Physical Chemistry Lecture 13 Carnot Cycles and Engines
advertisement
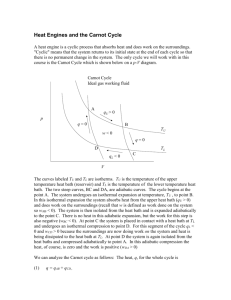
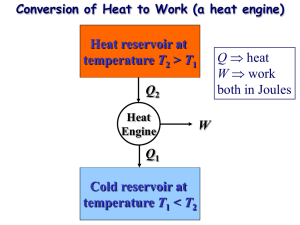
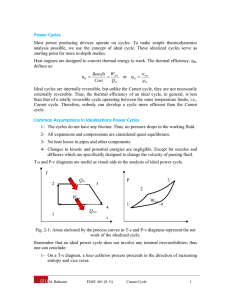
Physical Chemistry Lecture 13 Carnot Cycles and Engines Engines and cycles Want to transform heat into mechanical work Engine – a device that turns heat into work Carry out continuously Cycle – a sequence of events that carries a system away from a state and returns it to that state Cycles allow repetitive transfer of energy Types of cycles Otto cycle – commonly used in automobiles Diesel cycle – also used in automobiles Wankel cycle – used at one time in automobiles Carnot cycle – theoretical analysis Carnot cycle Theoretical analysis of the thermodynamics of transforming heat into work Sadi Carnot – French engineer who first proposed the concept Rudolf Clausius – German thermodynamicist who described the concept mathematically Carnot cycle with an ideal gas Remove heat from high-T reservoir Do work Dump heat in a lowT reservoir Involves four steps Carnot cycle Step 1: isothermal expansion Step 2: adiabatic expansion Step 3: isothermal compression Step 4: adiabatic compression Mathematics of the Carnot cycle with an ideal gas The points at intersections of the steps are related These relations determine the amount of heat absorbed and work done Work done V Heat Internal energy w = − R(Th − Tc ) ln A VB q = q h + qc ∆U = 0 Efficiency of a Carnot cycle Efficiency – determines how effectively heat is used as work −w ε = qh Using the determined values of these quantities for a reversible cycle ε reversible Th − Tc = Th The Otto cycle Used in automobile engines Four stroke cycle Adiabatic compression Isochoric heating Adiabatic expansion Isochoric cooling Efficiency less than for the ideal Carnot cycle Summary Carnot cycle – fundamental to understanding transformation of heat to mechanical work Other cycles are in use Ideal efficiency depends only on the temperature difference Can never be 1 (except in unusual circumstances)