Pre-equilibrium approximation Physical Chemistry Lecture 9
advertisement

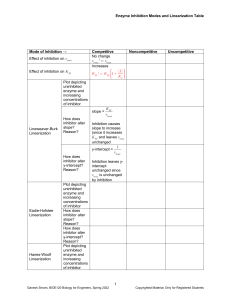
Pre-equilibrium approximation Product formed only by second step Physical Chemistry Elementary rate determined by intermediate’s concentration A B I I Assumption of rapid equilibrium Lecture 9 Kinetics of enzymatic reactions Substitution gives an expression for the rate in terms of reactant concentrations Effective rate constant depends on all three rates K eq B fast fast slower kf kr [I ] [ A][ B ] [ I ] K eq [ A][ B ] Effective activation energy depends on all elementary activation energies k d [ P] k2 [ I ] dt Gives the intermediate’s concentration in terms of reactant concentrations f I kr A kp P d [ P] K eq k p [ A][ B ] dt Example: formation of NO2 from NO and O2 Biological reactions Catalytic action Some reactions proceed with the presence of material that are not Usually occur in aqueous solution Generally multiple-step reactions Can be grouped in classes of reactions that happen by similar mechanisms Materials that affect a reaction, but are not among the reactants or products, are k A simple model A B I k A B Assumes the existence ofI k P a reactive intermediate I Requires a difference in time scale of the elementary processes f r p Substrate is a reactant k1 S C SC k 1 SC S C k2 SC P C Pre-equilibrium approximation Fast establishment of equilibrium Slower transformation of intermediate into product Requires a difference in activation energies of the steps Catalysts if they promote a reaction Inhibitors if they slow or stop a reaction Must interact with the reactants to influence the reaction’s progress Materials must not be used up in the reaction Tend to have small concentrations of catalysts compared to the reactants and products Simplest catalytic mechanism involves formation of a substrate-catalyst complex Enzyme catalysis Photochemically initiated reactions Electron transfer Reactants Products Intermediates in the sense of our definition fast fast slower Complex formation Unreactive complex dissociation Product formation Simple catalytic kinetics Complex treated as an intermediate Creation of product determined by last elementary step Final rate equation depends on two constants d[P] k2[SC] dt d[SC] k1[S][C] k1[SC] k2[SC] 0 dt [SC] k1 [S][C] k1 k2 d ]P] k2 [S][C] dt KM k1 S C SC k 1 SC S C k2 SC P C Complex formation Unreactive complex dissociation Product formation 1 Practical rate expression In general, one does not know the catalyst’s and the complex’s concentrations at any time Stoichiometry and conservation of matter specify relations among concentrations Restrict consideration to early times Small product concentration Quadratic term negligible Result is an expression for the initial rate in terms of the formal concentrations and rate constants Michaelis-Menten kinetics Enzyme Conservation of matter [ S ]0 [ S ] [ SC ] [ P ] [C ]0 [C ] [ SC ] Using the equilibrium relation K m [ SC ] [ S ][C ] [ S ]0 [ SC ] [ P][C ]0 [ SC ] which gives the quadratic equation [ SC ]2 [ P] [C ]0 [ S ]0 K m [ SC ] [ S ]0 [ P][C ]0 [ S ]0 [C ]0 v0 d [ P] dt 0 k2 [ S ]0 [C ]0 K m [C ]0 [ S ]0 Usual situation in catalysis [C]0 << [S]0 d [ P] dt 0 v0 k2 regenerate the enzyme and liberate product 0 Large excess of the substrate substrate complex Transformation to which gives Limiting behavior Formation of an enzyme 0 At early times, [ P] 0 and [ SC ]2 is negligible [C ]0 [ S ]0 K m [ SC ] [ S ]0 [C ]0 [ S ]0 K m E 1 k 2 [C ]0 S k1 ES ES E S ES k2 E k 1 Derivable from mechanism Defined in terms of maximum reaction rate (vmax) and MichaelisMenten constant (Km) Lineweaver-Burk plot Molecule that serves to catalyze biochemical reactions Activity usually interpreted in terms of the MichaelisMenten mechanism E E S k1 ES ES k 1 E S ES k2 E 1 v0 [S ]0 [S ]0 Km K 1 1 m vmax vmax [S ]0 S k1 ES ES E S ES k2 E k 1 P Inhibition Change of the enzyme’s activity by the presence of another molecule, inhibitor Three kinds of inhibition Competitive Noncompetitive Uncompetitive Inhibitor blocks the substrate by Formation of an enzyme- substrate complex Transformation to regenerate the enzyme and liberate product vmax [S ]0 [S ]0 Km Linear form Michaelis-Menten kinetics v0 k2[E]0 Rate equation Km 1 k 2 [C ]0 [ S ]0 Enzyme P Michaelis-Menten kinetics which is usually linearized as 1 v0 Molecule that serves to catalyze biochemical reactions Activity usually interpreted in terms of the MichaelisMenten mechanism binding at the active site Inhibitor changes the binding site structure to slow or prevent binding Inhibitor binds after the substrate and changes the activity P 2 Competitive inhibition Inhibition involves binding by inhibitor, I Two processes in parallel Lineweaver-Burk plot has different slope from uninhibited reaction Example: methotrexate inhibition of dihydrofolate reductase (1) E S ( 2) ES (3) E I k2 v0 1 v0 ES P E EI Biological systems control chemical reactivity through catalytic mechanisms [ S ]0 [ E ]0 [I ] [ S ]0 K m 1 Ki 1 vmax Summary [I] K m 1 K i 1 vmax [ S ]0 Enzymes Michaelis-Menten kinetics Inhibition A means of manipulation of enzyme activity Competitive Noncompetitive Uncompetitive Noncompetitive inhibition Noncompetitive binding involves two processes in parallel and in sequence Lineweaver-Burk plot has Different slope from uninhibited reaction Different intercept from uninhibited reaction (1) E S (2) ES P ES (3) E I EI (4) ES I EIS v0 k2 1 v0 E [ S ]0 [ E ]0 [I ] [I ] [ S ]0 1 K m 1 Ki Ki 1 [I] 1 K i vmax [I] K m 1 K i 1 [ S ]0 vmax Uncompetitive inhibition Inhibitor binds after the substrate is bound to the enzyme Lineweaver Burk plot Slope independent of inhibitor concentration Intercept depends on inhibitor concentration E S ES I EIS ES v0 k2 1 v [ S ]0 [ E ]0 [I ] 1 [ S ]0 K m K m 1 [I ] 1 vmax K i Km 1 vmax [ S ]0 3