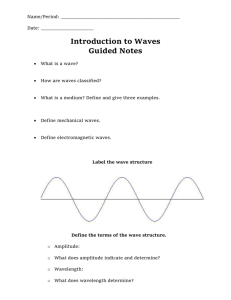
– longitudinal wave of compressions and rarefactions. Sound wave divide lines

Sound wave
– longitudinal wave of compressions and rarefactions.
What are those black circles?
divide lines
Speeds: of molecular motion; of the collective motion of the air; of the sound wave.
Normal conversation – speed of collective motion 45 m m/s.
Amplitude – 10 nm.
Pressure displacement
v
F m
Speed of a wave on a string
• Tension of the string, F , provides the restoring force
• Mass per unit length, m
= m / L , measures inertia of the string, that slows down any wave propagation v
B
B
P
V / V
Speed of sound ?
• Bulk modulus of elasticity, B , defines the restoring force
• Density of the medium,
, measures inertia of the medium that slows down any wave propagation
V / V is the fractional change of volume (can be measured in %)
Large bulk modulus of elasticity corresponds to a large change of pressure – vigorous restoring force – at small fractional change of volume
v
B
B
P
V / V
• A hard material with a low compressibility and low density -
Aluminum high speed of sound.
• A soft material with a high density – Lead – low speed of sound.
Speed of sound in a gas
B
v
V
B
P
/ V
Can we calculate the bulk modulus of elasticity, B, of a gas?
What process should we assume?
Sound waves are propagating quickly.
No time for heat exchange!
Adiabatic process.
PV
c
const P
cV
dP dV
cV
1
P
V
v
B
Speed of sound in a gas
B
P
V / V
V dP dV v
P
dP dV
P
V
B
P
= C p
/ C v in the gas
– the constant for an adiabatic process
P pressure of the gas
- density of the gas
v
Speeds of sound for different gases at normal conditions
= C p
/ C v in the gas
– the constant for an adiabatic process
P
P pressure of the gas
- density of the gas
For two different gases, the ratio of the sound speeds can be calculated as v
2
/ v
1
2
2
P
/
1
P
1
If the two gases are at the same pressure, P : v
2
/ v
1
1
2
1
2
Example: air and Helium at normal conditions
Ratio of the sound speeds: v
2
/ v
1
2
1
1
2
2
1
M
1
M
2
The volume occupied by one mole of a gas at the normal conditions is the same for all gases – 22.4 liters .
Therefore, the ratio of densities is equal to the ratio of molar weights.
(1) Air – a mixture of N
2
( M = 28 g/mole) and O
2
(
Average molar weight – M
1
= M air
Composed of diatomic molecules,
= 29 g/mole.
1
=
air
= 1.4
.
M = 32 g/mole)
(2) Helium M
2
= M
He
= 4 g/mole . A monatomic molecule,
2
=
He
= 1.67
.
v
2
/ v
1
1 .
67
1 .
4
29
2 .
94
4
In air, v
1
= 343 m/s.
In Helium, v
2
= 1008 m/s
I
1
2 s
0
P
0
Intensity of sound
I – average intensity s
0
– the amplitude of displacement of the air
P the amplitude of pressure variations
I
P
0
2
2
v
Expressions making a bit more sense:
Quadratic in the amplitude of pressure variations – proportional to potential energy of the gas deformations .
I
1
2 s
0
2 v
v
2 osc v
2 K
v
2
Quadratic in the amplitude of velocity of the oscillations of the gas and the gas density.
Proportional to kinetic energy of the gas motion and the wave speed .
I
10
6
W/m
2
,
v
343 m/s
v
osc
45
μm/s
Sensitivity of human ear..
Most sensitive at about 4000 Hz.
v / f
343 m/s
4000 Hz
8 .
6 cm
Eardrum - about 1 cm in diameter