Today’s Lecture Thermodynamics Temperature Scales Specific Heat
advertisement

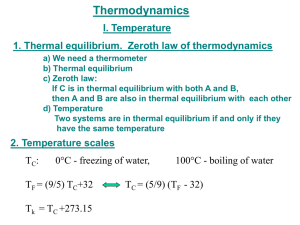
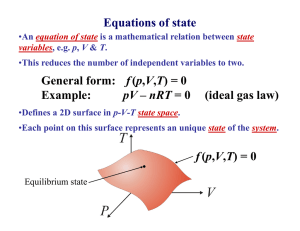
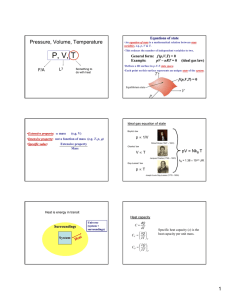
Today’s Lecture Thermodynamics Temperature Scales Specific Heat Heat Conduction Thermodynamics. Study of temperature, heat and related macroscopic properties of objects and matter. Specifically macroscopic properties: temperature, heat, pressure, internal energy… What do we mean by “Any macroscopic property”? Temperature, volume, pressure, electrical conductivity, length, color, etc. Why do we stress that we talk about MACROSCOPIC properties? The properties that we have mentioned have no meaning on a microscopic scale! Thermodynamics. Study of temperature, heat and related macroscopic properties of objects and matter. Specifically macroscopic properties: temperature, heat, pressure, internal energy… Temperature and Heat Thermodynamic equilibrium: two systems are in thermodynamic equilibrium with each other, when they are in thermal contact and no change in any macroscopic property occurs with time. What do we mean by thermal contact? Heating one results in macroscopic changes in the other. Two systems have the same temperature if they are in thermodynamic equilibrium. Two systems have the same temperature if they are in thermodynamic equilibrium. Zeroth law of thermodynamics: If two systems A and C each are in thermodynamic equilibrium with system B, then A and C are in thermodynamic equilibrium with each other. Sounds trivial, but gives a rationale for measuring temperature. Zeroth law of thermodynamics: If two systems A and C each are in thermodynamic equilibrium with system B, then A and C are in thermodynamic equilibrium with each other. B A C Liquid thermometers Based on thermal expansion of liquids. The liquids are held in little vessels at the bottom. At higher temperatures they expand and climb toward the top of narrow capillaries. Liquid thermometers Coefficients of thermal expansion ΔV / V β= ΔT β – fractional increase of volume per one degree Problems: 1. Liquid is a rather complex state of matter. Molecules in liquids are held together by cohesive forces, different for different liquids. 2. The coefficients of thermal expansion vary a lot between liquids, and may depend on temperature in an extreme fashion. 3. Liquids freeze at low temperatures and boil at high temperature. So the ranges of operation of the liquid thermometers are restricted. Gas Thermometer Gas Thermometer Gasses are much simpler than liquids. The molecules are moving freely most of the time, and only once in a while suffer short term collisions. The collision events are still different for different molecules…. BUT: when the gasses are rarified (low density) and the collisions are rare behavior of different gasses in the gas thermometer becomes very much the same – An Ideal Gas! In order not to change the gas density, it is preferable to keep the gas volume constant and to measure the gas pressure. Gas Thermometer Level of the mercury in the right tube is varied to keep the level in the left tube constant. Pressure of the gas is measured as P =ρgh The absolute temperature of the system is defined as: P T = 273.16 P3 P3 is pressure of the gas at a special reference point called “triple point”, which is unique and can be reproduced in every laboratory. Gas Thermometer To set the temperature scale we need some convenient reference points. 273.15 K, the same as 0°C is the temperature of ice melting at normal pressure; 373.15 K, the same as 100°C is the temperature of water boiling at normal pressure; 1K temperature difference is the same as 1°C temperature difference, but 0K corresponds to absolute zero or -273.15 °C. Temperature Scales Fahrenheit Celsius Lord Kelvin 9 TF = 32° + TC 5 TK = 273.15 + TC Temperature Scales What is normal body temperature (98.6oF) on the Celsius and Kelvin scales? If you have a fever of 101.6oF, how much has your temperature risen on each of these scales? The point here is that a change in oC or K is the same! Gas Thermometer A constant volume gas thermometer supports a 72.5mm column of mercury when it is submersed in liquid N2 at -196oC. What is the column height when the thermometer is submersed in molten Pb at 350oC? From the expression for the absolute temperature, we have: Converting to absolute temperature, oC to K, we find: Heat and Internal Energy Heat is energy being transferred from one object to another because of temperature difference alone. Heat is energy in transit! Internal energy relates to energetic contents of a body or a system. Whereas Heat is energy in transit! Nevertheless, both heat, ΔQ, and internal energy, U, are measured in the same energy units, Joules, J. Heat can be transferred to a body (or a system) that would cause growth of internal energy of the body, but not of its “heat”. Because Heat is not a material or a form of matter. Heat is energy in transit! When pouring water you transfer it from one vessel to another and you get more water in the second vessel. In thermodynamics you transfer heat but you usually end up having more or less internal energy. What are common results of heat transfer? 1. Increase in temperature. 2. A phase transition (melting ice). 3. Mechanical work. Cases #2 and #3 may not involve a change in temperature. Case #1, no phase transition or work done. How much does the temperature vary? Heat capacity of the object C, measured in J/K; tells you how many Joules of heat you need to transfer to increase the temperature of the object by 1K. ΔQ = CΔT ΔQ C= ΔT Heat capacity is an extrinsic parameter (depends on quantity): When you bring two objects together, heat capacity of the system of the two objects becomes the sum of the two individual heat capacities. It is convenient to introduce specific heat, c, an intrinsic parameter, which is heat capacity of a material per unit mass . Specific heat is measured in J/(K⋅kg). ΔQ = cmΔT ΔQ C c= = m mΔT Heat capacity of a water ball is large due to both high specific heat and large mass of the water inside the ball. Specific heats, c, of some Common Materials ΔQ = cmΔT where C ΔQ = c= m mΔT Specific heat is measured in J/(K⋅kg). Waiting to Take a Shower! The water temperature in the water heater is only 18oC. How much energy is required to heat its 150kg to 50oC? The water heater can supply 5kW of power. How long does it take to heat the water to 50oC? Equilibrium Temperature. Situation: Two objects with different temperatures, T1 and T2, are brought into thermal contact and eventually reach thermal equilibrium at a temperature T. Heat ΔQ1 is transferred to Object 1; heat ΔQ2 is transferred to Object 2. By energy conservation: By definition of heat capacity and specific heat: m1c1T1 + m2 c2T2 T= m1c1 + m2 c2 Equilibrium Temperature As an example: a piece of copper at 300oC is dropped into 1.0kg of water at 20oC. If the final equilibrium temperature is 25oC, what was the mass of the copper? Equating the energy gained by the water to that lost by the copper Solving for mCu Heat Transfer 1.Conduction 2. Convection 3. Radiation Heat Conduction A rectangular slab of thickness Δx and with an area A. The front side of the slab is at a temperature T; the back side has a somewhat different temperature, T+ΔT. We are trying to calculate the heatflow rate, the amount of heat flowing Heat flows from the hotter to the through the slab per unit time, cooler side of the slab H = ΔQ/Δt. We expect H to be proportional to the area, A, of the slab, the temperature difference, ΔT, between the back and the front and inversely proportional to the thickness of the slab, Δx. H should also depend of properties of the material the slab is made of… Heat Conduction Bringing all the parts together: ΔT H = − kA Δx Heat flows from the hotter to the cooler side of the slab The coefficient k reflects specific properties of the material of the slab and is called thermal conductivity H = ΔQ/Δt – heat-flow rate is measured in Joules/second, J/s, or Watts, W. Thermal conductivity, k, is measured in W/m⋅K. Thermal conductivities of different materials. Best heat conductor – Copper; use it when you build heat sink, as a material for pipes in your cooling system, a radiator. Worst heat conductors are the best insulating materials – air, fiberglass (layers in the walls of houses in cold regions), styrofoam (cups for your hot coffee). Heat Conduction – Example 1 ΔT H = −kA Δx A lake with a flat bottom and steep sides has a surface area of 1.5(km)2 and is 8m deep. In the summer the surface is 30oC while the bottom is 4oC. What is the rate of heat conduction in the lake? What is the significance of the minus sign? What about oC versus K? Heat Conduction – Example 2 ΔT H = − kA Δx An 8m x 12m house is built on a 23cm thick concrete slab. What is the heatloss through the floor if the interior is 20oC while the ground is at 10oC? Heat Conduction – Example 3 A pipe of length l and radius R1 is surrounded by insulation of radius R2 and thermal conductivity k. Find the expression for the heat loss through the insulation when the fluid in the pipe is at T1 and outside the insulation is at T2. At equilibrium the heat flow is uniform as a function of radius. Expressing this heat flow across a thin layer of insulation at the radius r yields: Integrating this expression results in the expression: Heat Conduction – Example 3 A pipe of length l and radius R1 is surrounded by insulation of radius R2 and thermal conductivity k. Find the expression for the thermal gradient, dT/dr, when the fluid in the pipe is at T1 and outside the insulation is at T2. From our solution for the heat flow we can write: In this geometry the thermal gradient is not uniform. Note that the larger the temperature difference the larger the thermal gradient and the larger the heat flow. Is the thermal gradient positive or negative? Which direction does the heat flow??