Final Exam
advertisement

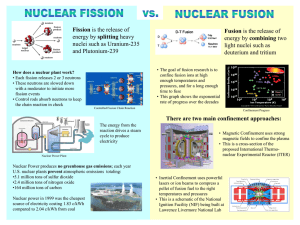
Final Exam • Wed Dec. 9 from11:30am-2:29pm York 2722 • Will cover all the course material including the last week • 25 questions –multiple choice. • You are allowed to bring 1sheet of paper with equations on both sides, scratch paper for calculation, calculator • You must bring a scantron form and a picture id. 10.1 Nuclear energy Nuclear Fission Nuclear Fusion Curve of the Binding Energy/nucleon Natural radioactivity fission Many elements found in nature are unstable and decay emitting radioactivity. fusion These include Uranium, 238U , Radon and Potassium 40K. Carbon 14C, 224Ra The half lives of natural radio-isotopes are long. Not useful as sources for power. Low Power output. Energy can be released by nuclear reactions of Fusion and Fission Induced Nuclear reactions Nuclear Power Can result in short half lives- fast reactions-high energy density • Nuclear power requires induced nuclear fission. • Nuclear fission can be induced by neutrons in a chain reaction. • Nuclear fusion can be induced by collisions at high temperature. Combining nuclei (Fusion) D + 21D ⎯⎯ → 31T + 11H 2 1 + Energy Neutron reactions (Fission) n+ 1 0 235 92 U ⎯⎯ → 141 56 Ba + 92 36 Kr + 3 01n + Energy 1 Fission of Uranium Fission of Uranium Strassman and Hahn (1939) Fermi (1936) Fast neutrons Slow neutrons Bombard uranium with neutrons Irradiated Uranium with neutrons Detected Barium Conclude Uranium nuclei splits into smaller fragments Found induced radioactivity Neutron Source (radon+Be) parafilm uranium “Transuranium elements” Liquid Drop model- n+ 1 0 235 92 U ⎯⎯ → 141 56 Ba + other _ products Nuclear Chain reaction Explained fission due to the instability of the higher larger nucleus. T1/2 ~10-12 s n+ 1 0 235 92 U ⎯⎯ → 236 → 92 U ⎯⎯ Lise Meitner Ba + products ∆E (average) =208 MeV Chain Reaction binding of 1 neutron releases ~3 neutrons Each neutron can initiate another reaction Two major isotopes of Uranium 235U Critical Condition for Chain reaction (0.7%) Fissionable upon neutron capture 235U (Fission) n 238U (99.3%) Non-Fissionable upon neutron capture n n 235U n (Escape) 238U (Capture) Nuclear reactor Reproduction constant K = no. of neutrons that produce a new fission event K=1 ( self –sustained reaction) 2 Enriched 235U Centrifuge separation of isotopes Natural Uranium is a mixture of 235U (0.7%) and 238U(99.3%) Most Uranium nuclear reactors use uranium enriched in (2-3%) 235U. Nuclear weapons used highly enriched 235U. (~90%) Enrichment done by mass separation. Gaseous diffusion Centrifuge process. Laser separation centrifugal separation gaseous UF6 Nuclear reactor Nuclear reactor fast neutrons must be slowed down to react efficiently. Moderator- slows neutrons to thermal velocities. Control rods- neutron absorbers to control the level of neutrons Critical condition. – When each neutron released initiates a new reaction. Plutonium Plutonium is a fissionable material created in a nuclear reactor. 238 92 − U + 01n ⎯⎯ → 239 94 Pu + 2e Question How many gallons of gasoline (1.3x108 J of energy/gallon) would be equivalent to a kg of Uranium? ∆E=208MeV No. of fissionable nuclei =N N = ( fraction235U)( ⎡ ⎤ 1kg m 22 ) = (0.0072)⎢ ⎥ = 1.8 x10 − 27 mass / atom ⎣ 238u(1.66 x10 kg / u) ⎦ Energy released = E = N(∆E) 239Pu can be made into nuclear bombs. Pu can be chemically separated from U in spent fuel rods from nuclear reactors. [ ] E = N( ∆E) = 1.8 x10 22 ( 208 x10 6 eV ) 1.6 x10 −19 J / eV = 6 x1011 J gallons of gasoline =V= E E / gallon E 6 x1011 J = = 4.6x10 3 gallons E / gallon 1.3 x10 8 J / gallon 3 Nuclear Fusion Fusion of small nuclei releases energy Nuclear Fusion High energy required to bring charged nuclei close together +Z D + D ⎯⎯ → He + n ∆E= 3.27 MeV D + 21D ⎯⎯ → 31T + 11H ∆E= 4.03 MeV D + 31T ⎯⎯ → 42 He + 01n ∆E=17.6 MeV 2 1 2 1 3 2 1 0 2 1 2 1 v +Z +Z +Z ~10-15 M Requirements for fusion High Temperatures (T~ 108 K) High density (n) for long time (τ) Lawson Criterion nτ > 1014 s/cm3 Plasma Fusion Magnetic Confinement Laser fusionInertial Confinement Plasma is a gas of ionized atoms Heated to high temperature Confined by magnetic forces Deuterium pellet T~108 K Princeton Tokomak long times low density Lawrence Livermore Lab Nova Laser Short times High density Prospects • Nuclear energy by fission is currently a source of much of the electrical power (~15% USA). • The problems with nuclear energy – Radioactive waste disposal – Atomic bomb threats • Nuclear fusion reactions promise an unlimited source of energy. – Controlled fusion reactions are not yet possible. 4