Assignment: Bonding Name: Click on this link to complete this activity.
advertisement

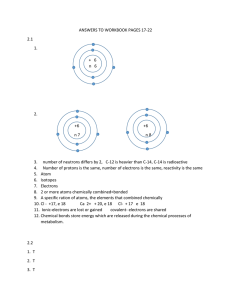
Assignment: Bonding Name: Click on this link to complete this activity. Covalent Bonding Follow the instructions closely as you move through this activity! There are some screens where you have to do something before you can move onto the following screen. Answer the following questions as you complete the Covalent Bonding activity. 1. What happens to the electrons in the Hydrogen atoms as they near the protons of the other atom? 2. How is the movement of the electrons different when the atoms are close? 3. What happens if you try to move the atoms very close to each other? 4. How are the atoms bonded together? 5. What type of elements form covalent compounds? 6. Which element is considered a non-metal, even though it is on the left of the periodic table? 7. What happens to the potential energy as the atoms move close together? 8. At what point of the graph is the bond the most stable? 9. What is the distance between the atoms called? 10. Complete the chart showing the solid lines between the atoms and the number of electrons being shared in the covalent compounds below: Number of Electrons Covalent Compound Drawing (Draw Lines) Shared H2 H H O2 O O N N2 N 11. Which bond above is the strongest type of bond? 12. Which bond above is the weakest type of bond? 13. Complete the chart showing the prefixes used for each number of atoms in the covalent compound Number Prefix 1 2 3 4 5 6 14. When do you never use the prefix “mono-“ when naming covalent compounds?