£ by Multimodal integration of EEG and MEG ... using minimum
advertisement

Multimodal integration of EEG and MEG data
using minimum £2-norm estimates.
by
Antonio Molins Jimenez
Telecommunications Engineer,
Universidad Polit6cnica de Madrid
Submitted to the Department of Electrical Engineering and Computer
Science
in partial fulfillment of the requirements for the degree of
Master of Science in Electrical Engineering and Computer Science
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
May. 2007 ET3' t( 200o74
@ Massachusetts Institute of Technology 2007. All rights reserved.
Author ......................................
Department of Electrical Engineering and Computer Science,
Harvard-MIT division for Health Sciences and Technology.
,/
May 8, 2007
Certified by
Emery N. Brown, M.D., Ph.D.
Professor of Health Sciences and Technology,
Professor of Computational Neuroscience
Thesis Supervisor
Certified by.. .'
Affiliat6culty, ,
MASSACHUS•TTS INSr
OF TECHNOLOGY
AUSG 1-.6 2007
b
.f..z
.......
Matti Haimiiliinen, Ph.D.
ll Scie ces and Technology
.fl
Z-hesis Supervisor
.................
Arthur C. Smith, Ph.D.
Chairman, Department Committee on Graduate Students
LIBRARIES
~r ,Ynr~i~~~
Multimodal integration of EEG and MEG data
using minimum 42-norm estimates.
by
ANTONIO MOLINS JIMENEZ
Telecommunications Engineer,
Universidad Politicnica de Madrid
Submitted to the Department of Electrical Engineering and Computer Science
on May 25, 2007, in partial fulfillment of the
requirements for the degree of
Master of Science in Electrical Engineering and Computer Science
Abstract
The aim of this thesis was to study the effects of multimodal integration of electroencephalography (EEG) and magnetoencephalography (MEG) data on the minimum
£2-norm estimates of cortical current densities. We investigated analytically the effect of including EEG recordings in MEG studies versus the addition of new MEG
channels. To further confirm these results, clinical datasets comprising concurrent
MEG/EEG acquisitions were analyzed. Minimum e2 -norm estimates were computed
using MEG alone, EEG alone, and the combination of the two modalities. Localization accuracy of responses to median-nerve stimulation was evaluated to study the
utility of combining MEG and EEG.
Thesis Supervisor: Emery N. Brown, M.D., Ph.D.
Title: Professor of Health Sciences and Technology,
Professor of Computational Neuroscience
Thesis Supervisor: Matti Him~ilinen, Ph.D.
Title: Affiliated faculty, Health Sciences and Technology
Acknowledgments
My advisor, Emery Brown, took me in his great lab when I most needed it, trusted
me for no reason at all, offered and explained an exciting project to me, and granted
me access to all the wonderful research resources without which no work would have
been accomplished.
Matti Haimiliiinen introduced me to the fundamentals of MEG and minimumnorm estimation, provided me with his wonderful software tools, and was always
there, even with a whole ocean between us, to solve my multiple questions with a
contagious smile.
Steve Stufflebeam was not only part of the admission committee that accepted
me into my program, but also gave very useful feedback for this thesis and guided me
through his exceptional clinical data.
David Cohen took time to share with me his insight in electromagnetism in
informal weekly meetings, thus giving me the opportunity to learn the fundamentals
of MEG from the very inventor.
The people at the Martinos Center were always available to me. Special thanks
go to Naoro Tanaka, who is responsible for some of the clinical data analysis used
in this thesis, and helped me with the subject selection and other technical details;
Daniel Wakeman, who helped me to navigate through the MEG lab at Charlestown;
and Fah-Sua Lin, who let me make use of his helpful Matlab@ scripts.
All the people at the Neurostatistics Lab were always helpful and supporting:
Camilo, Michelle, Anna, Patrick, Rob, Giulia, Maurizio, David, Sri, Ricardo, Sage, Julie... Working is much easier in a lab where a smile is the norm.
Outside the lab, lots of beautiful people made my time here much more fun
and memorable: Alina, Adam, Baldur, Andy, Miriam, Stephanie, Juliette,
Paulina, Xana, Rahul, Seann, Sachiko, Tom, Tania, Dani, Nico, Alex, Be,
Eli, Angelo, Christian, Gonzalito and many, many more helped me to relax and
enjoy the weather when possible, and more importantly, when impossible. You guys
have made of my stay in Cambridge a growing experience. I am so lucky to be surrounded by such wonderful people. With you as my friends, I know my remaining
time here can only get better...
Finally, my family deserves the greatest thanks of all. They have understood my
need to pursue my dreams, wherever these take me. Papa, Mamrni, Miguel, Luis
(you could not be included anywhere else), Alvaro, Piluca, Javi: I miss you every
second. You always give me the energy to carry on, the hope when I most need it,
the laugh that keeps me sane.
Gracias a todos.
Contents
1 Introduction
1.1 E/MEG Imaging
9
9
..................
...............
1.2
Physiological basis of E/MEG signal .......................
10
1.3
E EG . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12
1.4
ME G . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
12
1.5
Multimodal imaging: E/MEG .......................
13
1.6
The forward model ............................
14
1.7 The inverse problem
1.8
1.9
...................
........
Regularization ........................
. .....
16
.
17
1.8.1
M N E . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. .
18
1.8.2
LORETA
20
1.8.3
The equivalent current dipole model
.............................
........
. . . . . . .
20
Performance of the regularized solution . . . . . . . . . . . . . . . . .
21
1.9.1
Singular-value spectrum of the lead matrix . . . . . . . . . . .
21
1.9.2
Resolution matrix .........................
22
2 Objective of this thesis
27
3 Methods
29
3.1
Clinical data . . . . . . . . . . . ..
3.2
Data acquisition ...................
. . ......
. . .
29
3.3
Data processing ...................
. . ......
. . .
30
3.4
Noise covariance computation ...................
. . .
32
7
. . . . . . . . . . . . . . . . . . .
29
3.5
Forward M odel ..............................
32
3.6
Activity estimates .............................
33
3.7 Inverse operator analysis .........................
35
3.8
37
..
3.7.1
Eigenvalue spectra ......................
3.7.2
Resolution matrix metrics ...................
.
37
38
Analysis of the clinical data .......................
4 Results
39
4.1
Eigen-value spectra ............................
39
4.2
Resolution matrix metrics ........................
41
4.3
4.2.1
Dipole Localization Error
4.2.2
Spatial Dispersion ...................
4.2.3
Resolution Index .........................
Clinical data analysis . .........
.
...................
......
.
41
45
47
.......
.........
55
4.3.1
Equivalent current dipoles ................
4.3.2
Minimum £2-norm estimates . ..................
55
4.3.3
ECD/MNE comparison ......................
64
. . . .
5 Conclusions and future work
55
67
5.1
Conclusions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
67
5.2
Future work ................................
68
Bibliography
69
Chapter 1
Introduction
In the last few decades technical and mathematical advances have provided researchers
and clinicians with several tools to choose among for non-invasive functional brain
imaging, including functional magnetic resonance imaging (fMRI), positron emission
tomography (PET), single photon emission tomography (SPECT), electroencephalography (EEG), and magnetoencephalography (MEG). Each one of these techniques has
its own advantages and disadvantages, increasing the interest of multimodal studies
where the strengths of different modalities can be combined.
This thesis focuses on multimodal integration of EEG and MEG, globally known
as bioelectromagnetic (E/MEG) imaging, and its clinical applications.
1.1
E/MEG Imaging
Electrical activity of neurons generates electric and magnetic fields. Because of the
tissue properties, the skull and the scalp are relatively transparent to these signals.
With proper instrumentation, these fields can be recorded on or outside the head
surface. To estimate the distribution of the underlying currents, a mathematical inversion model is required. Since the electromagnetic inverse problem does not possess
a unique solution, suitable constraints have to be introduced. Research efforts aimed
at improving these estimates have produced plenty of estimation methods, some of
which will be discussed in this introduction.
EEG and MEG have been widely used in research and clinical studies since the
mid-twentieth century. E/MEG imaging is a powerful tool for studying neural processes
in the normal working brain that are of interest for neurophysiologists, psychologists,
cognitive scientists, and others interested in human brain function. The main reasons
of interest in E/MEG imaging are its time resolution and signal generation mechanisms.
Among the available functional imaging techniques, only E/MEG imaging have temporal resolutions below 100 ms, because the signals observed, unlike other brain imaging
modalities, are directly related to the electrical activity of the neurons (Horwitz and
Poeppel [2002], Nunez and Silberstein [2000]).
Clinical applications of EEG and MEG include improved understanding and treatment of serious neurological and neuropsychological disorders. A prominent example
is intractable epilepsy, where patients could benefit of improved localization of epileptic foci and vital cortical areas, minimizing the invasiveness of surgery and improving
the outcome (Baumgartner et al. [2000], Forss [1997], Mamelak et al. [2002]).
1.2
Physiological basis of E/MEG signal
When a neuron receives input from others, postsynaptic potentials (PSPs) are generated at its apical dendritic tree. In an excitatory PS, the apical dendritic membrane
depolarizes transiently, becoming extracellularly electronegative with respect to the
cell soma and basal dendrites. This potential difference creates a current flow from
the non-excited membrane of the soma and basal dendrites to the apical dendritic
tree sustaining the EPSPs Gloor [1985].
Some of the current, called primary current, takes the shortest route between
source and the by traveling within the dendritic trunk. Conservation of electric
charges imposes that the current loop be closed with extra-cellular currents flowing
across the entire volume conductor, forming a less compact current known as the
secondary (or volume) current.
The spatial arrangement of cells has a crucial role in the global addition process:
only ordered arrangements of synchronous firing simultaneously and close to each
other will produce a field detectable both in EEG and MEG. Thus, synchronously
activated large pyramidal cortical neurons are believed to be the main MEG and EEG
generators because of the coherent spatial distribution of their large dendritic trunks
locally oriented in parallel, and pointing perpendicularly to the cortical surface Nunez
and Silberstein [2000]. In addition, the postsynaptic currents generated in the dendritic trunks last longer than the typical action potential of cortical neurons, making
the temporal integration of generated fields much more effective. Furthermore, the
traveling action potential can be modeled with a pair of opposing currents sources
whose field is hardly detectable at a large distance.
The number of neurons needed to generate a measurable field outside the head has
been estimated in several publications. Himdilinen et al. [1993] used an approximate
model of the postsynaptic currents and concluded that approximately a million PSPs
might be required to produce a typical current detected in MEG. Later, Murakami
and Okada [2006] employed a more accurate neuronal model and were able to trim
down the estimate by almost an order of magnitude. Using estimates of macrocellular
current density given in Him~ilinen et al. [1993], a patch of 5 mm x 5 mm (40 mm2 in
the worst-case scenario) would create an extra-cranially detectable dipole of 10nA.m
(for MEG), consistent with empirical observations and invasive studies. EEG would
narrow down the needed surface area as reported in Nunez and Silberstein [2000],
but these estimates may be overly optimistic due to the limited resolution of the
source estimation methods and the different sensitivity profiles of magnetic and electric sensors (see for example Cohen et al. [1990]). The detection thresholds gives
the physiological resolution limit for both imaging modalities, although other facts
related to the estimation process lower the resolution as will be discussed later.
Finally, although EEG and MEG signals are believed to originate mainly in the
cortex, some authors have reported scalp recordings of deeper cortical structures
including the hippocampus, cerebellum and thalamus (see Baillet et al. [2001] for a
list of these findings).
Good reviews on the electrophysiological process associated with EEG/MEG signal generation can be found in Murakami and Okada [2006], Cohen and Halgren
[2003].
1.3
EEG
Although Richard Caton (1842-1926) is believed to have been the first to record the
spontaneous electrical activity of the brain, the term EEG first appeared in 1929 when
Hans Berger, a physicist working in Jena, Germany, announced to the world that
"it was possible to record the feeble electric currents generated in the brain, without
opening the skull, and to depict them graphically onto a strip of paper". Although the
technique has evolved with time, the basic principle remains unchanged: electrodes
are applied to the scalp, voltage difference between electrodes cause currents to flow
through the leads, and these currents are then simultaneously recorded and stored
for further processing.
EEG is currently common practice in the bedside, due to its simple set-up, immediate display of results, established interpretation, low price and non-invasiveness.
However, the source estimates discussed in this thesis are yet to be included in routine clinical practice; the standard EEG analysis approach is to study the voltage
traces themselves, where a trained neurophysiologist can identify indications of some
dysfunctions such as epileptic seizures.
1.4
MEG
The magnetic fields generated by neural currents are several orders of magnitude
smaller than the background fields in a typical laboratory, and thus elaborated interference blocking mechanisms and extremely sensitive sensors are needed. MEG imaging was made possible by the use of super-conducting quantum interference devices
(SQUID) and shielded rooms (Cohen [1970], Zimmerman [19771). The first SQUIDbased MEG experiment with a human subject was conducted at MIT by David Cohen
(Cohen [1968, 1972]), after the same technology was successfully applied to detect the
magnetocardiogram in 1969.
The first MEG experiments employed a single-sensor system and multiple acquisitions were needed to map the external magnetic field. Current MEG systems include
multiple 150-300 sensors in a helmet-shaped array (Bechstein et al. [2006], Schnabel
et al. [2004], Taulu et al. [2004]). External noise is dampened by using magnetically shielded rooms, gradiometric coil designs, reference sensor arrays, and software
noise cancellation techniques. The SQUID technology requires cryogenic temperatures, making MEG setups more expensive and bulkier as compared with the EEG
counterparts. Although this is not the focus of the thesis, good reviews of the hardware involved in MEG imaging can be found in Hfmlliiinen et al. [1993], Cohen and
Halgren [2003].
1.5
Multimodal imaging: E/MEG
EEG and MEG are concurrently aquired in modern systems as the one that will be
used in this thesis (see section 3.2), and techniques for combining both modalities in
a single estimate are already developed. As already stated in Wikswo et al. [1993],
the application of E/MEG as compared to single modality studies seems a promising
way to achieve more reliable estimates, as pointed out by Babiloni et al. [2001, 2004],
Yoshinaga et al. [2002].
Being governed by by the Maxwell's equations, (see section 1.6), EEG and MEG
are closely related. This relationship and the difference in equipment cost between
EEG and MEG modalities gave rise to a polemic that still persist in the scientific
arena: Is MEG, being more than one order of magnitude more expensive than EEG,
needed at all? (Cohen et al. [1990], Crease [1991], Wikswo et al. [1993]). The resolution of this problem is not straightforward because the quality of the source estimates
depends on several factors.
First, the orthogonality of the lead fields produced by MEG and EEG (see e.g.
figure 4 in Cohen and Halgren [2003]) is a powerful argument for the inclusion of EEG
and MEG channels in any study: inclusion of orthogonal information will increase
the rank of the lead field matrix relating the sources and measured signals and thus
the inversion will become less ill posed. More over, the advantages of including EEG
will affect different cortical areas in different ways. A notable example of this is
the inability of MEG studies to detect currents perpendicular to the scalp surface,
so that the sensitivity in the gyri decreases sharply. EEG has a more homogeneous
sensitivity map across all cortex (Malmivuo et al. [1997]) and can see these radiallyoriented sources. However, recent studies as Hillebrand and Barnes [2002] indicate
that radial sources, poorly resolvable in MEG, comprise only relatively small areas
of cortex at the crests of gyri and that source depth rather than orientation is the
prominent factor compromising the sensitivity.
However, these biophysical arguments have yet to be fully validated with clinical
data: No definitive report has been made of the improvement in current estimates accuracy when including simultaneous EEG and MEG recordings as compared to single
modality studies. However, non-concurrent EEG/MEG studies have been analyzed
in Babiloni et al. [2001], where the resolution matrix and its derived metrics were
used in 3 subjects data with encouraging results. Further simulations with varying
SNR were later reported in Babiloni et al. [2004].
1.6
The forward model
The relationships between the electrical activity in the brain and the recorded extracranial magnetic and electric fields are governed by the Maxwell equations. Furthermore, the quasi-static approximation applies in the case of MEG and EEG, as justified
by the head size and the values of the electromagnetic parameters in biological tissue
(Hdmdlmiinen et al. [1993]):
V E = p/co
V. E = p/co
Vox E = -B/9t
quasi-static
Vx E =O0
V-B=0
V-B=0
Vx B = I (J
+ coE/t)
0
VxB=
(= E = -V -V)
0J
Where E and B are the electric and magnetic fields, co and y 0 are the electric
permittivity and magnetic permeability in the vacuum, p is the free electric charge
density in that location, J is the free current density at that location, and V is the
electric potential that explains the conservative E field in the quasi-static approximation.
The current source JP (primary) generates a volume current J
as already dis-
cussed in section 1.2, so that charge is conserved. The total current J present in the
conductor is then:
J(r) = JP(r)+ J3'(r) = JP(r) + a(r) - E(r) = PR(r) - a(r) - VV(r)
(1.2)
Since, according to the fourth Maxwell's equation in Eqs. 1.1, the divergence
of the total current vanishes, we obtain a Poisson's equation governing the electric
potential:
V. (c(r)VV(r)) = V -j(r)
(1.3)
Which can be used to compute the potential distribution for an arbitrary conductivity distribution a(r) using numerical techniques. Once the total current is known
we can use the Ampere-Laplace law, solution of the quasi-static version of Maxwell's
equations, to calculate the magnetic field:
Br
(r) =
o/#
i(r) r x
47r fjJr
r - r'
r
r
- r'
v'
(1.4)
This equation can be expressed as a function of JP exclusively as explained in
Hiimliiuinen et al. [1993]:
- p
B(r) = L
47
((r)
+ V(r)V'u(r)) x
rr-r'
r-
Jr
-
r'
v'
(1.5)
Where the differential operator V' applies to the reference system of r'.
To solve using these equations, the conductivity distribution u(r) is needed. In
practice, the conductivity is often assumed to be piecewise constant. In this case, the
electric potential and the magnetic field can be calculated as a solution of integral
equations involving potential values on the surfaces separating compartments of different conductivities (Geselowitz [1967, 1970], Hdmmilinen et al. [1993]). The usual
practice is to construct a three-compartment model of the head, the cranial volume in
scalp, skull and brain. Sometimes a fourth compartment is used for the cerebrospinal
fluid (CSF) as well. The integral equations for B and V are discretized using triangular tessellations of the interface surfaces leading to a boundary-element model (BEM)
which can be solved using numerical techniques, see, e.g. Himiliinen and Sarvas
[1989]. For more details on the BEM method and on forward model calculations, see
Mosher et al. [1999].
1.7
The inverse problem
As discussed above, the main contribution to the EEG and MEG signals comes from
postsynaptic currents in the cortex. Therefore, the possible sources can be constrained
to the cortical surface resulting in a distributed dipole model. At each location, a
current dipole source represents the activity in the surrounding cortical area.
Because of the discrete number of measurements and sources, and the linearity of
Maxwell equations, one can construct a forward linear model that relates the activity
in the assigned locations x and the expected measurement data in the sensors b. To
complete the model, some measurement noise represented by n is included as follows:
A. x
n=b
(1.6)
The matrix A describing the linear transformation is often called lead field matrix.
The dimensions of A are Nm x Ns, being Nm the number of measurements, and N,
the number of sources. The element Ajk of the lead field matrix is the signal in sensor
j produced by a unit current dipole at location k.
The inverse problem for E/MEG is to estimate the current distribution originating
the recorded electric and magnetic fields registered by the sensors. Once the forward
model is known, this corresponds to solve for the vector of dipoles (x) in the equations:
LE x+n E = e
(1.7)
LM. x + nM =m
(1.8)
Sx +
Lm
=
n
u
e
(1.9)
m
Where LM and LE are the magnetic and electric lead field matrices, m and e are
the measured magnetic and electric data vectors, nE and n" are the detector noise
in the electric and magnetic readouts respectively. Eq 1.7, Eq 1.8 and Eq 1.9 are the
formulations in the EEG, MEG and E/MEG case, respectively. Since the structure of
the problem is the same in both modalities, saving the forward model computations,
we can use the general formulation A - x + n = b (Eq. 1.6).
1.8
Regularization
As already noted by von Helmholtz [1853], different source distributions can account
for the same observed fields outside the volume containing the currents. This fact
renders the inverse problem of E/MEG ill-posed in the sense of Hadamard. Therefore,
feasible constraints must be imposed on the current distribution to render the solution
unique. Furthermore, the solution may be very sensitive to small errors in the data.
Hence, regularization techniques are employed to stabilize the solution.
One approach to reqularize the solution is to require that a (weighted) £2-norm
of the currents is minimized while maintaining the consistency of the measured data
and that predicted by the forward model. In this case, the solution of the inverse
problem ^ can be formulated as:
= argmin {A.- x - bld
+
x2
(1.10)
Where Ial v = aTW-la is the W-weighted £2 -norm of the vector a, Wd is
the covariance matrix of the sensors noise, A is the regularization parameter, and
W, is the matrix that defines the weighting of the source components in the cost
function. Low values of the regularization parameter will prime the minimization of
the distance from observed and predicted data, whereas higher values will emphasize
the cost function.
Popular cost functions are e2-norm (MNE, Wang et al. [1992, 1993]) and the laplacian of the estimates (Low Resolution Tomography, LORETA, Pascual-Marqui et al.
[1994]), that will be explained subsequently. Is important to note that regularized
estimates are valid as far as the cost function has some practical or physiological
basis that can justify its use, which is not necessarily the case for any of the methods
commonly used, justified mainly by computational feasibility.
1.8.1
MNE
Minimum-norm estimation is well known in other fields as statistics (where is better
known by the name of Ridge regression) and linear algebra (where is better known
as Tikhonov regularization, Tikhonov [1963]). MNE has gained popularity as a regularization method for the E/MEG inverse problem (Hamtildiinen et al. [1993], Baillet
et al. [2001], Wang et al. [1992]). The solution of the inverse problem in this case
is given by the Eq. 1.10, whereW, is either an identity matrix or a diagonal matrix
where the individual variances of the currents are adjusted with help of other prior
information such as functional magnetic resonance imaging data.
The cost function minimized in MNE is then the e2-norm of the solution vector.
This is ad-hoc criteria makes the problem tractable, but is known to have some
problems such as superficial bias. This problem can be alleviated by depth-weighting
as analyzed in Lin et al. [2006b]. On the other hand, MNE offers a linear closed-form
solution that can be computed very efficiently, see section 1.9.1.
In a Bayesian sense, the MNE is the maximum a posteriori estimate under the
following assumptions:
1. The observed fields are generated by sources located only in the discrete source
space used to compute the lead-field matrix.
2. The amplitudes of the sources have a multi-variate Gaussian prior distribution
with a known source covariance matrix R'.
3. The measurement noise is additive Gaussian, with known noise covariance matrix C.
The e2-norm regularized inverse operator M can then be formulated as:
M = R/AT(AR/AT
+
C) -
1
(1.11)
Where A is the lead matrix, C is the data noise-covariance, and R' is the source
covariance matrix. Using the inverse operator, the expected value R of the current
amplitudes at time t is then given by k(t) = Mb(t).
In practice, the real covariance matrix of the sources is unknown, and a multiple
of a covariance template, R, is used. The covariance template is usually the identity,
implying that the different current sources are uncorrelated from each other. This
way, R' can be expressed as R' = R/A2 . The expression for the inverse operator is
now:
M = RAT(ARAT + A2 C) - 1
(1.12)
Eq. 1.12 gives a R(t) = Mb(t) that minimizes Eq. 1.10 with Wd = C and Wx =
R. This allows us interpret A-
1
as a multiplicative factor of the source covariance
in a Bayesian setting. Small values for A will result in more noisy estimates by
increasing the equivalent source covariance values (less regularized solution), and
large values of Awill produce smoother, smaller norm estimates where the equivalent
source covariance have smaller values (more regularized solution). The value of A is
then an important parameter that will affect the quality of the estimates, and its
optimum value will be a function of the measurable noise covariance matrix and the
unknown source covariance matrix.
MNE estimates can be normalized by their expected variance due to detector noise,
approximately yielding z-statistics instead of current estimates. The statistic maps,
often called dSPM, behave better in a number of ways, including a decreased depthbias, as analyzed in Dale et al. [2000]. This is because in the MNE solution sources
that are deep in the brain tend to have weights in the lead matrix that are much
smaller, so that the minimum-norm solution tend to assign the activity to locations
closer in the cortex that are more effective in generating the observed signal. When
normalizing by the variance as is the case in dSPM, this effect is balanced out by the
smaller variance of deep sources, as is discussed in Lin et al. [2006b].
1.8.2
LORETA
Other choices for W, are possible as well. For example, one can favor the smoothness
of the estimates in the spatial domain. Although this cannot be well justified by the
underlying physiological processes, it is nevertheless a way to make the solution to
the estimation problem unique. The cost function to be minimized is the Laplacian of
the currents, and the method that results from this choice has been named LORETA
(Low Resolution Tomography, Pascual-Marqui et al. [1994]). Although it does have
some nice properties regarding localization as pointed out in de Peralta Menendez
et al. [1997]), those results only examine the noiseless solution, and the statistics of
the estimates are not that favorable.
1.8.3
The equivalent current dipole model
Although is not a distributed current solution, and hence cannot be formulated with
the general formula provided here, the single equivalent current dipole (ECD) model
as a extreme case of regularization, where all the source is restricted to be one current
dipole somewhere in the brain. ECD can be a useful and accurate model in clinical
conditions such as early components of evoked potentials or localized epilepsy, where
the main source of E/MEG signal is effectively a single dipole much bigger than the
surrounding activity that can be considered background noise.
1.9
Performance of the regularized solution
An important problem on source estimation in E/MEG is assessment of resolution: to
which extent do estimated currents reflect the original ones? To quantify this, we
propose the use of two methods already found in the literature of inverse problems:
Singular-value spectra of the lead matrix and the resolution matrix. The fundamental
assumption in both cases is that the forward model is perfectly described, i.e., the
BEM model is completely accurate, the sources are only located in the discrete grid
of locations studied, and everything is perfectly co-registrated.
1.9.1
Singular-value spectrum of the lead matrix
Singular value analysis has been used for this purpose in ill-posed inverse problems as
optical tomography using Tikhonov regularization (Culver et al. [2001], Graves et al.
[2004]). The analysis establishes a link between the spectra of the singular values of
the whitened lead-field matrix and the resolution obtained in the estimation process,
which is a function of the parameters defining the forward solution as well as the
signal to noise ratio of the observations.
The withened lead field matrix can be computed as A, = C-1/ 2AR 1 /2 , where C
and R are as defined in 1.8.1.
Singular-value decomposition (SVD) of A yields a triplet of matrices: Aij =
•=ok(A) UikSkkVkj (A = USVT).
Here U and V are orthonormal matrices con-
taining the singular vectors of A, and S is a diagonal matrix that contains the singular
values of A. The rows of V correspond to image-space modes that can be used to
build up any spatial distribution of currents in the source locations. The columns
of U correspond to detector-space modes that can be used to build up any detected
pattern in the sensors due to a given spatial distribution of currents. The magnitudes
of the singular values in the diagonal of S provide a measure of the relative effects
of the image-space modes when transferred to detector-space modes in the process
of measuring. The singular values {Ai} are ordered to decrease in magnitude with
increasing image-space mode indices.
As advanced before, the MNE inverse can easily be computed once the SVD of
the lead matrix is now. Eq. 1.12 can be rewritten (using A = USVT) as:
M = RAT(ARAT + A2 C)= R1/ 2 &T(AAT +
1
A2I)-1C-1/
2
= R1/ 2AT(USSUT + A2 I)-1C-1/ 2
(1.13)
= R1/2V8S-1UC-1/2
= R1/ 2VrUTC-1/ 2
Where the elements of the diagonal matrix E are given by:
k
2=
Ak + A2
(1.14)
The values of the diagonal matrix r = OS- 1 can be then approximated as:
Akk
A-`,
A>A(1.15)
Ak<<A
This gives rise to a simple interpretation of the eigen-value spectra of Ai,{A} when
both C and R are diagonal: for a given A, the number of eigen-values above A fixes
the number of linearly independent image-space modes that can be retrieved by the
inversion in the minimum e2-norm case. As the number of independent image-space
modes is linked with the resolution of the estimate, and A is linked to the SNR as
discussed earlier, it follows that eigen-value spectra decaying slower with k will result
in higher resolution estimates for the same noise and activity conditions. Although
this is useful, no resolution estimate is given in image-space metrics, so singular-value
analysis is only useful in order to compare different estimates among them.
1.9.2
Resolution matrix
The resolution matrix has been proposed in inverse problems literature (de Peralta Menendez et al. [1997], Menke [1989]), providing resolution metrics more general
than singular-value analysis. It can be applied to any linear inverse problem, and
the metrics are constructed in image-space units (i.e. centimeters). Values for each
one of these resolution metrics can be obtained separately for each location. This
makes the resolution matrix a useful tool to quantify the goodness of the inverse operator.In E/MEG, the resolution matrix has been already used to assess resolution and
localization error in Babiloni et al. [2001, 2004].
The resolution matrix is constructed as follows: for a given estimation technique,
subject, sensor positions, source locations, and noise statistics, the regularized solution provides with an inverse operator that relates the observations b with the
estimates of x, and hence we have a relationship between the estimates * and the
underlying activity x using the forward model with the assumption of no noise:
x=Mb=MA x =Kx
(1.16)
active
measured
estimated
Ideally, Kjk
=
6jk, where 6 jk is the Kronecker delta function. This would imply a
perfect estimate for every source location.
The columns of K are the point spread functions (PSFs, see Fig. 1-1): the i-th
column (pi) indicates how the activity originally present in source location i is blurred
in space in the estimation process (see Fig. 1-1):
i= K.x= [pl,"" ,pNgJ'X=
Xi "Pi
(1.17)
i=l
The rows of K are the resolutionkernels (see Fig. 1-2): the i-th row (r[T ) indicates the
effect of activity at other locations on the estimated value at location i (see Eq. 1.17).
S=K
x=
xi= rT
Nm
.
(1.18)
"
rT
N,
X
Based on the PSFs, figures of merit for the resolution of the estimates can be
Estimate
Resolution matrix
K 1 ,2
"-
K1,N-1
Activity
K1,N
K2,N
KN,2
". KN, N-1
KN-1,N
0
KN,N
0
"Truth"
Estimate
Figure 1-1: Point spread functions: The first PSF explains the distribution of the
current estimates generated by a point-like activity located in the first source location.
constructed. For a given source location, the spatial dispersion (SD) is related to
the width of the PSF associated with that voxel, and the dipole localization error
measures the distance from the maximum PSF weight to the original location of the
voxel. Being dij the distance from voxel i to voxel j, SD and DLE are computed as:
SDi =
l(dkj - IIKkjl) 2
V EkN
IIKkjll
i = argmax {IIKk•j
k
(1.19)
(1.20)
DLEj = dij
Based on the RKs, a figure of merit for the resolution of the estimates can be
constructed. For a given source location, the resolution index (RI) scores, from 0 to
1, how much of the estimated value is due to close sources versus contribution from
source locations far from the estimated one. The formulation is such that a value of
Estim; ate
0
0
00
Resolution matrix
Activity
I
(
'1I
KN-1,1
/
\KN,1
Estimate
LN- 1,N
KN,2
."'
KN,N-1
LN,N
i
/
"Truth"
Figure 1-2: Resolution Kernels: The first RK explains how the estimate for the first
location is influenced by the activity in any other location, including the assigned
one.
0 reflects very poor performance, and a value of 1 is excellent:
D = max dj,
j = argmax ({IKikI
(D - dij) - IIKIIl
(1.21)
D - Kgij |
All metrics derived from the resolution matrix are spatially resolved, that is, each
source location has an assigned value, so that we can observe how the solution will
behaves for different areas of the brain. In this thesis, morphing will be applied to
generate spatially resolved average values for each metric across different subjects.
Chapter 2
Objective of this thesis
The aim of this work was to assess the improvement in localization and resolution of
simultaneous E/MEG studies as compared to EEG or MEG studies alone. The inverse
operators generated by clinical data will be examined by characterizing their eigenvalue spectra and associated resolution matrices, and the distribution of resolution
matrix derived metrics across the cortex will be characterized.
The results are confirmed by inspecting the estimated activity maps in situations
where the underlying activity can be well described, as is the case with median nerve
stimulation. We will try to find patients with different degrees of activity to recreate
in clinical conditions a dataset with different signal to noise ratios.
The results of the study provide further evidence of the need of E/MEG studies
versus single-modality ones in the standard clinical practice.
Chapter 3
Methods
3.1
Clinical data
Clinical data from four different E/MEG studies was used in the thesis. The subjects
were patients who presented with drug-resistant epilepsy undergoing median nerve
stimulation for the localization of the somatosensory hand areas. This information
is used along with the localization of the epileptic foci to evaluate the viability of
future surgery. All clinical data will be provided by Matti Hiimiliinen and Steven
Stufflebeam, and has been acquired at the MGH/MIT/HMS Athinoula A. Martinos
Center for Biomedical Imaging, with the approval of the Institutional Review Board.
3.2
Data acquisition
MRI scans were acquired in a 1.5 T MRI scanner (Siemens Medical Solutions, Erlangen, Germany). The exact composition of the MRI scans varied from patient
to patient according to clinical needs. However, for cortical surface reconstruction
and MEG/EEG forward modeling, Tl-weighted 3D MPRAGE data were acquired
from all patients. The FreeSurfer software was used to normalize and average several
sequences to get the best resolution possible across all tissue components.
Subsequent E/MEG studies were performed with a 306-channel MEG system with
70-channel EEG (Vectorview, Elekta-Neuromag, Helsinki, Finland).
The coils of
the MEG channels are arranged in a hemispherical mosaic sampled in 102 distinct
locations. Each of these locations contain three co-axial coils: one magnetometer
and two perpendicular gradiometers. The magnetometer is sensitive to the normal
component of the magnetic field at that given location, while the gradiometers register
the gradient of that normal component in two orthogonal directions in the coil plane.
The EEG electrodes were arranged approximately in the standard 20-20 layout defined
by the electrode cap. Bandwidth was set at 0.1 to 334 Hz, and data was digitized
at 1004 Hz. EEG and MEG traces were simultaneously recorded. About 127 stimuli
were delivered to each median nerve. The stimulus onset times were recorded on a
trigger channel to allow averaging of the epochs.
3.3
Data processing
Both EEG and MEG data were visually inspected for loose/disconnected electrodes
and noisy or dysfunctional SQUID sensors. In the actual data, only 4-5 EEG channels per acquisition were considered to be disconnected, and a typical example of the
channel selection can be seen in figure 3-1. The stimulus artifact (at 0 ms) is bigger
than in non-epileptic subjects, because the population under study is usually under
the effect of seizure-controlling drugs that increase the effective stimulation current
threshold. This increased artifact made unusable the first 5 ms of acquisition (typically), but did never impede the analysis of the physiological response because of the
propagation delay of the electrical signal through the nerve (more than 20 ms).
Signal-space projectors (Uusitalo and Ilmoniemi [1997]) were then applied. The
projectors corresponded to average reference electrode for EEG channels, and the first
three spatial components obtained by the principal component analysis (PCA) of the
magnetometer MEG channels readouts in the absence of subject. The data were not
low-pass filtered in the post-processing because the temporal scale of the expected
activity is in the order of milliseconds.
All signal processing and analysis was carried out in 64-bit AMD Opteron computers running Red Hat Enterprise Linux in the Neurostatistics lab at MIT. Matlab©
Figure 3-1: Example of visual inspection for disconnected/malfunctioning channels.
Only EEG channels are shown, and the ones marked as disconnected appear in red.
The vertical yellow lines correspond to stimulus presentation, median nerve stimulation. Stimulation-induced artifact is seen in all channels lasting around 1 ms after the
stimuli has been applied (0 ms to stimuli marked as vertical yellow lines).
(The Mathworks, inc) routines were used for analysis and representation of the findings.
3.4
Noise covariance computation
The noise covariance matrix for each acquisition was then computed from the signalspace projected data. The data was split in 100 ms epochs starting at each stimuli
onset. Baseline correction was performed in each epoch by subtracting the average
value registered in the time range [-100 ms, -10 ms] relative to stimulus onset. Let:
s,j p = 1... Pr, j = 1... Nr, r = 1... R
(3.1)
Be the baseline corrected epochs used to calculate the covariance matrix. In the
above, Pr is the number of accepted epochs in category r, Nr the number of samples
in the epochs of category r, and R is the number of categories. In our case, R = 2 (left
and right median nerve stimulation, respectively), Nr = 100 ms x 0.6 samples/ms,
and Pr will vary with each experiment. The covariance matrix was then computed
as:
R N, P,
C=1
-
s (r)) (s
-
(r)) T
(3.2)
•r=1j=l p=l
Where s - (r) and N, stand for:
Pr
s-(r)
p=l
3.5
(r)J, N =N
R
(Pr
r - 1)
(3.3)
r=l
Forward Model
Using the MRI data, the geometry of the gray-white matter was derived with an
automatic segmentation algorithm in the FreeSurfer software to yield a triangulated
model with approximately 340,000 vertices (Dale et al. [1999], Fischl et al. [1999,
2001]). The source space was created by decimation of the original triangulation to
a subset of vertices with a controlled average distance around 5 mm between nearest
dipoles, excluding sources located closer than 7mm to any EEG or MEG sensor.
Cortical patch statistics of the source space were then calculated as described in
Lin et al. [2006a] to obtain average normal directions, their standard deviation, and
approximate patch areas. Similar triangulated models were obtained for the skin
surface and the inner and outer skull surface. EEG sensors positions were digitized
at the time of acquisition by a 3D position scanner, and later co-registered with the
MRI data by means of a semi-automated routine that minimizes the distance from
the electrodes position to the reconstructed MRI skin surface. Registration with the
MEG was done by digitizing the position of three coils attached to the head of the
subject.
With the head geometry (skin and skull), the source space (pial), and the coregistered sensor positions, MEG and EEG forward models were calculated using a
3-layer BEM (Hfmaliiinen and Sarvas [1989], Oostendorp and van Oosterom [1989]).
The automated routines included in the MNE tools 1 software suite of the Martinos
Center were used for this purpose. The forward model considered only current sources
normal to the pial surface, and all the surfaces involved in the BEM computations
were decimated to include 5120 triangles for computational tractability. The accuracy of the forward model is critical for the reliability of the estimates, and hence,
visual inspection of the output surfaces produced by the automated procedure was
mandatory and was performed for every subject. A typical three-layer model of one of
the subjects under study can be seen in figure 3-2. The surfaces were also inspected
by using tkmedit, part of the FreeSurfer distribution, where the surfaces could be
visualized together with the MRI scan of the subject.
3.6
Activity estimates
Activity estimates were obtained with the MNE tools software for the MNE and
dSPM solutions. This was done before the subsequent analysis to test the goodness
'Information about MNE tools and used techniques, as well as download information can be
found at http://www.nmr.mgh.harvard.edu/martinos/userlnfo/data/sofMNE.php
BEM surfs for msthesisO6 - az:90 el:00
BEM surfs for msthesis06 - az:180 el:00
BEM surfs for msthesisO6 - az:OO0
el:90
BEM surfs for msthesisO6 - az:45 el:-22
Figure 3-2: Example of automatically extracted surfaces used in BEM model computations: triangular mesh for skin (black), outer skull (blue), and inner skull (red).
of the forward model and inverse operator. Inverse operators, lead matrices and the
SVDs discussed in section 1.9.1 were all stored for later analysis. As a result of the
estimation process, time-resolved current estimates after estimulation are obtained.
For the estimation process described above to be considered correct, the produced
current distributions have to be physiologically plausible. As reported elsewhere (see
Kakigi [1994] for an example), median nerve stimulation elicits a strong, dipole-like
activation 35 ms after stimulation localized in the posetior wall of the contra-lateral
post-frontal sulcus. This should be present not only in the current estimates, but also
in the EEG and MEG traces as a strong deflection positive or negative deflection at
that time point.
In all cases, focalized activity close to the central sulcus was obtained after the
inversion process. Because of the noise-normalized nature of the dSPM solutions, and
its better performance as reported elsewhere, dSPM operator was chosen for the rest
of the analysis.
3.7
Inverse operator analysis
For each subject, four different lead matrices and inverse solutions were generated by
selecting only certain channels out of the total acquired data:
1. EEG case - Only EEG channels are used in the inversion.
2. MEG case - Only MEG channels are used in the inversion.
3. EMEG case - All MEG and EEG channels are used in the inversion.
4. EMEG2 case - All EEG and most of the MEG channels are used in the inversion, so that the total number of channels used equals the total number of
MEG channels. In this case, only magnetometers were removed from the MEG
channels, and an effort was made to preserve the overall detector density as
compared with MEG alone. That is, for each EEG sensor a magnetometer was
removed as close as possible to the added electrode, as is exemplified in figure
3-3.
Figure 3-3: Example of MEG sensors removed for EMEG2 case: in blue, a surface
with a MEG sensor in each vertex. The position of each EEG sensor was projected in
this surface (red dots in the figure). Assignments of MEG/EEG pairs to be replaced
are depicted with green arrows.
36
EEG and MEG cases give a reference on the contribution to the spectra for EEG
and MEG alone. EMEG case give as much information as possible by using all
available channels, but is not readily comparable with MEG case as different number
of channels are used in the inversion. EMEG2 throws away some information of the
magnetometers, potentially the more collinear because of their sensitivity profiles,
and provides with a E/MEG model with same number of channels as the MEG case.
3.7.1
Eigenvalue spectra
MNE tools provide with the SVD decomposition of the lead matrix, that it later uses
to compute the inverse operator as described in the introduction. The eigen-values
of the SVD were plotted for each subject and channel selection to compare the global
effect of different channel selections in different subject scenarios.
Comparison of MEG and EMEG2 cases is the most informational when comparing
MEG to E/MEG: The number of channels is the same for both cases, so the differences
in eigen-value spectra have to be due to different degrees of co-linearity in the rows
of the inversion matrix. As low a degree of co-linearity as possible is desired to get
the best estimates. Ideally we would want as flat a spectrum as possible for a given
number of channels. The advantage of including EEG along with MEG measurements
should show up in a higher number of eigen-values above a given threshold for EMEG2
case as compared with MEG. This comparison is specially meaningful in the dSPM
case, where the different SNRs of MEG and EEG channels have been compensated in
the inversion matrix, and the effect of the regularization parameter is a function of the
eigen-values as was summarized in Eq. 1.15. In this case, the number of eigen-values
above A _ 1 can be linked to the resolution of the estimate for a fixed SNR value
as discussed in section 1.15.
3.7.2
Resolution matrix metrics
For each subject and channel selection, the different resolution matrix-derived metrics,
DLE, SD and RI, were displayed over the cortex surface. To better understand the
distribution and variation of different figures of merit, all cortices were morphed into
one of the subject's cortex, allowing for averages in a space-resolved manner across
different subjects.
3.8
Analysis of the clinical data
The obtained activity estimates were inspected from the clinical point of view, using
the resulting estimates for the four subjects in the dataset. For the clinician, localization accuracy and the reliability are capital. The estimates generated for the four
different channel selections were compared in a case-by-case basis, in time-points that
were showing dipole-like activity as seen in the EEG and MEG traces and the field
maps. For median nerve stimulation, a localized activity is expected and its position
is well described, so the subjects are effectively used as phantoms, well-known test
distributions in which to assess the goodness of the E/MEG imaging.
ECD fits at specific time points were compared with dSPM estimates using the
different channel selection cases. This way, the estimates are compared to a localization tool used in clinical practice. The goodness of fit of the dipole can then be
used to assess the dipole-like distribution of the actual activation pattern for the time
point under study.
Chapter 4
Results
4.1
Eigen-value spectra
Forward models were computed using MNE tools for each one of the channel selections
described in section 3.7. Four different subjects were studied, and the lead matrices for
each one of them reflect the different skin, skull and pial surface shapes, the different
electrode positions for the EEG, and the different placement of the MEG helmet
relative to the subject's head. In order to compare all cases, the source variance was
assumed to be the same.
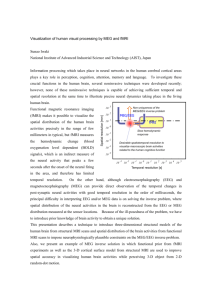
The results of the analysis can be seen in figure 4-1, showing a consistent trend
across subjects: EEG values have higher values than MEG for the first eigen-values,
reflecting their better SNR, but decay quicker than MEG indicating a higher degree
of co-linearity. Importantly, the spectra of EMEG2 case as compared with MEG case
shows higher eigen-values across all the spectrum, reflecting a resolution improvement
when replacing MEG magnetometers with EEG sensors. This implies that the rank of
the lead matrix is increasing, that is, EEG sensors sensitivity patterns are less linearly
independent of the remaining MEG sensors ones than the removed MEG sensors.
Hence, singular-value analysis alone suggests that it would be advantageous to
include the EEG sensors as compared with just adding more MEG channels to perform
the measurements.
10
Eigenvalues for msthesis03
Eigenvalues for msthesis04
10
8
8
10
10
10
106
Wl
tEEG
..
........
..............
M4
EG ....
.. .........
E"""
ME
4
10
-
EMEG
SEMEG2
2
10
0
10
100
200
Eigen-index
300
400
0
Eigenvalues for msthesis05
100
...
. ....
8
10
La10
10
300
400
Eigenvalues for msthesis06
10
10
10
200
Eigen-index
..
.............
6
.2
-
w
wJ
EEG
............... u EG ..........
EMEG
EMEG2
0
100
200
Eigen-index
300
400
0
100
200
Eigen-index
300
400
Figure 4-1: Eigen-value spectra for different subjects and different set of channels
included in the inversion. Although the forward matrices are different for each subject,
the behavior of each channel selection is consistent across subject and favors the
inclusion of EEG and MEG channels.
4.2
Resolution matrix metrics
For every subject and channel selection, inverse operators were generated for dSPM
using a SNR estimate of 3. This is a conservative estimate validated by clinical
practice in the Martinos Center. Resolution matrices corresponding to each subject
and condition were then computed, and values for DLE, SD and RI are found for
every source location.
As in the eigen-value spectra, the behavior of each metric across subjects was
reflecting similar behavior for a given metric and cortex location. To better reflect
the general trend rather than subject-specific phenomena, all maps are registered to
one cortex's geometry and then registered maps are averaged across subjects.
The average maps generated are topographically displayed over an inflated surface
generated by the FreeSurfer software starting from the pial surface detected in the
MRI scans. Gyri are represented in lighter gray, and the sulci in darker grey. Data is
displayed in a color-coded overlay.
Although topographic maps provide with space-resolved information, subtle differences in values were difficult to compare among some cases, specially EMEG versus
EMEG2. Use of cumulative histograms of the indices values proved valuable in reflecting these quantitative differences.
4.2.1
Dipole Localization Error
Cumulative histograms of DLE values for each subject are shown in Fig. 4-2. The
relative distribution of values for each case is preserved across subjects, and the
morphed and then averaged cumulative histogram (Fig. 4-3) shows the same trends
as individual subjects.
The distributions show a marked decrease in DLE values when including the MEG
sensors, but this might be a result of the increased number of channels. MEG and
EMEG2 cases show same distribution of values, suggesting no significant improvement in the localization error when including EEG channels.
Cumulative histogram of DLE values, msthesis04
Cumulative histogram of DLE values, msthesis03
100
0
4
DLE (cm)
2
6
8
2
0
4
DLE (cm)
6
8
Cumulative histogram of DLE values, msthesis06
Cumulative histogram of DLE values, msthesis05
100
0
6
4
DLE (cm)
2
8
2
4
DLE (cm)
6
8
Figure 4-2: Cumulative histogram of DLE values for each subject. Similar relative
distributions are seen in spite of forward model differences. Only EEG case shows a
clear disadvantage in localization, whereas MEG, EMEG and EMEG2 show similar
behaviors.
CiulanaveIstogam of DLEvalues,morphed
andaveraged
i
I
a
.3
$
0
1
2
3
4
DLE(cm)
5
6
7
8
Figure 4-3: Cumulative histogram of across-subjects average DLE values. Only EEG
case shows a clear disadvantage in localization, and the rest of cases show similar
behavior.
Average DLE for EEG case [cm]
0.5
1
1.5
2
2.5
3
3.5
Average DLE for MEG case [cm]
4
4.5
5
0.5
1
1.5
2
2.5
3
3.5
1.5
2
2.5
3
3.5
4
4.5
5
4
4.5
5
4
4.5
5
Averaae DLE for EMEG2 case rcml
Average DLE for EMEG case [cm]
0.5
1
4
4.5
5
0.5
1
1.5
2
25
3
3.5
Average DLE for MEG case rcml
Average DLE for EEG case [cm]
"";~
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
0.5
Averaae DLE for EMEG case fcml
1
1.5
2
Averana nLF fnr
1
1.5
2
2.5
3
FM•t
3.5
Pana&
Irnmi
""I-ZZA
-9~
0.5
2.5
3
3.5
4
4.5
5
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
Figure 4-4: Spatial distribution of average DLE values in the left hemisphere.
AvArmna
Average DLE for EEG case [cm]
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
0.5
1
1.5
2
2.5
3
3.5
4
4.5
5
0.5
1
1
1.5
2
2.5
3
3.5
4
4.5
5
0.5
Averaoe DLE for EMEG case reml
1.5
1
1.5
1
1.5
2
3
3.5
4
4.5
5
2
2.5
3
3.5
4
4.5
5
2
2.5
3
3.5
4
4.5
5
4.5
5
Averana ni F fnr EMFur) nsa reml
4=;W0.5
2.5
Average DLE for MEG case [cm]
Average DLE for EEG case [cm]
0.5
2
Average DLE for EMEG2 case [cm]
Average DLE for EMEG case [cm]
0.5
1.5
1
nL F fnr WFi nra., rtml
I~
2.5
3
3.5
4
4.5
5
0.5
1
1.5
2
2.5
3
3.5
4
Figure 4-5: Spatial distribution of average DLE values in the right hemisphere.
4.2.2
Spatial Dispersion
Cumulative histograms of SD values for each subject are shown in Fig. 4-6. The
relative distribution of values for each case is preserved across subjects, and the
morphed and then averaged cumulative histogram (Fig. 4-7) shows the same trends
as individual subjects.
Smaller values of the SDs imply better resolution, and across subjects, the distribution of values always reflect the same order: EEG presents the lower resolution
(larger SDs), then MEG, then EMEG2, and finally EMEG shows the best resolution
(smallest SDs). Because of the marked difference between MEG and EMEG2 cases,
these graphs suggest an improvement in resolution when including the EEG channels.
Moreover, the very small difference between EMEG and EMEG2 cases shows that
the increase in number of channels is not the main reason for the decrease in SD
values obtained when including the EEG channels.
The distribution of average SD values is plotted over the inflated cortices in Fig. 48 and Fig. 4-9. Here the effect on the SD values of including EEG measurements can
be seen by comparing MEG and EMEG cases: in the outer surface, the high SD values
for sources deep in the sulci decrease greatly; in the inner surface, the depth at which
SD values increase is increased. This effect is consistent with the slower decay of
EEG sensitivity with depth, and remains virtually unchanged in the EMEG2 case in
spite of the removal of the magnetometer readouts. This would explain the increased
resolution already noticed in the histograms.
To further quantify this effect, we compute the relative decrease of SD values when
replacing the MEG channels for the EEG ones, that is:
PC = 100
SDMEG - SDEMEG2
DME SDEMEG
SDMEG
(4.1)
Positive values of PC mean decreased SD and increased resolution in the considered
location when using the EEG channels. Topographical display of PC as seen in
Fig. 4-10 better delineates which areas would in average benefit most from the EEG
measurements inclusion. In the outer surfaces, source locations closest to the skin
Cumulative histogram of SDI values, msthesis03
0
2
4
SDI (cm)
6
Cumulative histogram of SDI values, msthesis04
0
8
2
4
SDI (cm)
6
8
Cumulative histogram of SDI values, msthesis05
Cumulative histogram of SDI values, msthesis06
0
0
2
4
SDI (cm)
6
8
2
4
SDI (cm)
6
8
Figure 4-6: Cumulative histogram of SD values for each subject. Similar relative
distributions are seen in spite of forward model differences. Smaller SDs, indicating
an increase in resolution, are seen in EMEG2 when compared to MEG in all subjects.
Cumulativehistogram
of SD values,
morphedandaveraged
1
a
SDI (cm)
Figure 4-7: Cumulative histogram of across-subjects average SD values. An increase
in resolution, reflected in overall smaller SDs, is achieved when including the EEG
channels, even when differences in channel numbers are accounted for.
surface get the biggest resolution increase, with only the very deep sources in the
bottom of the sulci and in the insular cortex showing decreased resolution. The central
and pre-central gyri are specially favored by the EEG inclusion, registering decreases
in SD values of up to 30%. In the inner part of both hemispheres, the inclusion of
EEG allow for better estimates of deep sources, as shown by a generalized decrease
in SD values.
4.2.3
Resolution Index
Cumulative histograms of RI values for each subject are shown in Fig. 4-11. The
relative distribution of values for each case is preserved across subjects, and the
morphed and then averaged cumulative histogram (Fig. 4-12) shows the same trends
as individual subjects.
Bigger values of the RIs imply better resolution, and across subjects, the distribution of values always reflect the same order: EEG presents the lower resolution
(smallest RI values), then MEG, then EMEG2, and finally EMEG shows the best
resolution (largest RI values). Because of the marked difference between MEG and
EMEG2 cases, these graphs suggest an improvement in resolution when including
the EEG channels. Moreover, the very small difference between EMEG and EMEG2
cases shows that the increase in number of channels is not the main reason for the
decrease in RI values obtained when including the EEG channels.
As can be seen in the topographical representation of Fig. 4-13 and Fig. 4-14,
the average RI values increase for virtually every location with the inclusion of EEG
measurements. This is made even clearer in Fig. 4-15, where the relative increase in
RI values for case EMEG2 compared to MEG is displayed. Almost all locations get
a better RI except for the ones located deep in the sulci or in the insular cortex.
Average SDI for MEG case [cm]
Average SDI for EEG case [cm]
0
0.5
1
1.5
2
2.5
3
3.5
4
0
0.5
Average SDI for EMEG case [cm]
0
0.5
1
1.5
2
2.5
3
0.5
1
1.5
2
2.5
3
3.5
4
0
0.5
0.5
1
1.5
2
2.5
3
2
2.5
3
3.5
4
1
1.5
2
2.5
3
3.5
4
3.5
4
3.5
4
Average SDI for MEG case [cm]
3.5
4
0
0.5
Averace SDI for EMEG case rcml
0
1.5
Average SDI for EMEG2 case [cm]
Average SDI for EEG case [cm]
0
1
1
1.5
2
2.5
3
Averaae SDI for EMEG2 case rcml
3.5
4
0
0.5
1
1.5
2
2.5
3
Figure 4-8: Spatial distribution of average SD values in the left hemisphere.
Averaae SDI for EEG case rcml
0
0.5
1
1.5
2
2.5
3
Averaae SDI for MEG case rcml
3.5
4
0
0.5
Average SDI for EMEG case [cm]
0
0.5
1
1.5
2
2.5
Averana SDI for FFG na
0
0.5
1
1.5
2
2.5
3
3.5
4
0
0.5
0.5
1
1.5
2
2.5
3
2
2.5
3
3.5
4
1
1.5
2
2.5
3
3.5
4
3.5
4
3.5
4
Average SDI for MEG case [cm]
3.5
4
0
0.5
Average SDI for EMEG case [cm]
0
1.5
Average SDI for EMEG2 case [cm]
rnmli
3
1
1
1.5
2
2.5
3
Averaae SDI for EMEG2 case fcml
3.5
4
0
0.5
1
1.5
2
2.5
3
Figure 4-9: Spatial distribution of average SD values in the right hemisphere.
Average SDI, 100*(MEG - EMEG2)/MEG [%]
Average SDI, 100*(MEG - EMEG2)/MEG [%]
-20
-10
0
10
20
-20
Average SDI, 100*(MEG - EMEG2)/MEG [%]
-20
-10
0
10
20
-10
0
10
20
Average SDI, 100*(MEG - EMEG2)/MEG [%]
-20
-10
0
10
20
Figure 4-10: Average decrease in SD values using EMEG as compared to MEG case.
Cumulative histogram of RI values, msthesis03
0
0.2
0.4
0.6
0.8
Cumulative histogram of RI values, msthesis04
1
0
Cumulative histogram of RI values, msthesis05
0
0.2
0.4
0.6
0.8
0.2
0.4
0.6
0.8
1
Cumulative histogram of RI values, msthesis06
1
0
0.2
0.4
0.6
0.8
1
Figure 4-11: Cumulative histogram of RI values for each subject. Similar relative
distributions are seen in spite of forward model differences. Larger RIs, indicating an
increase in resolution, are seen in EMEG2 when compared to MEG in all subjects.
Cumulatvehitogramof RI values,rorphed andaveraged
aS
at
0
0.1
0.2
0.3
0.4
0.5
RI
0.6
0.7
0.8
0.9
Figure 4-12: Cumulative histogram of across-subjects average RI values. An increase
in resolution, reflected in overall larger RIs, is achieved when including the EEG
channels, even when differences in channel numbers are accounted for.
Average RI for MEG case [cm]
Average RI for EEG case [cm]
.E,ý-r
0
0.2
0.4
0.6
0.8
0
1
Average RI for EMEG case [cm]
0.2
0.4
0.6
0.8
0
1
0 .8
1
0.2
0.2
0.4
0.6
02
0.4
0.6
0.4
0.6
0.8
1
0.8
1
0.8
1
Average RI for MEG case [cm]
0.8
0
Average RI for EMEG case [cm]
0
0.6
J
Average RI for EEG case fcml
0
0.4
Average RI for EMEG2 case [cm]
d
0
0.2
0.2
0.4
0.6
Average RI for EMEG2 case [cm]
0.8
0
1
0.2
0.4
0.6
Figure 4-13: Spatial distribution of average RI values in the left hemisphere.
52
Averaae RI for EEG case reml
tO
0.2
0
0.4
0.6
0.8
1
0
0.2
Average RI for EMEG case [cm]
0.4
0.8
0.6
1
Average RI for EMEG2 case [cm
I]
,I'
/
I
VP
w
-1 a.:
0
0.2
0.4
0.6
0.8
1
0
0.2
Average RI for EEG case [cm]
0
0.2
0.4
0.6
0.2
0.4
0.6
0.6
0.8
1
0.8
1
0.8
1
Average RI for MEG case [cm]
0.8
1
0
Average RI for EMEG case [cm]
0
0.4
0.2
0.4
0.6
Average RI for EMEG2 case [cm]
0.8
1
0
0.2
0.4
0.6
Figure 4-14: Spatial distribution of average RI values in the right hemisphere.
Average RI, 100'(MEG - EMEG2)/MEG [%]
Average RI, 100*(MEG - EMEG2)/MEG [%]
___
-20
-10
0
I
10
20
I
-20
Average RI, 100*(EMEG2- MEG)/MEG [%]
-10
0
10
20
Average RI, 100*(EMEG2 - MEG)/MEG [%]
i
-20
-10
0
10
20
-20
-10
0
10
20
Figure 4-15: Average increase in RI values using EMEG as compared to MEG case.
4.3
Clinical data analysis
4.3.1
Equivalent current dipoles
For all the four subjects under study, ECD fits at N20m times were computed for every
subject with the collaboration of Naoro Tanaka. The N20mrn time is characterized by a
deflection in the MEG and EEG traces changing from positive to negative close to the
somatosensory cortex about 20 ms after stimulation, time that takes for the action
potential to travel the arm's length and reach the cortex. The activity is localized in
the posterior wall of the contralateral central sulcus, in the area assigned to the arm in
the somatotopic map. One of the subjects, msthesis03, did not show N20m response
good enough for the ECD fit in the right somatosensory cortex, but localized activity
was seen in the MNE estimates. P30m activity was recovered instead, results of this
estimation for the different subjects can be seen in Fig. 4-16, Fig. 4-17, Fig. 4-18 and
Fig. 4-19.
4.3.2
Minimum (2-norm estimates
dSPM values resulting from the application of the minimum f 2 -norm inverse operator
to the collected data at the estimated N20m times can be seen in Fig. 4-20, Fig. 4-21,
Fig. 4-22 and Fig. 4-23. The four uppermost images in the panel correspond to right
median nerve stimulation, showing activity in the lateral side of the left hemisphere as
recovered by each of the four different channel sellections, while the lower four images
show the lateral side of the right hemisphere under left median nerve stimulation.
The threshold was set to half the maximum dSPM value registered across all the
hemisphere for each case. As can be seen in the figures, both EMEG and EMEG2
cases resulted in higher maximum dSPM values for all the four subjects, and the
distribution of values tended to be more concentrated in EMEG2 case as compared
to the MEG one.
331033tW1Mn
f
i (MInw rM
113nSW5SIllf
ai.
t
Sm
&fl
-27
m inYn-SAM-a
U03
~ ~r~
~~
AM~~~
owa00-M aVa3 ft&Va
3171
72.f U41r
4374
OU
IIO
I 11~I~M~
I=
W
am
it_ .7614913
ý"43 -43
4*W
nr:
.~~k-ao-l~
ac" n fttw"ka5wrr
62
543 4284
I~~
9 ni
P30m
)here]
[righthemisphere]
Digitalfliter:1.0- 300Hz.Scale:50fr/m, 100
ms.
P,
LOR
OPtMfUW-
=0 M8
a
*ft-10MA.-I40
krm~
0"•a_to10
fTAW
VW-•oD _ 7W
Ma-7413MJwI-IM.MI
Sc9b 20 M 00f~w m f
N20m
2 9mas
[left hemisphere]
[right hemisphere]
wDigtal
fit 1.0-300 H Sale: 50 flSom,100ms.
Figure 4-16: ECD fits for subject msthesis03. Right (top figure) and left (bottom
figure) median nerve stimulation at P30m and N20m times. See text for description.
J,,Lof P
.naL, awlItoll
aO
Ow. 1`1,
Ou -=
=
LA.
Amf -mo-
am -Tw : 7413w
'r=10.ta,i_M
M,~
50,,m
K
=
oor
50I
N20m
A
238ns
i
[left
hemisphere]
*i
]eehsmethgr
Digitalfilter:1.0- 300Hz.Scale:50ffT/cm,
100
waa=
at f:cM W
loii'm
war
An - GA- 230 w Ilialllr*
z NaidTawaVra
mis
u~Hw-tku
ar
31230 n 23 22 I 33 2 3L 42 4334L4U40 11 2342242
Taia
a- am =13I'T"aw-= a - MW
U0*L1I~nV1U
Omaimra
JIMf 90ff" 250n
[left hemisphere]
0 hemisphere]
taight
ms.
100
50fr/cm,
H,Scale:
1.0
-300
agithl
filter:
Figure 4-17: ECD fit for right (top figure) and left (bottom figure) median nerve
stimulation at N20m time for the subject msthesisO4. See text for description.
H
ft
23
413a"
23 -Wia
23 - =0 rO-?M
2 2 22- ft%
J1 kin
TIM-=*ID
II·
ý 2w so " 2w F
r
238nis
N20m
A
[lefthemisphere]
No
4i~~h00
[right hemisphere]
Digitalfilter:1.0-300 Hz Scale:50f/cm, 100
ms.
L
ý4m
ENQN=
4
w ac a& w (Mu=
#4M O-ar33.7 .
GWu
hee,
aoT dF~a.
6
m---ý r0
30Ok
W MSonlw IDAG-I~IPI
UM
"AMw -MO- Sao
EM
?WW
wM
tieiký aW 50"AM2 ITM~mr
mUPIUU
N20m
A
*0
24ms
[lefthemisphere]
[right hemisphere]
Digitalfiltr: 1.0-300Hz,Scale:50 f/cme,100ms.
Figure 4-18: ECD fit for right (top figure) and left (bottom figure) median nerve
stimulation at N20m time for the subject msthesis05. See text for description.
•A
Q'n •
~utllrr•.,
.O
_
P
.3
)r0:
-•aao_ •
-
LW
rr?0
n-law .IQ
0 "IM WS_Mo _W
Qsrta
LOW
a
. w ,ea M f
Said
M ?IWI
-im
....
22.ms
-11-.
111
[lefthemi sphere]
]
hgir[
misph
eht
Digitalfilter:
10- 300Hz.Scale:50f/me, 100
ms.
41P
Nwd~
T- 33,lL7
vaara LI,
•.rru•an lO.
vooe
e,u
IOD
he r--11
. 4000
G.L
Malm
,OO UOo
rr•L M
N20m
A
ci
[righthemisphere]
Digitalfiltr: 1.0-300Hz.Scale:50ff/em,100ms.
Figure 4-19: ECD fit for right (top figure) and left (bottom figure) median nerve
stimulation at N20m time for the subject msthesis06. See text for description.
MEG (max dSPM:5.562668)
EEG (max dSPM:8.166934)
-8
-6
-4
-2
0
2
4
6
8
0
-5
5
EMEG2 (max dSPM:7.5274471
EMEG (max dSPM:7.831937)
IL I
-6
-4
-2
0
2
4
-6
6
-4
EEG (max dSPM:9.414876)
-8
-6
-4
-2
0
IMF
r.(may dsPMu-1
-10
-5
0
2
4
0
2
4
---W,, ý
6
MEG (max dSPM:6.337901)
6
8
-6
o 79LAQ
5
-2
-4
-2
0
2
4
6
EMEG2 (max dSPM:10.861856)
10
-10
-5
0
5
10
Figure 4-20: Spatial distribution of dSPM values for N20m time in subject msthesisO3.
MEG (max dSPM:10.184804)
EEG (max dSPM:9.891609)
-8
-6
-4
-2
0
2
4
6
If~
8
-10
EMEG (max dSPM:15.040542)
-15
-10
-5
5
10
EMEG2 (max dSPM:13.426525)
5
0
0
-5
10
15
-10
-5
0
5
10
MEG (max dSPM:5.033584)
EEG (max dSPM:8.213980)
mw
-8
-6
-4
-2
0
2
4
6
8
EMEG (max dSPM:6.965190)
EMEG2 (max dSPM:6.825184)
If~i
-6
-4
-2
0
2
4
~
6
-6
-4
-2
0
2
4
I
6
Figure 4-21: Spatial distribution of dSPM values for N20m time in subject msthesisO4.
MEG (max dSPM:12.937396)
EEG (max dSPM:8.648503)
-6
-4
-2
0
2
4
6
-10
8
-5
-10
5
0
-6
-4
-2
0
10
2
4
-15
15
-10
-10
-5
0
5
10
-5
0
5
10
15
MEG (max dSPM:11.914299)
6
-10
8
-5
EMEG (max dSPM:15.011344)
-15
5
EMEG2 (max dSPM:15.955421)
FF:1 Imay dRsPU-SR7 •74 1
-8
0
-5
EMEG (max dSPM:17.590126)
-15
M,
"Al
I
-8
0
5
10
EMEG2 (max dSPM:13.614053)
10
15
-10
-5
0
5
10
Figure 4-22: Spatial distribution of dSPM values for N20m time in subject msthesis05.
MEG (max dSPM:1 1.899397)
EEG (max dSPM:9.562730)
-8
-6
-4
-2
0
2
4
6
-10
8
-5
0
-5
5
5
10
ffkl`ý
I
-10
EEG (max dSPM:10.965094)
-5
0
10
5
0
-5
5
10
MI
FMFUg (mavy dPUifln 77Inn7•,
E_-I
-6
-4
-2
0
2
4
0
-5
EMUG (may dSPM9.5786551
-8
-I
MEG (max dSPM:5.855110)
I--10
10
EMEG2 (max dSPM:13.681058)
EMEG (max dSPM:14.995072)
-10
0
6
8
-10
-5
0
5
10
Figure 4-23: Spatial distribution of dSPM values for N20m time in subject msthesis06.
Distance to ECD fit Dosition. dECD rmml
Absolute dSPM values, || cdSPM I
2
4
6
8
10
12
J
14
0
5
10
15
20
25
30
35
40
Figure 4-24: Typical spatial distribution of IjjdSPMII and corresponding dE D. We
want low values of dE CD to coincide in space with high values of IlkdSPMI, so that
source locations close to the ECD position get high dSPM values, and vice versa.
4.3.3
ECD/MNE comparison
The dSPM distributions for the N20m times were then compared with the ECD fits
using two metrics defined as follows:
dsPM
i= argmax I |jX
}
k
DLE
ECD
C
= dF
D
N
SDECD=EC(dcD.
SDECD ..
,k=l
i
IXdSPM||)2
I k
S11 ^dSPM 112
(4.2)
(4.3)
Where :dSPM is the dSPM value for the k-th source location at N20m time and dFC D
is the distance from the i-th location and the ECD fit location at N20m time, see
Fig. 4-24.
These two metrics are analogous to the SDs and DLEs defined in the resolution
matrix analysis. They use the ECD location as gold-standard considered to be true,
and then compute the SD and DLE for the column corresponding to the ECD location
of a hypothetical resolution matrix. An analog of the RI can not be computed, because
we don't observe activity in several locations but only in the zone activated for each
patient at the N20m time.
Results for these metrics can be seen in table 4.1 and 4.1. The trends observed in
the resolution matrix analysis can be again observed in the real data, although there
is more variation caused by a multiplicity of effects:
* The ECD fit is not perfect, the actual activity generating the measure can be
in a location nearby.
* The activity at the N20m time is not perfectly focal.
* The forward models generated contain positioning, registration and approximation errors.
* The SNR is not perfectly charaterized. In particular, is impossible to assess
what is the variance of the source.
In spite of all these effects, the conclusions of the previous theoretical analysis of
the inverse operator did hold for the clinical data.
DLEECD values for each subject are shown in Table 4.2. As expected, there was
no improvement in localization when moving from the MEG case to the EMEG2
case. Actually, an increase of 0.4 mm was observed. When looking at the values in
a subject-by-subject basis, though, it is clear that the reason for the negative result
is the high localization error for subject msthesis04 for all the selections making use
of the EEG sensors (EEG, EMEG, EMEG2). The reason for this might be a faulty
co-registration of the MEG and EEG data, that could already be noticed in the dSPM
estimates themselves as shown in Fig. 4-21: the activity is localized to the occipital
rather than the central area of the cortex, indicating some problem in the EEG data
that is not shown in the MEG-based estimates. Without this problematic acquisition,
the average difference in the DLEECD would be of 38 ym, almost nil. Including the
subject in the computation, the increase in DLEECD is still very small, 0.4mm.
SDECD values for each subject are shown in Table 4.1. Subjects msthesisO3 and
msthesisO3 both had decreased SDECD values. For subject msthesisO5 there was
an increase in SDECD values smaller than the decrease in the other two subjects.
As before, subject msthesis04 showed increased SDECD values for both hemispheres,
consistent with the already discussed DLEECD increase. In average, even including
msthesis04, the SDECD values decreased 2.1 mm when the MEG sensors were replaced
msthesis03
Hemisphere
EEG (mm)
MEG (mm)
EMEG (mm)
EMEG2 (mm)
MEG - EMEG2 (mm)
MEG - EMEG2 (%)
Left
-
-
Right
33.5
41.6
33.7
34.8
6.8
16.4
msthesisO4
msthesis05
Left
42.1
36.1
37.0
39.6
-3.5
-9.8
Left
42.0
36.3
33.6
36.6
-0.3
-0.9
Right
54.8
40.4
43.1
42.9
-2.4
-6.1
Right
43.0
27.8
30.8
31.0
-3.2
-11.5
Average
msthesis06
Left
40.9
42.1
36.6
36.4
5.7
13.5
Right
28.7
44.9
33.1
33.2
11.7
26.1
Left
41.7
38.2
35.7
37.5
0.6
1.6
Right
40.0
38.7
35.2
35.4
3.2
8.4
Both
40.7
38.5
35.4
36.3
2.1
5.5
Table 4.1: SDECD values for each subject at N20m times.
msthesis03
Hemisphere
EEG (mm)
MEG (mm)
EMEG (mm)
EMEG2 (mm)
MEG - EMEG2 (mm)
MEG - EMEG2 (%)
Left
-
Right
11.4
3.1
3.1
3.1
0.0
0.0
msthesisO~
Left
14.2
7.9
10.5
10.5
-2.6
-32.7
Right
68.2
11.3
5.9
11.3
0.0
0.0
msthesis05
Left
21.2
17.5
17.5
17.5
0.0
0.0
Right
13.2
5.0
10.0
10.0
-5.0
-99.6
Average
msthesis06
Left
11.6
12.8
7.3
12.8
0.0
0.0
Right
18.0
6.6
7.3
1.8
4.8
72.6
Left
15.7
12.8
11.8
13.6
-0.9
-6.8
Right
27.7
6.5
6.6
6.5
0.0
-0.7
Both
22.6
9.2
8.8
9.6
-0.4
-4.3
Table 4.2: DLEECD values for each subject at N20m times.
with EEG ones. Without including msthesis04, the average decrease would be of
4.2 mm, a 10% decrease on the average SDECD for the MEG case.
Chapter 5
Conclusions and future work
5.1
Conclusions
The inclusion of EEG channels in MEG-based minimum e2-norm current estimates
was analytically characterized using the eigenvalue spectra of the associated forward
models and the resolution matrices resulting of the combination of inverse operator
and forward model for four different clinical subjects. The E/MEG inverse operator
showed slower decay in the eigenvalue spectra, consistently better values for both
RI and SD, and no difference in DLEs for the four clinical subjects analyzed. No
inter-subject difference was noticed in the figures of merit in spite of differences in
geometry and source noise covariance matrices. These observations strongly suggest
an increase in estimation accuracy when including the EEG measurements in the
computation of the minimum 12-norm inverse operator.
Then, we analyzed the clinical datasets, using the N20m focal activation times
as focal events, and the ECD fits made by an expert operator as the position of the
source. Only well-defined N20m events were used for the analysis. The estimates
using the E/MEG data were always as good or better than the MEG ones, as measured
with metrics analog to the ones introduced in the theoretical analysis: The E/MEG
estimates showed less spread and similar localization accuracy when reconstructing
focal events even in the clinical case, where the number of approximation errors
substantially increase.
5.2
Future work
The encouraging results presented in this thesis suggest several possible extensions.
Some that would be of considerable interest would be:
* Repeat the study with more subjects to better characterize the behavior of the
studied metrics of resolution. As seen in this thesis, when only four subjects
are included, the results of having one misbehaving acquisition can be quite
noticeable in the outcome (see section 4.3.3).
* Study, the effect of varying the conductivity values of each compartment in the
accuracy of the MNE results as measured by the {SDECD, DLEECD} criteria.
* Study, the effect of different SNR values in the eigenvalue spectra and the resolution matrix, and validate this against other physiologically occurring focal
activation patterns, such as the N30p in median nerve stimulation, or subjects
that present different degrees of activation for the N20m event.
Bibliography
F. Babiloni, F. Carducci, F. Cincotti, C. Del Gratta, V. Pizzella, G.L. Romani, P.M.
Rossini, F. Tecchio, and C. Babiloni. Linear inverse source estimate of combined
EEG and MEG data related to voluntary movements. Human Brain Mapping, 14
(4):197-209, 2001.
F. Babiloni, C. Babiloni, F. Carducci, G.L. Romani, P.M. Rossini, L.M. Angelone,
and F. Cincotti. Multimodal integration of EEG and MEG data: A simulation
study with variable signal-to-noise ratio and number of sensors. Human Brain
Mapping, 22(1):52-62, 2004.
S. Baillet, J.C. Mosher, and R.M. Leahy. Electromagnetic brain mapping. Signal
Processing Magazine, IEEE, 18(6):14-30, 2001.
C. Baumgartner, E. Pataraia, G. Lindinger, and L. Deecke. Magnetoencephalography
in focal epilepsy. Epilepsia, 41(Suppl 3):S39-S47, 2000.
S. Bechstein, F. Petsche, M. Scheiner, D. Drung, F. Thiel, A. Schnabel, and
T. Schurig. Digitally controlled high-performance DC SQUID readout electronics for a 304-channel vector magnetometer. Journal of Physics: Conference Series,
43:1266-1269, 2006.
D. Cohen. Magnetoencephalography: Evidence of magnetic fields produced by alpharhythm currents. Science, 161(3843):784, 1968. pmid:68350989.
D. Cohen. Large-volume conventional magnetic shields. Rev. Phys. Appl, 5:53-58,
1970.
D. Cohen. Magnetoencephalography: Detection of the brain's electrical activity with
a superconducting magnetometer. Science, 175(4022):664, 1972.
D. Cohen and E. Halgren. Magnetoencephalography (neuromagnetism). Encyclopedia
of Neuroscience, pages 1-7, 2003.
D. Cohen, B.N. Cuffin, K. Yunokuchi, R. Maniewski, C. Purcell, G.R. Cosgrove,
J. Ives, J.G. Kennedy, and D.L. Schomer. MEG versus EEG localization test using
implanted sources in the human brain. Annals of Neurology, 28(6):811-817, Dec
1990.
R.P. Crease. Images of conflict: MEG vs. EEG. Science, 253(5018):374-375, Jul 26
1991.
J.P. Culver, V. Ntziachristos, M.J. Holboke, and A.G. Yodh. Optimization of optode
arrangements for diffuse optical tomography: A singular-value analysis. Opt.Lett,
26(10):701-703, 2001.
A.M. Dale, B. Fischl, and M.I. Sereno. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neurolmage, 9(2):179-194, 1999.
A.M. Dale, A.K. Liu, B.R. Fischl, R.L. Buckner, J.W. Belliveau, J.D. Lewine, and
E. Halgren. Dynamic Statistical Parametric Mapping combining fMRI and MEG
for high-resolution imaging of cortical activity. Neuron, 26(1):55-67, 2000.
R.G. de Peralta Menendez, O. Hauk, S.G. Andino, H. Vogt, and C. Michel. Linear
inverse solutions with optimal resolution kernels applied to electromagnetic tomography. Hum.Brain Mapp, 5:454-467, 1997.
B. Fischl, M.I. Sereno, and A.M. Dale. Cortical surface-based analysis II: Inflation,
flattening, and a surface-based coordinate system. Neurolmage, 9:195-207, 1999.
B. Fischl, A. Liu, and A.M. Dale. Automated manifold surgery: Constructing geometrically accurate andtopologically correct models of the human cerebral cortex.
Medical Imaging, IEEE Transactions on, 20(1):70-80, 2001.
N. Forss. Magnetoencephalography (MEG) in epilepsy surgery. Acta neurochirurgica.Supplement, 68:81-84, 1997.
D.B. Geselowitz. On bioelectric potentials in an inhomogeneous volume conductor.
Biophysical Journal, 7(1):1, 1967.
D.B. Geselowitz. On the magnetic field generated outside an inhomogeneous volume
conductor by internal current sources. Magnetics, IEEE Transactions on, 6(2):
346-347, 1970.
P. Gloor. Neuronal generators and the problem of localization in electroencephalography: Application of volume conductor theory to electroencephalography. Journal
of clinical neurophysiology : official publication of the American Electroencephalographic Society, 2(4):327-354, Oct 1985.
E.E. Graves, J.P. Culver, J. Ripoll, R. Weissleder, and V. Ntziachristos. Singularvalue analysis and optimization of experimental parameters in fluorescence molecular tomography. Journal of the Optical Society of America A, 21(2):231-241, 2004.
M.S. Hiimnilinen and J. Sarvas. Realistic conductivity geometry model of the human
head for interpretation of neuromagnetic data. IEEE transactions on bio-medical
engineering, 36(2):165-171, Feb 1989.
M.S. Hdmili5inen, R. Hari, R.J. Ilmoniemi, J. Knuutila, and O.V. Lounasmaa.
Magnetoencephalography-Theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics, 65(2):413-497,
1993.
A. Hillebrand and G.R. Barnes. A quantitative assessment of the sensitivity of wholehead MEG to activity in the adult human cortex. Neuroimage, 16(3 Pt 1):638-50,
2002.
B. Horwitz and D. Poeppel. How can EEG/MEG and fMRI/PET data be combined?
Human brain mapping, 17(1):1-3, 2002.
R. Kakigi. Somatosensory evoked magnetic fields following median nerve stimulation.
Neurosci Res, 20(2):165-74, 1994.
F.H. Lin, J.W. Belliveau, A.M. Dale, and M.S. Hiimiiliinen. Distributed current
estimates using cortical orientation constraints. Human brain mapping, 27(1):1-13,
Jan 2006a.
F.H. Lin, T. Witzel, S.P. Ahlfors, S.M. Stufflebeam, J.W. Belliveau, and M.S.
Hdimi1iinen. Assessing and improving the spatial accuracy in MEG source localization by depth-weighted minimum-norm estimates. Neurolmage, 31(1):160-171,
May 15 2006b.
J. Malmivuo, V. Suihko, and H. Eskola. Sensitivity distributions of EEG and MEG
measurements. IEEE transactionson bio-medical engineering, 44(3):196-208, Mar
1997.
A.N.
Mamelak,
N.
Lopez,
M.
Akhtari,
and
W.W.
Sutherling.
Magnetoencephalography-directed surgery in patients with neocortical epilepsy.
Journal of neurosurgery, 97(4):865-873, Oct 2002.
W. Menke. Geophysical Data Analysis: Discrete Inverse Theory. Elsevier, 1989.
J.C. Mosher, R.M. Leahy, and P.S. Lewis. EEG and MEG: forward solutions for
inverse methods. Biomedical Engineering, IEEE Transactions on, 46(3):245-259,
1999.
S. Murakami and Y. Okada. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. The Journal of physiology,
575(Pt 3):925-936, Sep 15 2006. PUBM: Print-Electronic; DEP: 20060413; JID:
0266262; 2006/04/13 [aheadofprint]; ppublish.
P.L. Nunez and R.B. Silberstein. On the relationship of synaptic activity to macroscopic measurements: Does co-registration of EEG with fMRI make sense? Brain
topography, 13(2):79-96, Winter 2000.
T.F. Oostendorp and A. van Oosterom. Source parameter estimation in inhomogeneous volume conductors of arbitrary shape. IEEE transactions on bio-medical
engineering, 36(3):382-391, Mar 1989.
R.D. Pascual-Marqui, C.M. Michel, and D. Lehmann. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain.
Internationaljournal of psychophysiology : official journal of the InternationalOrganization of Psychophysiology, 18(1):49-65, Oct 1994.
A. Schnabel, M. Burghoff, S. Hartwig, F. Petsche, U. Steinhoff, D. Drung, and
H. Koch. A sensor configuration for a 304 SQUID vector magnetometer. Neurology and Clinical Neurophysiology, 2004:70, 2004.
S. Taulu, J. Simola, M. Kajola, E.N. Oy, and F. Helsinki. MEG Recordings of DC
Fields Using the Signal Space Separation Method (SSS). Neurology and Clinical
neurophysiology, 2004:35, 2004.
A.N. Tikhonov. Solution of incorrectly formulated problems and the regularization
method. Sov.Math., Dokl., 5:1035-1038, 1963.
M.A. Uusitalo and R.J. Ilmoniemi. Signal-space projection method for separating
MEG or EEG into components. Medical biological engineering computing, 35(2):
135-140, Mar 1997.
H. von Helmholtz. Uber einige Gesetze der Verbeitung elektrischer Strdme in koperlichen Leitern, mit Anwendung auf die thierisch-elektrischen Versuche. Ann. Phys.
Chem, 89:211-233, 1853.
J.Z. Wang, S.J. Williamson, and L. Kaufman. Magnetic source images determined by
a lead-field analysis: The unique minimum-norm least-squares estimation. IEEE
transactionson bio-medical engineering, 39(7):665-675, Jul 1992.
J.Z. Wang, S.J. Williamson, and L. Kaufman. Magnetic source imaging based on
the minimum-norm least-squares inverse. Brain topography, 5(4):365-371, Summer
1993.
J.P. Wikswo, A. Gevins, and S.J. Williamson. The future of the EEG and MEG.
Electroencephalographyand clinical neurophysiology, 87(1):1-9, Jul 1993.
H. Yoshinaga, T. Nakahori, Y. Ohtsuka, E. Oka, Y. Kitamura, H. Kiriyama, K. Kinugasa, K. Miyamoto, and T. Hoshida. Benefit of simultaneous recording of EEG
and MEG in dipole localization. Epilepsia, 43(8):924-928, Aug 2002.
J.E. Zimmerman. SQUID instruments and shielding for low-level magnetic measurements. Journal of Applied Physics, 48:702, 1977.