Periodic Table 2013 Page 1
advertisement

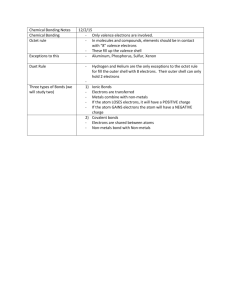
Periodic Table 2013 1 Columns in the periodic table are called A B C D 2 We can say what about groups of elements in the periodic table? A B C D 3 1 2 4 8 How many electrons can go in the second shell? A B C D 7 The number of electrons The atomic mass The number of electron shells Chemical and physical properties of the atom How many electrons can go in the first shell? A B C D 6 Periods Groups Metals Non-metals Periods tell us what about the periodic table? A B C D 5 They are different in physical and chemical properties Groups are only metals Groups are only non-metals Elements in the same group have similar physical and chemical properties Rows in the periodic table are called A B C D 4 Periods Rows Groups Stairstep 1 2 4 8 What is in the nucleus of the atom? A B C D Protons and electrons Electrons and neutrons Protons and neutrons Protons 11/12/2013 7:12:05 AM Page 1 Periodic Table 2013 8 What orbits around the nucleus in shells? A B C D 9 What are valence electrons? A B C 10 12 13 The innermost electrons in an atom The middle electrons in an atom The outermost electrons in an atom Electronegativity is A B C D 11 Electrons Protons Neutrons Isotopes The ability of an element to obtain or steal an electron The ability of an element to donate or give an electron How negatively charged an ion is A song by Justin Timberlake. Period A B C D E F Region where electrons are found Dull, Brittle Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity A B C D E F Region where electrons are found Dull, Brittle Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity Shell Metals A B C D E F Region where electrons are found Dull, Brittle Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity 11/12/2013 7:12:05 AM Page 2 Periodic Table 2013 14 Non-metals A B C D E F 15 Metalloids A B C D E F 16 17 18 Region where electrons are found Dull, Brittle, and sometimes a gas Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity Region where electrons are found Dull, Brittle Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity Ionization Energy A B C D E F Region where electrons are found Dull, Brittle Metallic and Non-metallic in character Horizontal Rows Increases from lower left to upper right Shiny and conduct electricity A B C D E F The number of electrons in the first shell The number of electrons in the second shell Increases from upper right to lower left The outermost electrons The ability or an atom to attract electrons Vertical columns A B C D E F The number of electrons in the first shell The number of electrons in the second shell Increases from upper right to lower left The outermost electrons The ability or an atom to attract electrons Vertical columns 2 8 11/12/2013 7:12:05 AM Page 3 Periodic Table 2013 19 Groups A B C D E F 20 Atomic Radius A B C D E F 21 The number of electrons in the first shell The number of electrons in the second shell The size of the atom The outermost electrons The ability of an atom to attract electrons Vertical columns Electron affinity A B C D E F 22 The number of electrons in the first shell The number of electrons in the second shell Increases from upper right to lower left The outermost electrons The ability or an atom to attract electrons Vertical columns The number of electrons in the first shell The number of electrons in the second shell Increases from upper right to lower left The outermost electrons The ability or an atom to attract electrons Vertical columns Valence Electrons A B C D E F The number of electrons in the first shell The number of electrons in the second shell Increases from upper right to lower left The outermost electrons The ability or an atom to attract electrons Vertical columns 11/12/2013 7:12:05 AM Page 4