*. Bureau o f Mines
Petroleum K esearch C enter
Laramie, Wyoming
_______ through API K esearch P r o jec t 52
Total Ionization for Compound
Purity:
71
85
99
113
4 i» /a ir re * /e ir re e e p # rr
127
100.0
5 4 .6
3 6 .2 8 .3
5 .0
3 .5
141
155
160
183
IQT
226
2.8
2 .4
1.8
0.6
2.8
4 i » / Ie e W e Z e irre e e p irr
XiMitionaI Information:
Sensitivity for Base Peak:
rfiw/Iaetrfe Zeirreeaprn
M\ » M1KI l IiMMI Il Il
Maker and Model
Ionizing
C o n so lid a te d Xl mle I No. 2 1-103 (A Io Iifird )
C olin tor
Ion Chamber
Slit Width
Temfirnitunr
Temper Iliire
240 V
SO air re,.,art i
270 V
Sample P ressu re and ILis is o f Xnisurem ent: G la ss c a p i ll a r y
dipper (.ilmul one m ir r n lile r s o Iumf I
_____________ _______
I. AIKIl IAl I)l IX Viuon U tl Company o f (^i 11 fo rm a
Ilrsrarrh ("enter
Ilrea . Ca 11 fo rm a
P ilr of Mf isiin m il t Septem her 12.
I Of,2
Si rial No. 1907
- 614 MASS SPECTRAL DATA
A m erican P clioleum In ililu le R eio o rch Projecl 44
C ollege Slolion, Texoi
T exoi A&M U n iv e n ily
Cntitiilfiitr.J |»v IIir t'nion Uil V.iim|i.iny o f C alifom ij, Hrra1 Cali fomie
I>| I a l l i r
Of
to
Charyr
In I r n « .
P r I .,I I t r
II %
Al n r n f l I f f I f Iil
O
f
70
I OHI 11* , r o l l
•«•••«
0 .0 4
0 .2 0
2 . Il
4 .2 9
5 .6 6
0 .2 2
0 .0 8
0 .0 2
0.0 1
0.0 1
0 .0 2
36
37
3 7 .5
38
3 8 .5
39
3 9 .5
40
4 0 .5
41
42
4 2 .7 5
43
43.25
4 3 .7 5
44
45
4 5 .5
0 .1 0
1 .6 1
0 .0 5
2 .8 7
0 .0 3
5 .7 5
0 .0 1
0 .5 9
0 .0 3
0 .4 9
0 .1 2
0 .0 2
0 .0 9
0 .0 7
0 .0 5
0 .0 4
0 .0 1
0 .0 2
48
4 8 .5
49
0 .0 6
0 .0 2
0 .7 6
I n I r n « 11 v
firM
of
nf
h i-
of
u .» .
( Iiaryr
70
tell.
i ill 11
24
25
26
27
28
29
30
31
32
33
34
Al Ma fnr i
0.3 3 8 . B a p r r r I
0 . 3 3 8 7 y « r # f #•
In r
O ctohrr 31, 1963
S r r m l No. 1908
3 *Mr thy I m l" Ir
4 9 .5
so
5 0 .5
51
5 1 .5
52
5 2 .5
53
54
55
56
5 6 .5
57
5 7 .5
58
5 8 .5
59
60
61
6 1 .5
62
6 2 .5
63
6 3 .5
64
6 4 .5
65
6 5 .5
66
6 6 .5
67
72
I o il i
0 .0 3
I «r
I n ir n s iI y
I)..3197...,,»,,,
73
74
75
7 5 .5
76
7 6 .5
77
78
79
80
81
82
83
84
85
86
87
88
89
8 9 .5
90
91
92
93
97
98
99
100
101
102
103
104
131.17
C9 H9N
Al
Of
0 .5 3
3 .8 0
4.2 7
0 .0 2
4 .0 3
0 .0 3
17.41
2 .7 2
0 .2 5
0 .0 6
0 .0 4
0 .1 3
0.0 7
0 .0 5
0 .1 7
0 .3 6
0 .6 0
0 .4 1
1 .1 9
0 .0 7
0 .7 1
0 .2 6
0 .0 4
0 .0 1
0.1 1
0 .5 9
0 .3 2
0 .2 5 .
1.67
6.3 1
10.01
2 .0 9
fir|J of
For loniiinr toll «r<i e f
Charge
ir 111
70
105
106
0 .1 5
0 .0 1
HO
111
112
113
114
115
116
117
118
119
120
0 .0 1
0 .0 2
0 .0 2
0 .0 3
0.1 1
0 .1 9
0 .1 4
0 .0 8
0 .0 3
0.0 1
0 .0 1
123
124
125
126
127
128
129
m
111 p
132
133
134
0 .0 2
0 .0 1
0 .0 2
0 .1 3
0 .2 9
2 .7 9
3 .4 3
to I II
1 P Q .P .0 .
57 .5 6
5.3 2
0.2 7
0.0 1
STANDARDS
■ HEXADECANE
d-BUTANE
Approximate
Approximate Approximate
Boiling Point I reezing Point
llcnsilJ ,.,
..
'c
•c
•c
Magnetic Field:
Magnetic Field:
Total Ionization:
Total Ionization:
4
4
23.011
ie/e Itre*/e irre«nee#r<
Semi-structural Formula:
4
Sensitivity at
0 &
Vi,
charge
4 i» /e ir ro * /e e c r * * ie e r #
jir/aicron/mierrmpere
V l,Z le B 6 V « / e i t r e w ,# r #
4 i i / l « e 6 4 r f / e i < r * ^ p r r#
//vI
15 .6
ftrlalirr Imlenitlr
57
71
85
99
113
127
Purity:
Total Ionization for Com|*ounJ:
0 .4 6 5 eager#*
V i,/le e f c V e /e ie r o « e p r r <
l»/leel «.'eirrwwp#r
Source: t). S . Bureau o f Mines
Petroleum B esearch C enter
Laram ie. Wyoming
________ thronr.h API Hnsrarrh P r o te c t 52
M ifn r I r
0. 3387fleprrrI
MtW fl
COMPPl NI)
Name: 3 -Mcthy I in d o le
Molecular
Molecular
Weight
Formula
IV I*i I vr I li I r n s i I v
IU I,
.....
f or ient » me ml lrerr of
70 iof li
irlli
0 .1 9
6 .5 3
0 .1 8
11.59
0 .5 3
5 .2 2
0 .2 6
1.0 9
0 .3 6
0 .0 4
0 .0 3
0 .0 4
0 .0 6
0 .1 3
0. 14
0 .0 9
0 .0 2
0 .1 0
1.0 4
0 .0 1
2 .4 3
0 .0 5
4 .9 3
0 .5 1
2 .7 5
5 .5 5
1 0 .4 5
6 .5 3
0 .7 5
0 .0 3
0 .0 5
I Ii I
Al rarnriir firld nf
100.0
5 4 .6
3 6 .2
8 .3
5 .0
3 .5
111
-SiM
141
2.8
155
2 .4
169
183 _
1Q7*
1.8
226
0.6
2.8
4 « w /le e W e /e if r < » « e p r r #
XdJitionaI Information:
Sensitivity for Uase Peak:
Vl»/IemkJe/«ir» o«»p#f #
MXxs M 'l.riUPM I Il Il
Maker and Mo'h i CotisnI n l.iI n l My,It I No. 21-103 (Xknl i f ir I)
Vapor
Ionizing
hm ( hatrlw r
( olh i lor
Mil Width
Currrnl
Temprmiurc
Trmprralwrr
45 . . r „ . . „ „ .
:? o *c
S .m |i|r P i r s s u r r and I k ii s is nI Vrasurrn-Tiit: C la s s c a p i ll a r y
'lil I ■ I I ■I • hi
" 11 r ••I 11 »• r •IU
LAIHMIA IUUY: I n ion Oi I Con pani o f Ca I I fo i n ia
Bvsrarr Ii O n t r r
U rea, Ca 11 Iornta
I *.il I- of
Mn-n r -IiI
Si- p
Serial No. I'«18
M A SS S P tX T H A L D A TA
A m c iiC A n P e t r o l e u m I n s ti t u te R e s e a r c h P r o j e c t 4 4
P i t t s b u r g h , Pa.
C a m e g i e In e ti t u to o f T e c h n o l o g y
C o n lr ib u t e J by th e A t l a n t i c R e f in in g Company, P h ila d e lp h ia , P e n n s y lv a n ia
I ^ -D iT ie th y l in d o le
MasvCharge
Ratio
Tync
(n 't)
Vnk
3 3 .6
36
37
3 7 .5
38
39
40
41
U
4 2 .5
43
4 3 .5
44
4 4 .5
45
4 5 .5
46
4 6 .5
47
4 7 .5
48
4 8 .5
49
4 9 .5
50
5 0 .5
51
5 1 .5
52
5 2 .5
53
5 3 .7 5
54
5 4 .5
55
5 5 .5
56
5 6 .5
57
5 7 .5
58
(g a s)
November 3C, 1951
S e r ia l No. *329
Relative Intensities
for iomimg vollncvt of
rolls
7 0 r o ll.
.o n
.0 4
.8 8
.0 1
2 .0 8
6 .6 1
.9 7
1 .6 0
2 .9 6
.0 1
.3 2
.0 2
1 .8 3
.0 3
.0 6
.0 5
.0 2
.0 2
.0 1
.0 1
.0 6
.0 1
.3 6
.0 7
4 .6 7
.0 8
7 .8 2
.0 9
2 .5 2
.0 1
.8 2
.0 1
.2 3
.0 2
.2 4
.0 4
.1 8
.0 8
.4 0
.9 4
.4 7
d
d
d
d
d
d
d
d
d
d
d
d
d
d
d
d
Mass-Charge
Ratio
Type
( m -r )
Peak
5 8 .5
59
5 9 .5
60
61
62
63
6 3 .5
64
6 4 .5
65
6 5 .5
66
67
68
6 8 .5
69
6 9 .5
70
7 0 .5
71
7 1 .5
72
7 2 .5
73
7 3 .5
74
75
76
77
78
79
7 9 .5
80
81
82
83
84
85
86
67
ADDITIONAL
d
d
d
d
d
«
d
d
d
d
d
d
d
Relative Intensities
for iorming volumes of
vo/f#
VO voffs
1 .7 0
.2 6
• 10
.0 5
.7 1
2 .0 7
4 .8 5
.0 3
1 .4 5
.2 0
2 .1 2
.1 0
.5 7
.1 3
.1 1
.0 1
.0 4
.0 3
. 06'
1 .0 0
.6 0
3 .4 7
4 .2 0
7 .4 5
1 .1 3
.0 1
2 .7 1
3 .6 3
3 .4 9
1 1 .2
2 .0 7
.2 6
.0 1
.0 5
.0 3
.0 2
.0 2
.0 5
.2 2
.4 8
.8 0
MasvChargc
Ratio
Type
(mU)
Peak
Relative Intensities
for iontiint v(-Itare* of
volts
88
89
90
91
92
93
94
95
96
97
98
99
10 0
101
102
10 3
104
105
10 6
107
10 8
109
no
in
112
113
114
115
11 6
117
118
11 9
120
121
12 2
123
124
I
7 0 i offs
.6 0
1 .9 2
.8 0 *
2 .4 7
.3 2
.0 5
.0 2
.0 2
.0 2
.07
.3 6
.2 8
.33
2 .0 8
6 .1 7
5 .8 6
1 .4 2
.1 6
.1 0
.0 4
.0 1
.03
.0 6
.0 6
.0 9
.3 9
.6 5
5 .6 9
1 .8 6
1 .4 7
.65
.0 6
.0 2
.0 1
.0 1
.0 2
.0 1
.02
S ensitivity for base peak
nt dirwto** p#r micron
144
N FORMATION
62
Sensitivity for n-Butane
43
3 9 .4
R elative Intensities for n-Butane
15
27
29
Vapor T e n p era tu re - 560»r
M agnet C u rren t - .6 8 am peres
43
58
SYMBOLS:
p —parent peak
i=isotopc peak
r—rearrangement
m =m etastnble ion
(HifIiKe peak)
d=doubly-charged ion
4 4 .2
1 0 0 .0
1 0 .4
66
S o r i 11 No.
I . I-D im v th y l in -lo le In i'.)
Mfl-AChnr^o
Tvnc
(wi'r)
Teak
326
327
128
129
130
131
132
133
134
D5
136
139
140
141
142
143
144
JU 5—
IlvlaliVP I nlvttNitie*
f o r HWiiniiK w ollB i-es o f
r o //i
IOvoUs
.0 6
.7 6
3 .5 5
.60
.0 6
.03
.0 5
.0 5
.0 3
.0 3
.2 3
.4 6
2 .3 6
8 .7 7
100.
U6
147
149
153
154
155
156
157
158
159
160
161
165
166
167
.3 7
.0 5
.01
.01
.01
.01
.02
.1 3
.2 6
.0 3
.0 3
.01
.01
.03
M«vv Vhitrgv
IliltlO
(m /r )
Tyne
of
Teak
Itelalive Iiili nvilua
f o r IO iim nic v u h » i:v s o f
M.i.Vi C h a rg i
R atio
( m /z )
Ty lx*
of
Pvak
H cla liw IntviiMtiv*
- 6? M ASS SPCC TnA L DATA
A m e ii c a n P e tio le u m Institute R e se a r c h Project 4 4
P i t t s b u r g h . Pa.
C a r n e g i e I n s t i t u t e of T e c h n o l o g y
C o r t r i b u t o J by t h e At h in t i£ K e f in ng Ctmt any. Phi l. i d el ph i. i, P e n n s y lv a n i a
Mass-Charge
Katio
T ype
7 0 vo/fx
• 28
2 .9 7
.1 0
4 .2 6
.0 3
6 .3 6
.9 2
.1 4
.7 5
.4 2
.0 1
.0 1
1 .1 2
.0 3
2 .4 8
.0 1
.0 8
.0 1
.0 2
.1 7
.0 6
1 .7 8
.4 7
1 2 .7
.3 9
1 7 .9
.8 4
4 .7 2
d
d
d
d
d
d
d
d
.1 9
.0 8
.1 3
.0 2
.6 5
.2 8
1 .7 5
.0 3
d
.0 7
5 .7 6
.2 8
d
6 2 .5
63
6 3 .5
M.iss-Chaigv
Kalio
Kvlatixc Iniensitivs
fm limiting xultegi of
Vrak
( m 'r )
?o
37
3 7 .5
3B
3 3 .5
39
40
< 0 .5
<1
42
< 2 .3
< 2 .6
<3
4 3 .5
44
4 4 .5
45
<6
47
48
4 8 .5
49
4 9 .5
50
5 0 .5
51
5 1 .5
52
53
54
55
56
57
58
59
60
61
6 1 .5
November 3 0 , 1951
S e r ia l No. 626
Q u in o lin e Ig is J
(m /c)
Tyne
of
Peak
volts
d
6 4 .5
65
6 5 .5
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
Masv Chargi
Ratio
Relative Intensities
ftr i,mixing voltages of
d
101
1C2
103
Relative Intvnsitiea
for IOlIitmg
Tvnr
Peak
rJQVOlti
7 0 vo/fx
( "i > )
— 164
2 .4 9
.5 4
5 .4 2
.0 5
1^5
.0 8
1 .0 8
106
.0 2
in ?
.0 3
108
.C l
.2 2
.0 2
10 9
.1 2
.0 2
HO
.0 8
.0 7
111
.0 3
112
.0 3
.0 5
.0 2
.0 6
113
.0 7
.1 2
114
115
.0 4
1 .0 5
6 .0 8
116
.C l
117
.C l
9 .3 0
.0 1
118
1 0 .5
119
.0 3
3 .4 7
.0 2
120
2 .4 8
.7 8
12 1
.0 2
122
.0 2
.2 0
.1 0
.4 2
123
.2 0
.0 4
124
.0 8
.0 2
125
.1 0
126
.1 0
.2 6
127
2 .0 9
123
.4 3
1 6 .5
ICO
12-7
.8 7
P
130
1 0 .5
.4 0
.4 7
.6 7
131
132
.
.0 3
.1 5
.0 9
.0 3
133
.0 1
134
.0 5
.1 7
.6 8
135
.3 4
.0 5
.0 7
.2 4
.9 2
.8 5
1 .0 0
Sensitivity for base peak
5 .8 2
t* t/iVuioM*
auVrva
2 2 .5
129
7 .3 7
prr
Sensitivity for n-Butane
ADDITIONAL INFORMATION
4 ^ .1
43
Relative Intensities for n-Butane
IS
27
Vapor T e c p e r a tu r e - 560»?
M agnet C u rr e n t - , 88 an p o res
58
d - doubly-charged ion
P = p a re n t peak
i = isotope peak
SYMBOLS:
m.-=mctastable ion
(H.itTuse peak)
M ASS S PECTROM LTKH
COMPOVND
Model:
^ em e * Q u ln o lin o
CEC 2 1 -1 0 1 (K c d lM e d )
Klectrim current ( c a tc h e r
Molecular
weight
1 2 9 .1 5
Molecular
Formula
C9 H7N
Semi structural Formula
Ion accelerating
v o lta g es:
NiCHijfCCiCHi3CM
Purity
Source:
):
(m 'r )
57
9
microamjicrcx
In V I
1880
20 0
530
TemjHrature of ionization cham ber:
Basis of pressure m easurem ent:
Eastman KoJik Company
mo/r prrrrnf
LAHOKATOItY:
3 7 .3
4 4 .1
1 0 0 .0
1 0 :4
FVT H ea eu ren o n t
Date of measurement
The A t l a n t i c R e f in in g Cocpany - P h i la d e l p h i a , P e n n s y lv a n ia
3/1751
- 68 -
MASS SPECTRAL DATA
A m e n c a n P e t r o l e u m I n s ti t u te I t o s e a r c h P r o j ec t 4 4
P i t t s b u r g h , Pa.
C a i n o g i e I n s t i t u t e of T e c h n o l o g y
Conl r i but-vt b> t hv Union O il Cprrp .in y o f C a l i f o r n i a , B rea , C a l i f o r n i a
G-V. tt'v ’ c u i n o li n o
Mass Chargi
Ratio
Tvn
f HI V )
Peak
V r iiI
Ih I.itive Intensities
I..I Ionmng vntlugr* of
7 . VftIlK
I ■ .5 9 0
7.)
rolls
Mass-Charge
Ratio
No. IbvO
of
Peak
( Oi Ir)
0 .4
0 .3
■1.3
2 .9
7 .1
7 .3
3 -9
25
26
27
28
29
30
31
32
0 .3
3 .5
5 .7
2 9 .5
0 .8
0 .8
0 .3
4 .4
0 .4
4 .7
7 .3
3 0 .5
0 .9
1 .0
0 .3
4 .4
36
37
38
39
40
41
42
<3
44
0 .1
2 .6
5 .6
1 5 .4
2 .5
2 .0
1 .6
1 .2
1 .6
0 .3
33
6 .7
1 6 .9
2 .7
2 .2
1 .7
1 .2
1 .7
48
49
50
51
0 .3
1 .0
7 .0
0 .3
1 .1
8 .2
9 .8
4 .0
1 .5
0 .4
0 .6
0 .9
52
53
54
55
56
3.4
5 7 .5
58
5 6 .5
2 .4
7 .6
70 I offs
I • .5 9 0
I ■ .7 5 0
12
13
14
15
16
17
16
O cto b er 3 1 . 19!«8
Mass O iargi
Ratio
Relative Intensities
for ioniitn? v.V.legcs of
70
rolls
Relative Intensities
f
for ionizing VVlUKMO
T
Peak
<in Se)
70
59
60
61
62
63
64
65
66
67
68
0 .6
0 .3
2 .7
7 .0
1 4 .4
4 .4
5 .1
2 .5
0 .6
0 .2
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
66
87
88
89
90
91
92
93
94
95
96
97
98
99
4 .5
5 .4
0 .6
0 .7
3 .6
4 .3
3 .0
2 .6
1 .4
1 .0
0 .5
0 .2
rolls
I ■ .5 9 0
I * .7 5 0
0 .2
0 .3
1 .1
1 .9
3 .0
2.6
8 .5
2.6
1.1
1.6
1.7
0.2
70 volts
I • .7 5 0
•
100
101
102
103
104
105
106
107
108
109
HO
111
112
H3
114
115
116
117
118
119
120
121
0 .5
0 .8
0 .4
1 .9
6 .4
0 .8
0 .2
0 .3
0 .3
0 .4
2 .1
3 .2
1 6 .3
6 .2
4 .4
0 .4
0 .1
0 .4
0 .8
127
128
0 .5
0 .8
134
135
0 .6
0 .5
140
141
142
4 .8
1 1 .7
4 4 .8
m e
5 .9
143
P
144
145
0.4
"Ho in t e r f e r e n c e
Sensitivity for base peak
0 .1
INQIVlilON
0.6
7 6 .4
143
ADDITIONAL INFORMATION
Sensitivity for n-Butane
43
1 7 9 .8
Relative Intensities for n-Butane
1 0 .7 0
4 5 .0 0
4 4 .4 0
IS
h3 c X
^
M
SYMBOLS:
p—parent peak
i = isotope peak
_________________
r ---rearrangement
in—mctastable ion
M
27
29
43
100.00
58
1 1 .9 0
d=doubly-charged ion
( 'I itT u s e p ^ a k )
MASS SPKCTROMKTER
Model:
6 -M eth yl Qui n o I in e
CCC 2 1 -1 0 2 (M o d ifie d )
Klcctron current (
10
microamperes
Semi structural Formula
See a d d it io n a l
inform al ion c o l umn
Purity
Jon accelerating
voltages:
I .
Temperature of ioniration chamber:
270 "C
Ilaxis of pressure measurement:
API R esea rch P r o j e c t 52
molr prrrrnt
LABORATORY:
M icromanometer
Date of measurement
U nion O il Company o f C a l i f o r n i a , R esea rch C e n te r , B rea, C a l i f o r n i a
J u ly 17. 19b8
S e r i a l No. 1500
-
69
-
MASS SPECTRAL DATA
A m e r ic a n P e l io l e n m I n s tit u te R e s e a r c h P r o j e c t 4 4
C a r n e g ie I n s t it u t e of T e c h n o l o g y
P itt s b u r g h . P a.
C o n tr ib u te - by th e Un Ion Ci I f o - n my o f C i l if o r n i.- i , B r e t , C a l i f o r n i a
7-V r-thylo i r n c l in e
M assriinrgi
Katin
T- t t
(m'c)
Peak
Iu-Iatiw Intm sit -'S
f"i Iiirumg \oliaei - of
volts
2
.* 2
25
26
27
28
29
30
31
32
33
36
37
38
39
.ill
3 .8 6
6 . Ik
2 k . *.9
1 .C 2
.1 0
.1 5
3 .8 0
.0 9
.2 0
2 .9 2
5 .2 9
1 2 .8 1
1 .7 5
1 .7 5
.3 5
l.k 9
.1 7
•57
.2 3
.1 5
.1 6
.Ok
.Ok
.2 6
1 .0 9
.1 2
8 .3 8
.Ok
9 .3 6
.0 6
3 .2 7
.Ok
1 .0 6
.2 5
.0 3
Io
Ul
U2
1*3
1-3.5
kit
U .5
15
16
W
IO
1.9
1 9 .5
50
5 0 .5
51
5 1 .5
52
5 2 .5
53
$u
5 k .5
55
5 5 .5
56
^6. S
C v to h er ' I ,
S eria l No. 14IV
( n 's )
7C oil*
Maits-Vliargi
Kntio
(nrr)
Tvm
Kvlativc Intensities
for ioniring voltage* of
of
Peak
volts
Co
61
62
63
6k
65
66
67
68
69
6 9 .5
70
7 0 .5
71
7 1 .5
72
73
.67
7k
3 .9 k
k .6 k
2 .9 7
75
76
77
.87
.5 7
.25
2.27
78
79
80
81
82
83
8k
85
66
87
88
89
90
.1 .1 7
.k l
.1 5
.1 5
.1 2
.1 5
.2 9
1 .0 6
1 .9 0
92
.k l
2.67
2 .k 8
7.86
(m'c)
Peak
Kelative Intensities
fin ienmng vi llage of
7 0 volts
9k
95
96
97
93
99
100
101
1 02
103
IOk
1 05
106
107
103
109
HO
JJl
112
113
Hk
u s
Ho
H7
HS
119
120
121
122
123
32k
.1 3
.lb
.0 9
.2 6
.6 6
3 .6 9
2 .k 2
5 -3 5
2 .k 6
•51
.8 2
.8 0
.2 3
.1 9
.1 5
.2 0
.2 8
.2 9
.2 9
2 .0 6
3 .2 8
1 6 . kk
5 .6 0
5 .2 6
.kk
.5 8
.2 2
.0 9
.Ok
.1 2
.0 1
126
12 7
123
129
130
131
.0 9
.5 0
.9 2
.1 6
.1 0
•3k
2 .k 2
Sensitivity or base pea
1* divmost p e r m i n on
8 5 -9
U 3
.26
ADDITIONAL
TY.m
7 0 roll*
1 .6 0
2 .3 k
•kk
7 .3 9
.kk
.2 3
2 .k 3
6 .0 6
U .9 C
3 -6 7
3 .k 8
.5 8
.2 8
.2 2
.2 6
.1 3
.3 2
k .9 k
.2 3
k .9 1
• 35
57
5 7 .5
53
5 8 .5
59
M ass Chnrgi
Ratio
19b7
N FORMATION
Sensitivity for n Butane
43
1 6 8 .3
H
Relative Intensities for n-Butane
O U r ^ x
M
SYMBOLS:
r
p --parent peak
i= iso to p c peak
rvarr.InVrmviit
last.Able inn
(iliffnsv peak)
1rr
x x H
15
27
29
43
58
M
f -Mcth y I q u i n o l in e
ik i.ie
C in A v 1
C o n s o lid a te d # 2 1 - 1 0 3 Ilodli- I c l
Klertron current (
Svnii-atructural lurm uln
5 pi- iitrlI I ion 11
•fOfrr.it inn ro ! urtn
Purity
Bureau o f M in es, Laram ie
k k .2
1 0 0 .0
1 1 .9
M A SS S I*KCTKOMKTKK
Model:
Molecular
Formula
k7.6
d —doubly-chargvd ion
COMPOUND
Name:
Molecular
Weight
was n o t scanned
):
IbOO
TvrniHrature of ionization cham ber:
liaxia of pressure mvaaurvment:
LAliURATOKY:
10 micronIiiiicrrs
I Ml 'l' )
Ion nccvlvrating
voltages:
50
rolls
1112
2/C" C
hlcroTanomclcr
Dale nf measurement
Union O il Company j f C a l i f o r n i a , r e s e a r c h C e n te r , D rca, C a l l V c m l a
A p r il 1 0 , 1957
Serial No. 1419
- TO 7-Vf’t k v I r j '
I
Of
(W e)
Pvak
fui ionilinf Vull*;i%of
13.3
.1,
I !■
.C-’1
135
1 36
] 1.0
.02
U .39
1 1 .1 5
P
I
I
I
M iis v C h a r K - -
(m/#)
.1 3
.1 7
ip
141
112
i'. 3
ITT
U5
U6
1;:8
^.--r I 'I \ r .
I T>r I
Uvlative Intviisitiis
MassCharg*
Katio
U3.15
o.l.,
ICO. CO
.U
.1 5
.0 9
T!,r
I'rak
Kvlativc
IiitiiiMtivs
for iomimf vvll»r«iof
CharE1
Kaliu
(m /e )
Ty Iiv
of
Peak
! I !° . r 'n-'
Kvlatixv IntvnaitiVA
for ioiiiting ioltegrsof
- TlMASS SPECTRAL DATA
A m e r ic a n P e tr o le u m I n s tit u te R e s e a r c h P r o je c t 4 4
C a r n o g io I n s t it u lo o f T e c h n o l o g y
P itt s b u r g h , P a .
C o n tr ib u te d by th e A t l a n t i c K c f i n ir g Company. P h ila d e lp h ia ,
2 , 6 - D im e t h y lq u in c lin e ( g a s )
.Maas-Chnrgt
Ratio
S e r i a l No. 63 1
Relative Inti iisitiea
T P,
for iont/inp V v lie c n of
Peak
36
37
3 7 .5
38
3 C. 5
39
40
U
42
43
4 3 .5
44
4 4 .5
45
4 5 .5
46
48
49
4 9 .5
50
5 0 .5
51
5 1 .5
52
5 2 .5
53
54
5 4 .5
55
5 5 .5
56
5 6 .5
57
5 7 .5
5ft
5 8 .5
59
60
61
6 1 .5
62
A? A
P e n n s y lv a n ia
d
d
d
d
d
d
d
d
d
d
d
Mass-Chai g<
Ratio
7 0 ro/fa
(rnU)
.0 3
.7 4
.0 1
2 .0 0
.0 1
7 .9 8
1 .1 7
1 .0 7
.9 9
.3 8
.0 2
2 .2 0
.0 3
.0 5
.0 1
.0 1
.0 2
.2 8
.0 7
4 .2 0
.0 8
7 .1 7
.1 5
2 .8 0
.1 5
1 .2 1
.2 3
.0 6
.2 9
.0 6
.1 4
.1 1
.4 3
1 .3 5
.2 9
.0 7
.0 2
.0 4
.7 3
.0 3
2 .6 8
63
6 3 .5
64
6 4 .5
65
6 5 .5
66
67
68
6 8 .5
69
6 9 .5
70
7 0 .5
71
7 1 .5
72
73
74
75
7 5 .5
76
7 6 .5
77
7 7 .5
78
7 8 .5
79
7 9 .5
80
81
82
83
84
85
86
87
88
89
8 9 .5
90
I?
of
Peak
November 3 0 , 1951
Mass-ChargRatio
Relative Intensities
ionizing vultegvs of
for
d
d
d
d
d
d
d
d
d
d
d
d
Ql
7 0 vo/fs
(m /e)
6 .9 5
.5 8
3 .2 7
1 .0 7
5 .8 5
2 .1 2
.6 1
.1 0
.0 6
.0 1
.0 8
.0 7
.1 0
1 .1 3
.3 2
.0 4
.0 2
.2 0
1 .8 8
2 .4 6
.1 5
1 .8 0
.9 1
4 .9 8
2 .2 4
3 .2 9
5 .6 1
.9 6
.0 7
.0 9
.0 4
.0 3
.0 5
.0 5
.3 3
.7 0
1 .2 0
1 .1 8
4 .4 6
.0 5
3 .1 8
.8 9
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
107
108
109
HO
111
112
113
IU
115
116
117
118
119
120
121
122
123
12 4
125
126
127
128
129
Relative Intensities
for ionizing vo lieg rs of
Peak
volts
7 0 volts
.1 5
.0 5
.0 4
.0 2
.0 2
.0 8
.2 9
.3 1
.3 1
.7 6
1 .3 6
1 .3 1
.6 0
.1 2
.0 7
.0 5
.0 1
.0 4
.0 8
.1 1
.1 1
1 .0 5
1 .8 2
9 .9 5
4 .0 5
.7 8
.1 6
.0 2
.0 2
.0 2
.0 2
.0 3
.0 2
.1 7
.5 9
2 .9 3
4 .0 2
2 .3 4
Sensitivity for base peak
i* d tru io n e
micron
5:
157
ADDITIONAL INFORMATION
Sensitivity for n-Butane
43
3 8 .3
Relative Intensities for n-Butane
15
27
5 6 0 °?
V ap or T ernperaiura
K a rn et C u rr e n t - .8 8 ejnperee
3 7 .3
4 4 .2
1 0 0 .0
1 0 .4
68
SYM H O LS:
r —rearrangement
m =m ctaatable ion
(ditfusc peak)
p —parent peak
i= is o to p e peak
d = d o u b ly -c h a rg ed ion
COMPOUND
MASS SPKCTKOMKTKH
Name:
Model:
2 , C -D laxtth yl Q u ln o l Ine
M oletular
Weight
Klvctron current ( c a tc h e r
) :
9
Svm i-struclural hormula
Molvciilar
Kormula
NC(CH3 ) |C H) zCC(CH)?C(CH3 ICH
3 5 7 .2 1
CtC 2 1 -1 0 1 (M o d ifie d )
C iiH u *
Purity
Source:
Lastnvm Kodak Company
voltages:
1880
Tcm ptrature of ioniration chamber:
Beoia o f pressure measurement:
mo/r prrfrnt
57
micron in prrcs
volts
(m V )
200
•c
j-VT K o ee u r e o e o t
530
72
?.v-i-'--v-l IOu rv I r.. !Iv I
Iielntixr Iiitrn silv s
Nlnss l haryr
[Maxi O .argr
ItVVC
fur io n iiiu y t viler? v s n(
Iin IlO
___ Ivatio___
of
7d"T7i77 ( m 'r )
Vcak
r o lls
(•>iU)
130
131
132
2 .3 7
133
134
135
136
.0 2
.01
.02
.01
137
.02
138
.06
.95
.14
.25
139
140
141
3.05
3 .1 0
8.80
142
1.59
.13
.01
143
144
145
149
150
151
152
153
154
155
.01
.02
.09
.32
.66
3.12
5. 02
32.6
156
____
1>8
1(0
P
100.
12.8
159
1.08
.22
1(1
.10
.05
1(2
1(3
1(5
.01
.01
1(6
.01
K7
168
1(9
170
171
172
173
176
177
178
179
180
181
.0 4
.04
.03
.10
.36
.04
.01
.01
.0 5
.09
.49
.10
.05
S -r : 1 1
Tync
of
Vcak
Iivlalivv IntvnsitM's
fvr iuniririf v.ilL e rv * of
.M.xs.s(nnryi Tyre
Italic
of
(m /< )
Vcak
» <1
Hvlaltxv Intensities
for in m im g w -Iibk m of
- 73 -
MASS SPECTRAL DATA
A m e ii c a n Petroleum Institute H esearch P roject 4 4
P i t t s b u r g h . Pa
C a r n e g i e I n a l il u t e of T e c h n o l o g y
C o n t r i b u t e d by th« At ’ n i t ic R’vf ini.-.g Cimpjry. f>i i lad*! ph ia, P e n n s y lv a n i a
Ma<* Chargi
Ratio
(m tf)
Peak
Ma-?- Charge
Relative Intensities
> of
Ratio
f.ii biiuimi*
7 0 volts
( in V)
volt*
.0 3
.2 9
.8 7
.0 3
1 1 .1
.0 7
2 .1 3
.0 7
1 6 .6
.0 9
5 .5 6
4 .1 0
5 .0 6
.2 1
.0 3
.0 3
,u
.0 3
.1 0
.0 4
.0 4
.0 8
.0 1
.6 1
.0 1
1 .9 5
.0 3
1 .3 9
.1 0
4 .1 8
.1 3
5 .1 7
.1 4
5 .5 4
.0 8
6 .3 2
2 .2 8
.0 3
.3 5
.1 4
.1 3
36
37
38
d
39
3 9 .5
40
4 0 .5
41
4 1 .5
42
43
44
45
4 5 .5
46
d
d
d
d
d
47
4 7 .5
48
4 8 .5
49
4 9 .5
50
5 0 .5
51
5 1 .5
52
5 2 .5
53
5 3 .5
54
5 4 .5
55
5 5 .5
56
57
5 7 .5
56
5 8 .5
59
November 30. 1951
S e r i a l No. GPb
Pecahydroquinol ii.e (g is)
d
d
d
d
d
d
d
d
d
d
d
of
Peak
7 0 l-o/f.v
d
5 9 .5
60
6 0 .5
61
6 1 .5
62
63
6 3 .5
U
6 4 .5
65
6 5 .5
66
6 6 .5
67
68
69
70
71
7 1 .5
72
73
74
75
76
77
78
79
80
BI
82
83
84
85
86
87
88
89
90
91
92
d
d
d
d
d
.
Relative ntensitu’s
fur Ivnmng volucii of
T, T
Peak
( i/i r)
.0 5
.CS
.0 3
.0 7
.0 1
.1 8
.5 5
.0 3
.2 7
.1 2
1 .3 4
.1 6
.8 6
.2 7
5 .9 0
5 .7 5
2 .5 1
2 .5 3
.4 2
.0 2
.0 7
.0 6
.1 4
.2 0
.1 9
1 .7 2
.7 5
2 .4 3
1 .9 5
3 .2 2
8 .2 0
6 .6 4
1 .1 7
.2 4
.0 5
.0 5
.0 4
.1 4
.L2
*81
.3 6
d
d
Mass-Chnrgi
R itio
Relative Intensities
for IOiiiiinir Xnll*,;. s of
70 volts
volt*
.9 7
1 .4 4
1 .7 0
100.
_
• 6 .4 1
.8 5
.1 6
.0 8
.1 0
.2 1
.2 0
.3 7
.9 2
.9 4
.6 1
1 .3 4
1 .6 7
1 0 .5
i.n
.1 6
.0 4
.0 2
.0 8
.0 6
.4 1
.4 5
.1 6
.2 2
.2 9
.4 6
.2 1
.7 3
.1 0
.0 3
.0 4
.1 4
.5 7
.3 7
93
94
95
«6
97
98
99
100
IC l
10 2
103
104
1C5
IOc
107
108
109
HO
111
112
11 3
IH
115
116
117
HS
H9
120
121
122
1 23
124
125
126
127
128
129
130
Sensitivity for base peak
t* divtiieni per tnieron
60
96
Sensitivity for n-Eutane
ADDITIONAL INFORMATION
Relative Intensities for n-Butane
15
27
Vapor Temperature - 560°F
Hflgnet Current - .88 acperea
37.3
23
U.l
43
100.0
SS
r —rearrangement
m = m etastab le ion
('l:lTu<e p«,ak)
p— parent peak
isotope peak
SYMBOLS:
1 0 .4
d= doubly-charged ion
MASS SPECTRO M FTFR
COMPOUND
M odel:
Name: D e c a h y d r o q u ln o lin o
CEC 21-101 (Modified)
Kleetron current ( c a t c h e r
M olecular
weight
1 3 9 .2 3
Molecular
Formula
C9 H17N
Semi structural Iorm ula
I
“
I
Nh (CM2 ) 3 CHCM(CH2 ) 3CH2
Purity
S u u ice:
Ion accelerating
v o lta g es:
mirrocmyrrr.i
I <3/f.v
(m e I
vo/f.i
57
1880
200
530
T< mptrature of ionization cham ber:
Basis of pressure m easurem ent:
Eastman Kodak Company
moiV percent
LABORATORY:
)'•
(m /f )
The A t l a n t i c R e f in in g Company, P h l le d e l p h l a , I e n n s y lv a n la
FYT Kg QBlUTCLCiitDate of measurement
3 /1 /5 1
LITERATURE CITED
1.
Adkins , H., Coonradt, H. L., "The Selective Hydrog.enation of
Derivatives of Pyrrole, Indole, Carbazole and Acridine", Journal
of The American Chemical Society, Vol. 63, 1563-1570, June, 1941.
2.
Ahuja, S . P., Derrien, M. L,, LePage, J. F., "Activity and
Selectivity of Hydrotreating Catalysts", Industrial and
Engineering Chemistry Product Research and Development, Vol. 9,
272-281, Fall 1970.
3.
Boyd, R. H., Morrison, R. T., Organic Chemistry, Allyn and
Bacon, Inc., Boston 1966.
k.
Brown, D., Earnshaw, D.G., McDonald, F.R., Jensen, H.B., "GasLiquid Chromatographic Separation and Spectrometric Identification
of Nitrogen Bases in Hydrocracked Shale Oil Naphtha", Analytical
Chemistry, Vol. 42, 146-151, February, 1970.
5-
Budzikiewicz, H., Djerassi, C., Williams, D. H., Mass Spectrometry
of Organic Compounds, Holden-Day, Inc. , San Francisco, 1967.
6.
Catalog of Mass Spectral Data, American Petroleum Institute Research
Project 44, Carnegie Institute of Technology, Pittsburgh, Pa.
7.
Cook, G. L., "Oil Shale - An Impending Energy Source", Journal of
Petroleum Technology,, Vol. 24, 1325-1330, November, 1972.
8 . Damon, R. A. , "Hydrogenolysis of Quinoline - Constructed Synthetic
Shale - Oil", PhD. Thesis in Chemical Engineering, Montana State
University-, i960.
9.
Elderfield, R. C., Heterocyclic Compounds, Vol. IV, John Wiley and
Sons, Inc. , New York , 1952.
10.
Falkenberry, H. L., Slack, A. V., Gartrell, F. E., "Control of
Fossil Fuel Power Plant Stack Gas Effluents", Combusion, Vol. 44,
9-15, October, 1972.
11.
Fedoruk, A. R . , "Nickelous Chloride - Gaseous Hydrochloric Acid as
a Petroleum Hydrodenitrogenation Catalyst", M. S. Thesis in Chemical
Engineering, Montana State University, 1973.
12.
Flinn, R. A., Larson, 0. A. ,' Beuther, H., "How Easy is Hydrodenitrogenation?", Hydrocarbon Processing and ^troleum Refiner,
Vol. 42, 129 - 132, September, 1963.
- 75 -
13.
Gould, E . S . , Mechanism and Structure in Organic Chemistry, Holt,
Rinehart and Wilson, Inc., Hevr Y o r k , 1959.
14.
Hartung, G. K. ,. Jewell, D. M., Larson, 0. A., Flinn, R. A.,
"Catalytic Hydrogenation of Indole in Furnace Oil", Journal of
Chemical and Engineering Data, V o l . 6 , 477-^80, July, 19 6l.
15»
Hatch, L.' F., "A Chemical View of Refining", Hydrocarbon Processing,
V o l . 48, 77-88, February, 1969.
16.
Hindin, S. G., Weller, S . W., Mills, G. A., "Mechanically Mixed
Dual Function Catalysts", Journal of Physical Chemistry, Vol. 62,
244-245, February, 1958.
17.
Houlihan, W. J. (ed.), Indoles Parts' One and T w o , Wiley-Interscience
C o . , New York, 1972.
18.
Lake, 0. R., McCut chan, P. VonMeter, R., Neel, J. C., "Effect of
Digestion Temperature on Kjeldahl Analyses", Analytical Chemistry
V ol. 23, 1634^1638, November, 1951.
19.
McCandless, F. P., "Catalytic Hydrodenitrogenation in the Presence
of Chlorides", PH..D. Thesis in Chemical Engineering, Montana State
University, 1966.
20.
McCandless, F. P., Berg, L. "Hydrodenitrogenation of Petroleum
Using.a Supported Nickelous Chloride - Gaseous Chloride Catalyst
System", Industrial and Engineering Chemistry Process Design and
Development, Vol. 9, 110-115, January, 1970. ^
21.
Morrison, R. T., Boyd, R. N., Organic Chemistry, Allyn and Bacon,
Inc., Boston, 1966.
22.
Palmer, M. H . , The Structure and Reactions of Heterocyclic
Compounds, Edward Arnold Ltd., London, 19 67.
23.
Paquette, L. A . , Principles of Modern Heterocyclic Chemistry, W. A.
Benjamin, Inc. , New Gork, 19^8".
24.
Poulson, R. E . , "Stationary Phases for Separation of Basic and
Nonbasic Nitrogen Compounds or Hydrocarbons by Gas-Liquid
Chromatography", Jovurnal of Chromatographic Science, Vol. 7,
152-157, March, 19 69.
25. Ryffel, J. R. , "Reaction Kinetics of the Destructive Catalytic
Hydrogenation of Quinoline", Ph.D. Thesis in Chemical Engineering,
Montana State University, i960.
- 76 26.
Smith, E. D. , Radford, R. D . , "Modification of Gas Chromatographic
Substrates for the Separation of Aliphatic Diamines", Analytical
Chemistry, Vol. 33, Il60-ll62, August, I 96I.
27.
Smith, J.M., Chemical Engineering Kinetics
1970.
28.
Snyder, L, R., "Nitrogen and Oxygen Compound Types in Petroleum",
Analytical Chemistry, V o l . 4l, 314-323, February, 19 69.
29.
Snedecor, G. W., Cochran, W. G . , Statistical Methods,Iowa State
University Press, Ames, Iowa, 19^7^
30.
Sundberg, R. J., The Chemistry of Indoles, Academic Press,
New York, 1970.
31.
Turner, D. ¥., Andrews, R. L., Siegmund, C. W . , "Influence of ■
Combustion Modification and Fuel Nitrogen Content on Nitrogen
Oxides Emission from Fuel Oil Combustion", Combustion, Vol. 44,
21-29, August, 1972.
32.
Veening, H., Dupre, G. D., "Gas Chromatographic Separation and
Determination of Molar Response Factors of Basic Nitrogen
Compounds and Hydrocarbons", Journal of Gas Chromatography,
McGraw Hill, New York,
V o l . 4, 153-155, April, 1966.
33.
Walton, H. F., Introductory Quantitative Analysis, PrenticeHall, Englewood Cliffs, New Jersey, 1968.
1762 10013142 2
N378
B873
cop. 2
I.!
Buller, Thomas J
Hydrodenltropenation "
o f i n d o l e a n d quinoline
w i t h a nickelous
chl o r i d e ...
S. k UACLOJ dL~
^fjtrnp
-
d / s n
6 ^ 3
f nlteqe
Plnre
Rinrier'/