dQ dS T
advertisement

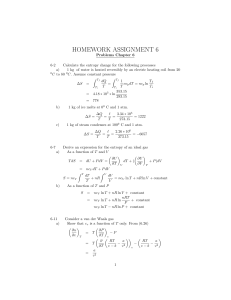
VIII. Entropy 1. Macroscopic definition of entropy dQ dS T for a reversible process at constant T dQ is path dependent dS is path independent S is additive function S is function of state 2.The second law of thermodynamics dQ dS T for any process (including irreversible) For closed, isolated system (dQ = 0): dS 0 any reversible cycle: ΔS=0 any irreversible process in closed isolated system: ΔS>0 Example 1: This P-V diagram represents a system consisting of a fixed amount of ideal gas that undergoes three different processes in going from state A to state B. Rank the change in entropy of the system for each process. P State A 2 ΔS1 = ΔS2 = ΔS3 = SB - SA 3 I State B V The same as: ΔT1 = ΔT2 = ΔT3 = TB - TA ΔU1 = ΔU2 = ΔU3 = UB - UA Example 2: Which of the following statements is false? A. The change of entropy in a cyclic process is zero B. The change of entropy for any adiabatic process is zero C. The change of entropy for any isothermal process is zero D. Entropy for a closed, isolated system is constant E. Entropy of a system can decrease Example 3: 50.0 kg of water is converted to ice at 0.0ºC. What is the change in entropy of water? m = 85.0 kg T = 0.0ºC L = 334*103 J/kg ΔS - ? Q mL S T T 50.0kg 334 103 J / kg S 61kJ / K 273K Example 4: The isolated system is 50.0 kg of ice at 0 ˚C plus the temperature reservoir at slightly above 0 ˚C that is used to melt the ice. What is the change in entropy of the system when the ice is melted ? Solution: •The system is isolated: Qice = -Qreservoir •The ice and the reservoir are at almost the same temperature: Tic = Treservoir=T •The system consists of both the ice and the temperature reservoir: S system S ice S reservoir Qice Qreservoir Qice Qice 0 T T T T ΔS = 0. Therefore the process is reversible! 3. Entropy of ideal gas dQ dU dW nCV dT nRTdV V dT dV dS nCV nR T T T T V dU nCV dT dW PdV nRTdV V T2 V2 S S 2 S1 nCV ln nR ln T1 V1 3a. Free expansion of ideal gas •A given amount of an ideal gas undergo free expansion from volumeV1 to V2 •Gas forms a closed and thermally isolated system. Because of that: W=0, Q=0 ΔU=0 ΔT=0 T1 = T2 •General equation for the entropy change of any ideal gas: T2 V2 S nCV ln nR ln T1 V1 V2 S nR ln 0 V1 Closed and thermally isolated system with ΔS>0: the process is irreversible! Example: Two moles of an ideal gas undergo an adiabatic free expansion from V1 = 1.00 L to V2 = e1.00 L = 2.72 L. (The gas is an isolated system). The change in the entropy of the gas is __ J/K. S 2.00mol 8.31J / mol K ln 2.72 16.6J / K 0 The process is irreversible! 3b. Reversible isothermal expansion of ideal gas Ideal Gas : T V Sgas nCV ln 2 nR ln 2 T1 V1 Reversible Isothermal Expansion (V2 V1 ) T2 T1 T , so ln(T2 / T1 ) 0 and Sgas V2 nR ln V 1 But Sgas 0, so doesn't this mean that the process is not reversible? Answer: No. One must consider only isolated systems to answer that. Isolated system = gas + T reservoir (heat source) The heat absorbed by the gas is V Qgas Wgas nRT ln 2 . V1 The change in entropy of the gas is Sgas V nR ln 2 , which agrees T V1 Qgas with our previous result. The heat absorbed by the reservoir is Qreservoir Qgas . The change in entropy of the system is Qgas Qgas Ssystem Sgas Sreservoir 0. T T Thus the process is reversible. 3c. Reversible Adiabatic Expansion of Ideal Gas Ideal Gas : Sgas For an ideal gas, T V nCV ln 2 nR ln 2 T V 1 (1) 1 Reversible Adiabatic Expansion (V2 V1 ) Here the gas is an isolated system (W Q 0). Therefore we expect Sgas 0. Adiabatic: TV 1 1 constant = T1V1 V T2 V1 1 1 T1 V2 V2 1 1 V 1 2 V1 T V ln 2 (1)ln 2 T V 1 1 T2V2 1 Cp CV 1 CV R . Therefore, CV CV R R 1 and CV CV , so CV ( 1) R. (3) Substituting Eq. (3) into (2) gives and Sgas 0 . 1 1 V ( 1)ln 2 . V1 Substituting this expression into Eq. (1) gives V Sgas n CV ( 1) R ln 2 . V1 (2) Thus the process is indeed reversible. 4. Microscopic interpretation of entropy 2 S k B ln 2 k B ln 1 k B ln 1 S kB ln number of possible microscopic states Example 6: A thermally insulated box is divided by a partition into to compartments, each having volume V. Initially one compartment contains n moles of an ideal gas at temperature T, and other compartment is evacuated. We then break the partition, and gas expands to fill both compartments. What is the entropy change in this freeexpansion process? V2 2V1 n2 n1 n S ? V1 V2 2V1 N nN A 1 2 2N1 kB N A R 2 S k B ln k B ln 2 N k B N ln 2 nR ln 2 1